Physical, Chemical, and Immunohistochemical Investigation of the Damage to Salivary Glands in a Model of Intoxication with Aluminium Citrate
Abstract
:1. Introduction
2. Methods
2.1. Experimental Animals
2.2. Aluminum Citrate Intoxication and Experimental Groups
2.3. Surgical Procedures
2.4. Sample Digestion and Quantification of Al by Graphite Furnace Atomic Absorption Spectrometry (GF AAS)
2.4.1. Instrumentation
| Samples | (Al Levels—ppm) ± Standard Deviation |
|---|---|
| Submandibular (control) | 278.7 ± 32.2 |
| Submandibular exposed to Al | 397.3 ± 15.3 |
| Parotid (control) | 202.2 ± 0.1 |
| Parotid exposed to Al | 274.9 ± 0.1 |
2.4.2. Reagents and Solutions
2.4.3. Procedures
2.4.4. Evaluation of the Accuracy of the Procedure
2.5. Perfusion, Histological Processing and Immunohistochemical Analysis
2.6. Statistical Analysis
3. Results
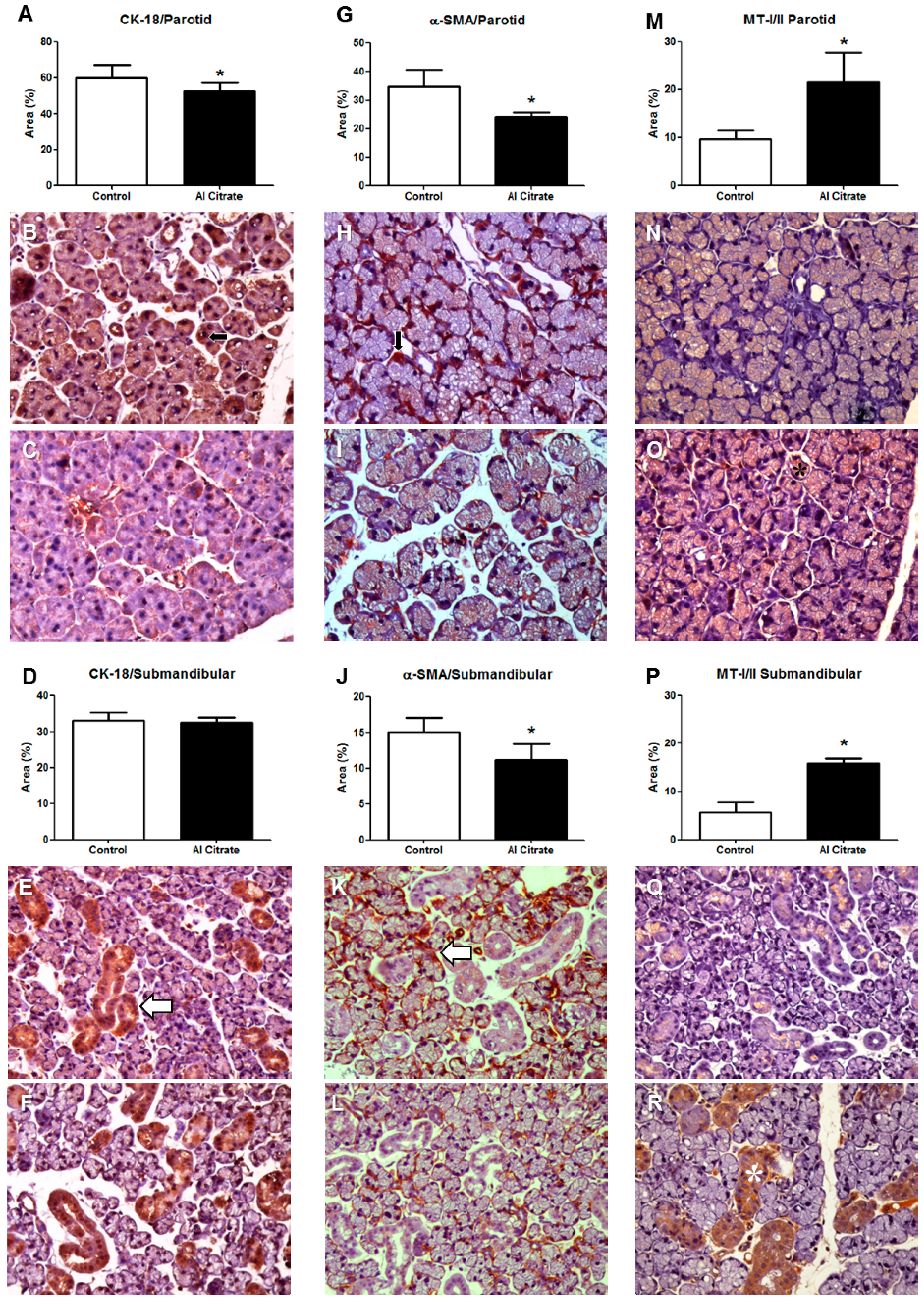
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Exley, C. Human exposure to aluminium. Environ. Sci. Processes Impacts 2013, 15, 1807–1816. [Google Scholar] [CrossRef]
- Lévesque, L.; Mizzen, C.A.; McLachlan, D.R.; Fraser, P.E. Ligand specific effects on aluminum incorporation and toxicity in neurons and astrocytes. Brain Res. 2000, 877, 191–202. [Google Scholar] [CrossRef]
- Nayak, P. Aluminum: Impacts and disease. Environ. Res. 2002, 89, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Polizzi, S.; Ferrara, M.; Bugiani, M.; Barbero, D.; Baccolo, T. Aluminium and iron air pollution near an iron casting and aluminium foundry in Turin district (Italy). J. Inorg. Biochem. 2007, 101, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Ščančar, J.; Milačič, R. Aluminium speciation in environmental samples: A review. Anal. Bioanal. Chem. 2006, 386, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Vike, E. Air-pollutant dispersal patterns and vegetation damage in the vicinity of three aluminium smelters in Norway. Sci. Total Environ. 1999, 236, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Kiss, T. Interaction of aluminum with biomolecules—Any relevance to Alzheimer’s disease? Arch. Gerontol. Geriatr. 1995, 21, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Barreto, F.C.; Araujo, S.M. Aluminium intoxication in chronic kidney disease. J. Bras. Nefrol. 2011, 33, 21–25. [Google Scholar] [PubMed]
- Centers for Disease Control and Prevention (CDC). Elevated serum aluminum levels in hemodialysis patients associated with use of electric pumps—Wyoming, 2007. MMWR Morb. Mortal. Wkly. Rep. 2008, 57, 689–691. [Google Scholar]
- Davis, K.; Aslam; Pejovic-Milic, A.; Chettle, D.R. In vivo measurement of bone aluminum in population living in southern Ontario, Canada. Med. Phys. 2008, 35, 5115–5123. [Google Scholar] [CrossRef]
- Peto, M.V. Aluminium and iron in humans: Bioaccumulation, pathology, and removal. Rejuvenation Res. 2010, 13, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Sargazi, M.; Roberts, N.B.; Shenkin, A. In-vitro studies of aluminium-induced toxicity on kidney proximal tubular cells. J. Inorg. Biochem. 2001, 87, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Bagavant, H.; Nandula, S.R.; Kaplonek, P.; Rybakowska, P.D.; Deshmukh, U.S. Alum, an aluminum-based adjuvant, induces Sjogren’s syndrome-like disorder in mice. Clin Exp. Rheumatol. 2014, 32, 251–255. [Google Scholar] [PubMed]
- Darbre, P.D.; Mannello, F.; Exley, C. Aluminium and breast cancer: Sources of exposure, tissue measurements and mechanisms of toxicological actions on breast biology. J. Inorg. Biochem. 2013, 128, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Suarez, I.; Naves, M.; Diaz-Corte, C.; Fernandez-Martin, J.L.; Menendez-Rodriguez, P.; Cannata-Andia, J.B. Effect of aluminium on calcium-sensing receptor expression, proliferation, and apoptosis of parathyroid glands from rats with chronic renal failure. Kidney Int. Suppl. 2003, 63, S39–S43. [Google Scholar] [CrossRef]
- Konduracka, E.; Krzemieniecki, K.; Gajos, G. Relationship between everyday use cosmetics and female breast cancer. Pol. Arch. Med. Wewn. 2014, 124, 264–269. [Google Scholar]
- Mannello, F.; Ligi, D.; Canale, M. Aluminium, carbonyls and cytokines in human nipple aspirate fluids: Possible relationship between inflammation, oxidative stress and breast cancer microenvironment. J. Inorg. Biochem. 2013, 128, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Smans, K.A.; D’Haese, P.C.; Van Landeghem, G.F.; Andries, L.J.; Lamberts, L.V.; Hendy, G.N.; De Broe, M.E. Transferrin-mediated uptake of aluminium by human parathyroid cells results in reduced parathyroid hormone secretion. Nephrol. Dial. Transplant. 2000, 15, 1328–1336. [Google Scholar] [CrossRef]
- Denisov, A.B. Morphological and functional state of major salivary glands under conditions of aluminum chloride excess in drinking water. Bull. Exp. Biol. Med. 2009, 148, 940–942. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Iglesias, S.; Soto-Otero, R.; Iglesias-Gonzalez, J.; Barciela-Alonso, M.C.; Bermejo-Barrera, P.; Mendez-Alvarez, E. Analysis of brain regional distribution of aluminium in rats via oral and intraperitoneal administration. J. Trace Elem. Med. Biol. 2007, 21, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Rondon-Barragán, I.S.; Ramírez-Duarte, W.F.; Barato, P.; Eslava-Mocha, P.R. Importancia del ciclo biogeoquímico del aluminio (Al) con relación con la acidez de los suelos en la producción piscícola y la salud pública ¿cuál sería el caso de la Orinoquia? Revista ORINOQUIA 2007, 11, 81–94. [Google Scholar]
- Zatta, P.F.; Cervellin, D.; Zambenedetti, P. Effects of the aluminium speciation on the morphology of rabbit erythrocytes: A toxicological model. Toxicol. In Vitro 1998, 12, 287–293. [Google Scholar] [CrossRef] [PubMed]
- BAST, C.B. Toxicity Summary for Aluminum; Oak Ridge Reservation Environmental Restoration Program: Oak Ridge, TN, USA, 1993. [Google Scholar]
- Bondy, S.C. The neurotoxicity of environmental aluminum is still an issue. Neuro Toxicol. 2010, 31, 575–581. [Google Scholar]
- Fimreite, N.; Hansen, O.Ø.; Pettersen, H.C. Aluminum concentrations in selected foods prepared in aluminum cookware, and its implications for human health. Bull. Environ. Contam. Toxicol. 1997, 58, 1–7. [Google Scholar] [CrossRef]
- Roberts, N.B.; Clough, A.; Bellia, J.P.; Kim, J.Y. Increased absorption of aluminium from a normal dietary intake in dementia. J. Inorg. Biochem. 1998, 69, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Siany da Silva, L. Efeitos do Estradiol Sobre Alterações Provocadas Por Alumínio Nos Níveis de Peptídeo Natriurético Atrial e Nitrato em Ratas Castradas. Available online: www.bc.ufpa.br/Portal/DTC/Ciencias_Biologicas/Ciencias_Biologicas_2003/LIBERAL.htm (accessed on 24 July 2014).
- Barreto, F.C.; Holanda Almeida Araújo, S.M. Intoxicação alumínica na DRC. J. Bras. Nefrol. 2008, 30, 18–22. [Google Scholar]
- Martin, R.B. Aluminum: A neurotoxic product of acid rain. Acc. Chem. Res. 1994, 27, 204–210. [Google Scholar] [CrossRef]
- Tapparo, A.; Solda, L.; Bombi, G.G.; Zambenedetti, P.; Zatta, P.F.; Bertani, R.; Corain, B. Analytical validation of a general protocol for the preparation of dose-controlled solutions in aluminum toxicology. Analyst 1995, 120, 2425–2429. [Google Scholar] [CrossRef] [PubMed]
- Yokel, R.A.; Allen, D.D.; Ackley, D.C. The distribution of aluminum into and out of the brain. J. Inorg. Biochem. 1999, 76, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Cunat, L.; Lanhers, M.C.; Joyeux, M.; Burnel, D. Bioavailability and intestinal absorption of aluminum in rats: Effects of aluminum compounds and some dietary constituents. Biological trace element research 2000, 76, 31–55. [Google Scholar] [CrossRef]
- Martin, R.B. The chemistry of aluminum as related to biology and medicine. Clin. Chem. 1986, 32, 1797–1806. [Google Scholar] [PubMed]
- Meshitsuka, S.; Aremu, D.A. 13C heteronuclear NMR studies of the interaction of cultured neurons and astrocytes and aluminum blockade of the preferential release of citrate from astrocytes. J. Biol. Inorg. Chem. 2008, 13, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.B.; Day, J.P.; Taylor, G.A.; Ferrier, I.N.; Fifield, L.K.; Edwardson, J.A. Absorption of aluminium-26 in Alzheimer’s disease, measured using accelerator mass spectrometry. Dement. Geriatr. Cogn. Disord. 2000, 11, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.G.; Weiss, L.M. Keratin expression in human tissues and neoplasms. Histopathology 2002, 40, 403–439. [Google Scholar] [CrossRef] [PubMed]
- De Azevedo, F.A.; da Matta Chasin, A.A. Metais: Gerenciamento da Toxicidade; Atheneu Editora: São Paulo, Brazil, 2003. [Google Scholar]
- Domingues, M.G.; Jaeger, M.M.M.; Araujo, V.C.; Araujo, N.S. Expression of cytokeratins in human enamel organ. Eur. J. Oral Sci. 2000, 108, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Thirumoorthy, N.; Shyam Sunder, A.; Manisenthil Kumar, K.T.; Senthil Kumar, M.; Ganesh, G.N.K.; Chatterjee, M. A review of metallothionein isoforms and their role in pathophysiology. World J. Surg. Oncol. 2011, 9. [Google Scholar] [CrossRef]
- Vasak, M.; Meloni, G. Chemistry and biology of mammalian metallothioneins. J. Boil. Inorg. Chem. 2011, 16, 1067–1078. [Google Scholar] [CrossRef]
- Babula, P.; Masarik, M.; Adam, V.; Eckschlager, T.; Stiborova, M.; Trnkova, L.; Skutkova, H.; Provaznik, I.; Hubalek, J.; Kizek, R. Mammalian metallothioneins: Properties and functions. Metallomics 2012, 4, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Higashimoto, M.; Isoyama, N.; Ishibashi, S.; Inoue, M.; Takiguchi, M.; Suzuki, S.; Ohnishi, Y.; Sato, M. Tissue-dependent preventive effect of metallothionein against DNA damage in dyslipidemic mice under repeated stresses of fasting or restraint. Life Sci. 2009, 84, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Cherian, M.G.; Jayasurya, A.; Bay, B.H. Metallothioneins in human tumors and potential roles in carcinogenesis. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2003, 533, 201–209. [Google Scholar] [CrossRef]
- Pedersen, M.Ø.; Larsen, A.; Stoltenberg, M.; Penkowa, M. The role of metallothionein in oncogenesis and cancer prognosis. Prog. Histochem. Cytochem. 2009, 44, 29–64. [Google Scholar] [CrossRef] [PubMed]
- Bharathi; Vasudevaraju, P.; Govindaraju, M.; Palanisamy, A.P.; Sambamurti, K.; Rao, K.S. Molecular toxicity of aluminium in relation to neurodegeneration. Indian J. Med. Res. 2008, 128, 545–556. [Google Scholar]
- Han, S.; Lemire, J.; Appanna, V.P.; Auger, C.; Castonguay, Z.; Appanna, V.D. How aluminum, an intracellular ROS generator promotes hepatic and neurological diseases: The metabolic tale. Cell. Biol. Toxicol. 2013, 29, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Mahieu, S.; Contini Mdel, C.; Gonzalez, M.; Millen, N. Melatonin reduces oxidative damage induced by aluminium in rat kidney. Toxicol. Lett. 2009, 190, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Singla, N.; Dhawan, D.K. Zinc protection against aluminium induced altered lipid profile and membrane integrity. Food Chem. Toxicol. 2013, 55, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Singla, N.; Dhawan, D.K. Zinc modulates aluminium-induced oxidative stress and cellular injury in rat brain. Metallomics. 2014, 6, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.O.; Kroncke, K.D.; Buchczyk, D.P.; Sies, H. Role of copper, zinc, selenium and tellurium in the cellular defense against oxidative and nitrosative stress. The Journal of nutrition 2003, 133, 1448S–1451S. [Google Scholar] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, N.M.M.d.; Correa, R.S.; Júnior, I.S.M.; Figueiredo, A.J.R.; Vilhena, K.F.B.; Farias-Junior, P.M.A.; Teixeira, F.B.; Ferreira, N.M.M.; Pereira-Júnior, J.B.; Dantas, K.D.G.F.; et al. Physical, Chemical, and Immunohistochemical Investigation of the Damage to Salivary Glands in a Model of Intoxication with Aluminium Citrate. Int. J. Environ. Res. Public Health 2014, 11, 12429-12440. https://doi.org/10.3390/ijerph111212429
Costa NMMd, Correa RS, Júnior ISM, Figueiredo AJR, Vilhena KFB, Farias-Junior PMA, Teixeira FB, Ferreira NMM, Pereira-Júnior JB, Dantas KDGF, et al. Physical, Chemical, and Immunohistochemical Investigation of the Damage to Salivary Glands in a Model of Intoxication with Aluminium Citrate. International Journal of Environmental Research and Public Health. 2014; 11(12):12429-12440. https://doi.org/10.3390/ijerph111212429
Chicago/Turabian StyleCosta, Natacha M. M. da, Russell S. Correa, Ismael S. M. Júnior, Adilson J. R. Figueiredo, Kelly F. B. Vilhena, Paulo M. A. Farias-Junior, Francisco B. Teixeira, Nayana M. M. Ferreira, João B. Pereira-Júnior, Kelly Das Graças F. Dantas, and et al. 2014. "Physical, Chemical, and Immunohistochemical Investigation of the Damage to Salivary Glands in a Model of Intoxication with Aluminium Citrate" International Journal of Environmental Research and Public Health 11, no. 12: 12429-12440. https://doi.org/10.3390/ijerph111212429
APA StyleCosta, N. M. M. d., Correa, R. S., Júnior, I. S. M., Figueiredo, A. J. R., Vilhena, K. F. B., Farias-Junior, P. M. A., Teixeira, F. B., Ferreira, N. M. M., Pereira-Júnior, J. B., Dantas, K. D. G. F., Silva, M. C. F. d., Silva-Junior, A. F., Alves-Junior, S. D. M., Pinheiro, J. D. J. V., & Lima, R. R. (2014). Physical, Chemical, and Immunohistochemical Investigation of the Damage to Salivary Glands in a Model of Intoxication with Aluminium Citrate. International Journal of Environmental Research and Public Health, 11(12), 12429-12440. https://doi.org/10.3390/ijerph111212429







