Patient-Reported Outcome Measures and Risk Factors in a Quality Registry: A Basis for More Patient-Centered Diabetes Care in Sweden
Abstract
:1. Introduction
1.1. Objectives
- To develop a questionnaire so that patients' abilities and their judgments of diabetes care can be measured on a scale suitable for detecting and quantifying change.
- To describe a Swedish diabetes patient sample in terms of their abilities and judgments together with registry data on their risk factors, and to study associations between different patient abilities, judgments and risk factors.
- To characterize groups of patients with low abilities, low judgments, or high risk factor levels; namely, groups with a need for improvement. The intention here was to illustrate how the questionnaire can be used in practice, and to see what groups could be identified using ad hoc levels of abilities, judgments, or risk factors. In practice, detecting such patients might trigger some kind of response in the form of an intervention. In the following, we refer to these levels as ad hoc response levels.
1.2. Previous Work
2. Methods
- Diabetes self-management or self-care ability (items 1–4): the patients are able to manage their diabetes treatment, or take any other necessary actions, by themselves. Patients with a good ability should be less limited by diabetes, and thus be more able to live the life they desire. This is an important determinant of diabetes-related quality of life [10,11,21,22]. Poor self-management has also been found to be associated with depression [23,24].
- Ability to carry out daily activities such as social activities (items 29–30) and work-related activities (items 26–28). A good ability indicates a well-functioning patient who has overcome any limitations imposed by the disease, which is an important determinant of good health-related quality of life [25,26,27,28]. Further, the ability to carry out work-related activities is a determinant of costs from a societal perspective as it affects costs of lost production due to illness [29].
- The quality of the service provided (items 9–10, 19–22); for example, did the staff give the patient a good reception? Did they understand the patient's situation? This would affect the patient’s confidence in healthcare and their compliance to treatment and lifestyle advice. Good communication should also make healthcare receptive to the individual needs of the patient.
- The quality of the information given by the staff (items 11–14). The visit to healthcare should be an important source of diabetes-related information for the patient. Poorly informed patients may do worse, as described above.
- Access to physicians and nurses (items 5–8). Is it easy for the patients to get an appointment with their physician or diabetes nurse when they wish? A timely response to any problem that occurs should be beneficial for the patient.
- Involvement in decisions regarding diabetes care (items 15–18), for example regarding medical treatment or diabetes self-care. This should build up the patient’s confidence in healthcare and could be a cornerstone of developing their self-management ability.
2.1. Patient Questionnaire
2.2. IRT Model
2.3. Methods for Analyzing Outcome Measures
3. Material
| Main Study Sample | Validation Sample | |||
|---|---|---|---|---|
| Type 1 diabetes | Type 2 diabetes | Type 1 diabetes | Type 2 diabetes | |
| Age, years | 49 (15, 18–80) | 66 (10, 21–80) | 50 (16, 18–90) | 66 (12, 21–95) |
| Age at diagnosis, years | 25 (16, 0–76) | 56 (11, 5–79) | 26 (17, 0–81) | 54 (14, 5–89) |
| Diabetes duration, years | 24 (15, 0–66) | 10 (8, 0–54) | 24 (15, 0–71) | 12 (10, 0–71) |
| Male / female | 50% / 50% | 56% / 44% | 50% / 50% | 57% / 43% |
| HbA1c, mmol/mol | 63 (14, 32–136) | 55 (14, 26–139) | 63 (13, 27–124) | 58 (14, 28–133) |
| Systolic blood pressure, mmHg | 128 (15, 90–185) | 137 (17, 85–210) | 127 (16, 85–200) | 134 (16, 80–206) |
| LDL cholesterol, mmol/l | 2.5 (0.8, 0.7–5.2) | 2.6 (0.9, 0.5–6.6) | 2.7 (0.7, 0.5–6.2) | 2.6 (0.9, 0.5–6.2) |
| Number of patients | 1 124 | 1 792 | 1 656 | 1 431 |
4. Results
4.1. IRT Scales
- Self-Management Skills (items 1,2): the patient's ability to self-manage diabetes.
- Self-Management Ability (3,4): the patient's emotional ability to cope with diabetes.
- Sense of Security (24, 25): the patient’s lack of worries related to diabetes comorbidities.
- Social Activities (29, 30): the patient’s ability to carry out social activities.
- Work Activities (26–28): the patient’s ability to carry out work-related activities.
- Access (5–8): the patient’s experience of access to healthcare staff.
- Service & Information (9–14, 19–22): the patient’s experience of healthcare service and information provided.
- Involvement (15–18): the patient’s judgment of how well they could participate in decisions regarding diabetes treatment and care.
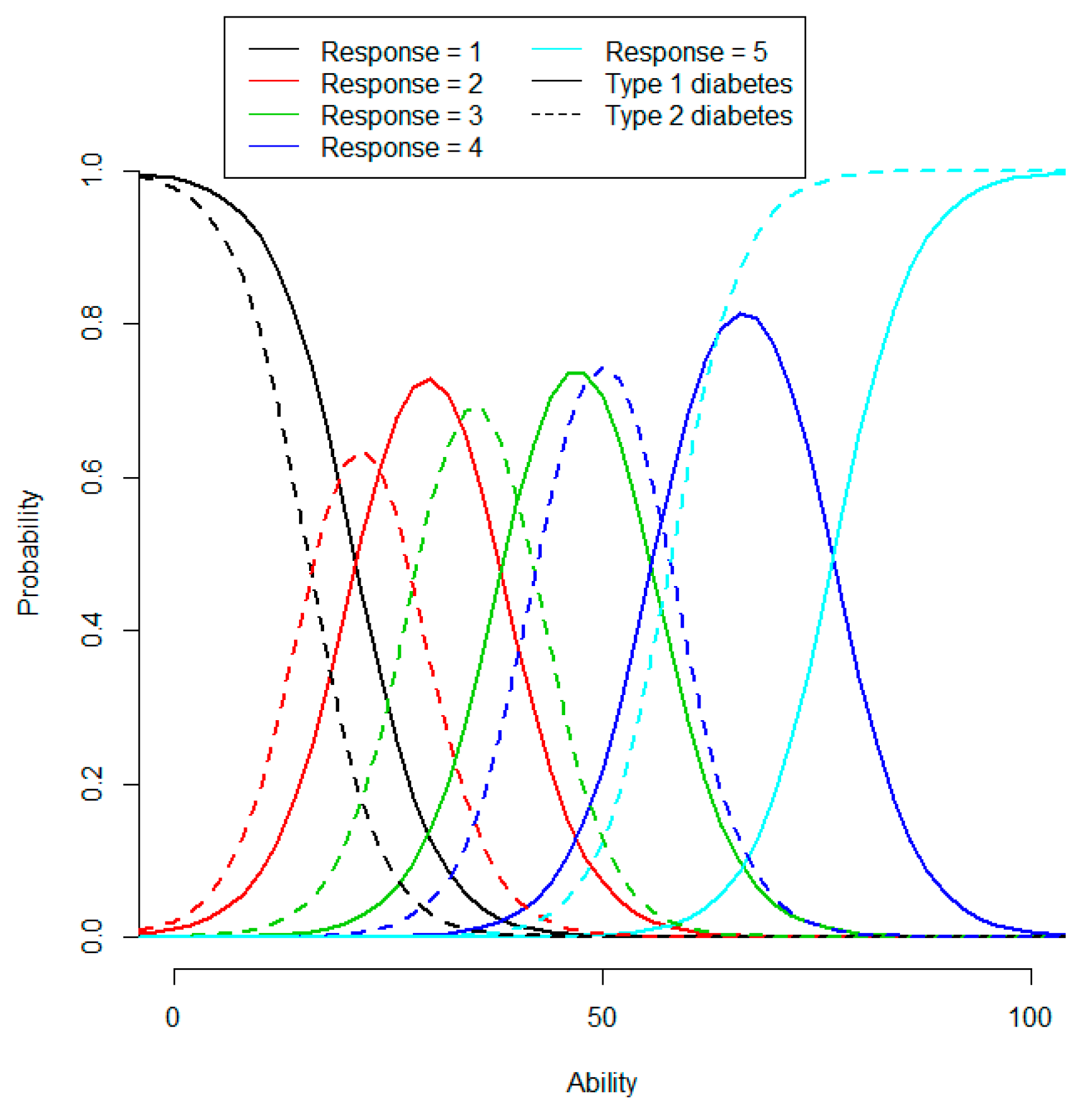
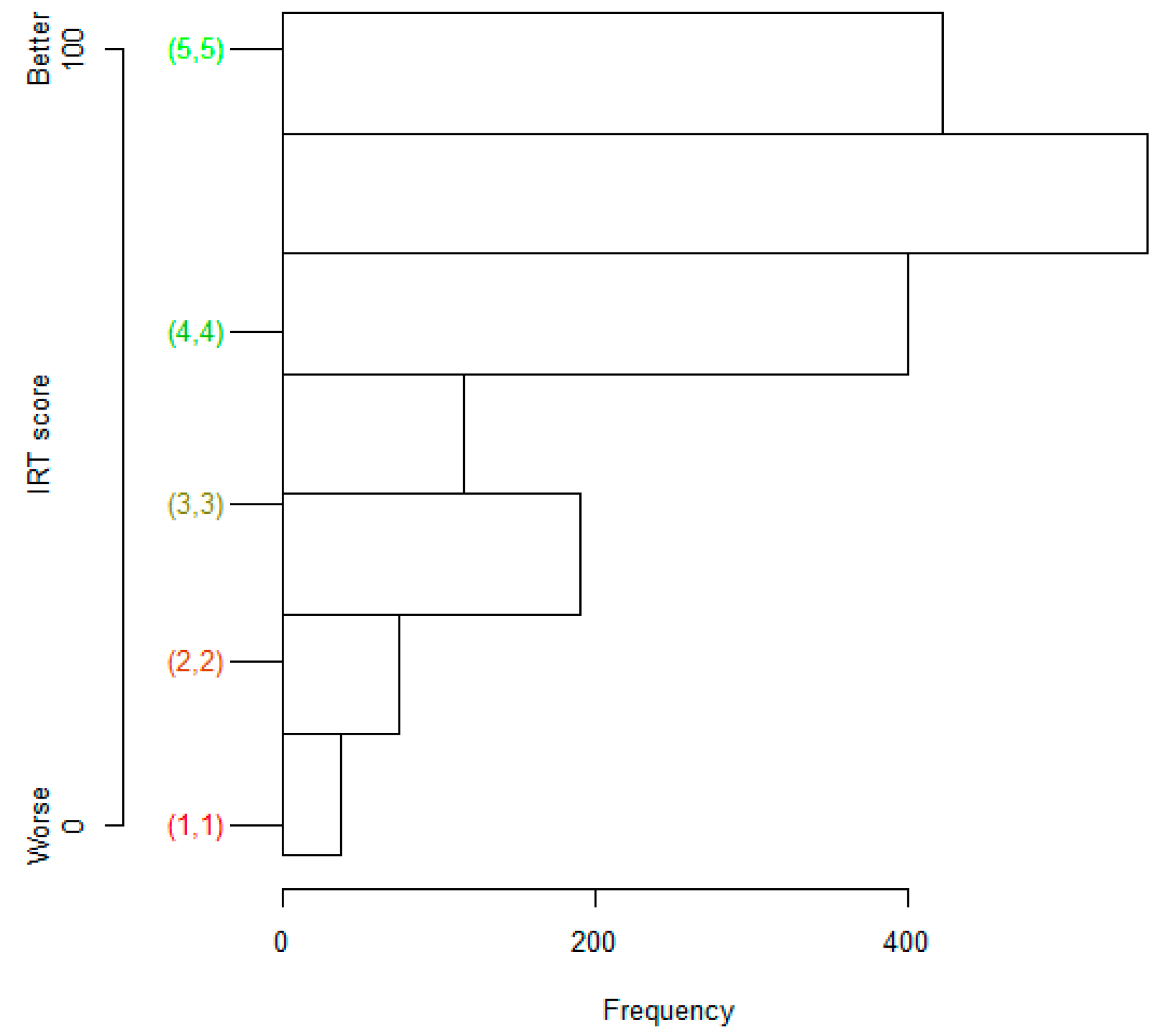
4.2. Patient Abilities and Judgments, EQ-5D Index, and Some Determinants

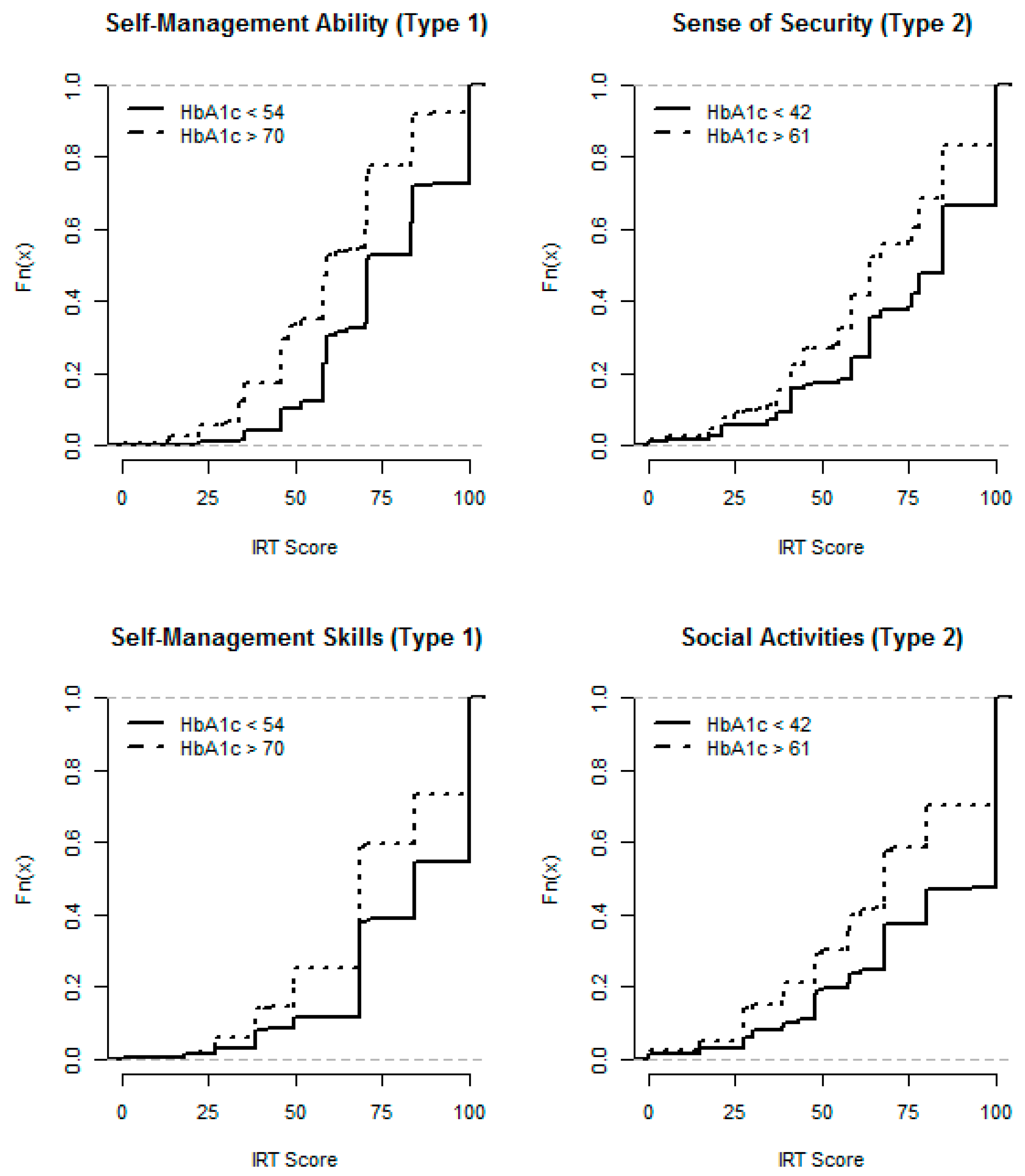
4.3. Analysis of Ad Hoc Response Levels
| S-M Skills | S-M Ability | Sense of Security | Social Activities | Work Activities | Access | Service & Infomation | Involvement | HbA1c | LDL | SBP | EQ-5D Index | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (a) Type 1 diabetes. | ||||||||||||
| S-M Skills | 1.00 * | |||||||||||
| S-M Ability | 0.60 * | 1.00 * | ||||||||||
| Sense of Security | 0.33 * | 0.48 * | 1.00 * | |||||||||
| Social Activities | 0.30 * | 0.43 * | 0.48 * | 1.00 * | ||||||||
| Work Activities | 0.26 * | 0.40 * | 0.43 * | 0.62 * | 1.00 * | |||||||
| Access | 0.18 * | 0.32 * | 0.25 * | 0.14 * | 0.12 | 1.00 * | ||||||
| 0.37 * | 0.41 * | 0.32 * | 0.27 * | 0.21 * | 0.55 * | 1.00 * | ||||||
| Involvement | 0.44 * | 0.39 * | 0.27 * | 0.25 * | 0.19 * | 0.41 * | 0.70 * | 1.00 * | ||||
| HbA1c | −0.17 * | −0.26 * | −0.11 | −0.08 | −0.08 | −0.10 | −0.06 | −0.09 | 1.00 * | |||
| LDL | −0.02 | −0.02 | −0.03 | −0.07 | −0.08 | −0.04 | −0.02 | −0.00 | 0.13 * | 1.00 * | ||
| SBP | 0.06 | 0.13 * | 0.06 | 0.04 | 0.05 | −0.00 | 0.02 | 0.04 | 0.04 | 0.04 | 1.00 * | |
| EQ-5D Index | 0.21 * | 0.34 * | 0.37 * | 0.06 | 0.14 * | 0.13 * | 0.42 * | 0.38 * | −0.13 * | −0.04 | −0.09 | 1.00 * |
| (b) Type 2 diabetes. | ||||||||||||
| S-M Skills | 1.00 * | |||||||||||
| S-M Ability | 0.73 * | 1.00 * | ||||||||||
| Sense of Security | 0.40 * | 0.49 * | 1.00 * | |||||||||
| Social Activities | 0.36 * | 0.46 * | 0.54 * | 1.00 * | ||||||||
| Work Activities | 0.33 * | 0.42 * | 0.48 * | 0.73 * | 1.00 * | |||||||
| Access | 0.38 * | 0.44 * | 0.33 * | 0.26 * | 0.28 * | 1.00 * | ||||||
| 0.47 * | 0.51 * | 0.34 * | 0.32 * | 0.32 * | 0.68 * | 1.00 * | ||||||
| Involvement | 0.51 * | 0.49 * | 0.31 * | 0.28 * | 0.27 * | 0.58 * | 0.76 * | 1.00 * | ||||
| HbA1c | −0.10 * | −0.11 * | −0.14 * | −0.04 | −0.04 | −0.03 | −0.15 * | −0.11 | 1.00 * | |||
| LDL | −0.03 | −0.04 | 0.01 | −0.02 | −0.04 | −0.00 | 0.01 | 0.06 | −0.04 | 1.00 * | ||
| SBP | 0.06 | 0.08 | 0.06 | 0.05 | 0.02 | −0.00 | 0.03 | −0.01 | 0.08 | 0.06 | 1.00 * | |
| EQ-5D Index | 0.24 * | 0.32 * | 0.31 * | 0.24 * | 0.22 * | 0.20 * | 0.42 * | 0.49 * | −0.06 | 0.04 | 0.06 | 1.00 * |
5. Discussion
6. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- United Nations (UN). UN Resolution 61/225: World Diabetes Day; UN: New York, NY, USA, 2006. [Google Scholar]
- Nationella Diabetesregistret. Nationella Diabetesregistret. Årsrapport. 2010 års Resultat.; Västra Götalandsregionen: Göteborg, Sweden, 2011. [Google Scholar]
- Nationella Diabetesregistret. Nationella Diabetesregistret. Årsrapport. 2011 års Resultat.; Västra Götalandsregionen: Göteborg, Sweden, 2012. [Google Scholar]
- Gudbjornsdottir, S.; Cederholm, J.; Nilsson, P.M.; Eliasson, B. The national diabetes register in sweden: An implementation of the st. Vincent declaration for quality improvement in diabetes care. Diabetes Care 2003, 26, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, A. The doctor as double agent: Information asymmetry, health insurance, and medical care. J. Health Econ. 1991, 10, 411–432. [Google Scholar] [CrossRef] [PubMed]
- Sheaff, R.; Pickard, S.; Smith, K. Public service responsiveness to users’ demands and needs: Theory, practice and primary healthcare in england. Pub. Admin. 2002, 80, 435–452. [Google Scholar] [CrossRef]
- Brasil, F.; Pontarolo, R.; Correr, C.J. Patient reported outcomes measures (PROMS) in diabetes: Why are they still rarely used in clinical routine? Diabetes Res. Clin. Prac. 2012, 97, e4–e5. [Google Scholar] [CrossRef]
- Cederholm, J.; Eeg-Olofsson, K.; Eliasson, B.; Zethelius, B.; Gudbjornsdottir, S. A new model for 5-year risk of cardiovascular disease in type 1 diabetes; from the swedish national diabetes register (NDR). Diabet. Med. 2011, 28, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Zethelius, B.; Eliasson, B.; Eeg-Olofsson, K.; Svensson, A.M.; Gudbjornsdottir, S.; Cederholm, J. A new model for 5-year risk of cardiovascular disease in type 2 diabetes, from the Swedish national diabetes register (NDR). Diabetes Res. Clin. Prac. 2011, 93, 276–284. [Google Scholar] [CrossRef]
- Nam, S.; Chesla, C.; Stotts, N.A.; Kroon, L.; Janson, S.L. Barriers to diabetes management: Patient and provider factors. Diabetes Res. Clin. Prac. 2011, 93, 1–9. [Google Scholar] [CrossRef]
- Thorpe, C.T.; Fahey, L.E.; Johnson, H.; Deshpande, M.; Thorpe, J.M.; Fisher, E.B. Facilitating healthy coping in patients with diabetes: A systematic review. Diabetes Educ. 2013, 39, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Fidler, C.; Elmelund Christensen, T.; Gillard, S. Hypoglycemia: An overview of fear of hypoglycemia, quality-of-life, and impact on costs. J. Med. Econ. 2011, 14, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Strachan, M.W. Fear of diabetes complications. Diabetes/Metab. Res. Rev. 2005, 21, 262–263. [Google Scholar] [CrossRef]
- Zoffmann, V.; Harder, I.; Kirkevold, M. A person-centered communication and reflection model: Sharing decision-making in chronic care. Qual. Health Res. 2008, 18, 670–685. [Google Scholar] [CrossRef] [PubMed]
- Executive summary: Standards of medical care in diabetes—2012. Diabetes Care 2012, 35, S4–S10.
- Myndigheten för Vårdanalys. Patient-Centeredness in Sweden’s Health System. An Assessment and Six Steps for Progress. Available online: http://www.vardanalys.se/Global/Rapporter%20pdf-filer/2013/2012-7-Patientcenteredness-v7%200-web.pdf (accessed on 20 July 2012).
- Glenngard, A.H.; Anell, A.; Beckman, A. Choice of primary care provider: Results from a population survey in three swedish counties. Health Policy 2011, 103, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Janlöv, N.; Rehnberg, C. Uppföljning av Husläkarsystemet Inom Vårdval Stockholm—år 2010; Karolinska Institutets folkhälsoakademi: Stockholm, Sweden, 2011. [Google Scholar]
- Lundstrom, M.; Pesudovs, K. Catquest-9sf patient outcomes questionnaire: Nine-item short-form rasch-scaled revision of the catquest questionnaire. J. Cataract. Refract. Surg. 2009, 35, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, E.; Fitzpatrick, R. A Structured Review of Patient-Reported Outcome Measures (PROMS) for Diabetes. Report to the Department of Health; Patient-Reported Outcome Measurement Group; University of Oxford: Oxford, UK, 2009. [Google Scholar]
- Wikblad, K.; Leksell, J.; Wibell, L. Health-related quality of life in relation to metabolic control and late complications in patients with insulin dependent diabetes mellitus. Qual. Life Res. 1996, 5, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Leksell, J.K.; Sandberg, G.E.; Wikblad, K.F. Self-perceived health and self-care among diabetic subjects with defective vision: A comparison between subjects with threat of blindness and blind subjects. J. Diabetes Complicat. 2005, 19, 54–59. [Google Scholar] [PubMed]
- Johnson, B.; Eiser, C.; Young, V.; Brierley, S.; Heller, S. Prevalence of depression among young people with type 1 diabetes: A systematic review. Diabet Med. 2013, 30, 199–208. [Google Scholar] [CrossRef] [PubMed]
- DeJean, D.; Giacomini, M.; Vanstone, M.; Brundisini, F. Patient experiences of depression and anxiety with chronic disease: A systematic review and qualitative meta-synthesis. Ont. Health Technol. Assess. Ser. 2013, 13, 1–33. [Google Scholar] [PubMed]
- Mayfield, J.A.; Deb, P.; Whitecotton, L. Work disability and diabetes. Diabetes Care 1999, 22, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Von Korff, M.; Katon, W.; Lin, E.H.; Simon, G.; Ciechanowski, P.; Ludman, E.; Oliver, M.; Rutter, C.; Young, B. Work disability among individuals with diabetes. Diabetes Care 2005, 28, 1326–1332. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, B.; Oftedal, B.; Bru, E. The relationship between clinical indicators, coping styles, perceived support and diabetes-related distress among adults with type 2 diabetes. J. Adv. Nurs. 2012, 68, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Schokker, M.C.; Stuive, I.; Bouma, J.; Keers, J.C.; Links, T.P.; Wolffenbuttel, B.H.; Sanderman, R.; Hagedoorn, M. Support behavior and relationship satisfaction in couples dealing with diabetes: Main and moderating effects. J. Family Psychol. 2010, 24, 578–586. [Google Scholar] [CrossRef]
- Drummond, M. Methods for the Economic Evaluation of Health Care Programmes, 2nd ed.; Oxford University Press: Oxford, UK, 1997; p. 305. [Google Scholar]
- Hornsten, A.; Lundman, B.; Selstam, E.K.; Sandstrom, H. Patient satisfaction with diabetes care. J. Adv. Nurs. 2005, 51, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Al Shahrani, A.; Baraja, M. Patient satisfaction and it’s relation to diabetic control in a primary care setting. J. Fam. Med. Prim. Care 2014, 3, 5–11. [Google Scholar] [CrossRef]
- Polonsky, W.H. Emotional and quality-of-life aspects of diabetes management. Curr. Diab. Rep. 2002, 2, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Duangdao, K.M.; Roesch, S.C. Coping with diabetes in adulthood: A meta-analysis. J. Behav. Med. 2008, 31, 291–300. [Google Scholar] [CrossRef] [PubMed]
- The World Health Organization (WHO). International Classification of Functioning, Disability and Health (ICF). 2014. Available online: http://apps.who.int/classifications/icfbrowser/ (accessed on 5 March 2014).
- Berne, C.; Fritz, T. Diabetes mellitus. In Läkemedelsboken; Medical Products Agency: Uppsala, Sweden, 2012. [Google Scholar]
- Amsberg, S.; Wredling, R.; Lins, P.E.; Adamson, U.; Johansson, U.B. The psychometric properties of the swedish version of the problem areas in diabetes scale (swe-paid-20): Scale development. Int. J. Nurs. Stud. 2008, 45, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Leksell, J.; Funnell, M.; Sandberg, G.; Smide, B.; Wiklund, G.; Wikblad, K. Psychometric properties of the swedish diabetes empowerment scale. Scand. J. Car. Sci. 2007, 21, 247–252. [Google Scholar] [CrossRef]
- Wredling, R.; Stalhammar, J.; Adamson, U.; Berne, C.; Larsson, Y.; Ostman, J. Well-being and treatment satisfaction in adults with diabetes: A swedish population-based study. Qual. Life Res. 1995, 4, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E.; Snow, K.; Kosinski, M. Sf-36 Health Survey Manual and Interpretation Guide; The Health Institute, New England Medical Center: London, UK, 1993. [Google Scholar]
- EuroQol Group. Euroqol—A new facility for the measurement of health-related quality of life. The euroqol group. Health Policy 1990, 16, 199–208. [Google Scholar]
- Hays, R.D.; Morales, L.S.; Reise, S.P. Item response theory and health outcomes measurement in the 21st century. Med. Care 2000, 38, II28–II42. [Google Scholar] [CrossRef] [PubMed]
- Cella, D.; Chang, C.H. A discussion of item response theory and its applications in health status assessment. Med. Care 2000, 38, II66–II72. [Google Scholar] [CrossRef] [PubMed]
- Van der Ark, L.A. Mokken scale analysis in r. J. Stat. Softw. 2007, 20, 1–19. [Google Scholar]
- Edelen, M.O.; Reeve, B.B. Applying item response theory (IRT) modeling to questionnaire development, evaluation, and refinement. Qual. Life Res. 2007, 16, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Revelle, W. Psych: Procedures for Psychological, Psychometric, And Personality Research; Northwestern University: Evanston, IL, USA, 2011. [Google Scholar]
- Orlando, M. Critical issues to address when applying item response theory (irt) models. In Advances in Health Outcomes Measurement: Exploring the Current State and the Future of Item Response Theory, Item Banks, and Computer-Adaptive Testing; National Cancer Institute and Drug Information Association: Bethesda, MD, USA, 2004. [Google Scholar]
- Samejima, F. Graded response model. In Handbook of Modern Item Response Theory; van der Linden, W.J., Hambleton, R.K., Eds.; Springer: New York, NY, USA, 1997; pp. 85–100. [Google Scholar]
- Orlando, M.; Thissen, D. Likelihood-based item-fit indices for dichotomous item response theory models. Appl. Psychol. Meas. 2000, 24, 50–64. [Google Scholar] [CrossRef]
- Kang, T.; Chen, T.T. Performance of the generalized s-x2 item fit index for the graded response model. Asia Pac. Educ. Rev. 2010, 12, 89–96. [Google Scholar] [CrossRef]
- Maydeu-Olivares, A.; Garcia-Forero, C. Goodness-of-fit testing. Int. Encycl. Educ. 2010, 7, 190–196. [Google Scholar]
- Hooper, D.; Coughlan, J.; Mullen, M.R. Structural equation modelling: Guidelines for determining model fit. Electron. J. Bus. Res. Methods 2008, 6, 53–60. [Google Scholar]
- Milfont, T.L.; Fischer, R. Testing measuring invariance across groups: Applications in cross-cultural research. Int. J. Psychol. Res. 2010, 3, 111–121. [Google Scholar]
- Burstrom, K.; Sun, S.; Gerdtham, U.G.; Henriksson, M.; Johannesson, M.; Levin, L.A.; Zethraeus, N. Swedish experience-based value sets for eq-5d health states. Qual. Life Res. 2013, 23, 431–442. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria, 2009. [Google Scholar]
- Rizopoulos, D. Ltm: An r package for latent variable modeling and item response theory analyses. J. Stat. Softw. 2006, 17, 1–25. [Google Scholar] [CrossRef]
- Scientific Software International. Irt pro: User’s Guide; Scientific Software International, Inc.: Lincolnwood, IL, USA, 2011. [Google Scholar]
- Peterson, A.; Bojestig, M.; Gudbjörnsdottir, S.; Löfgren, U.B.; Schiöler, L.; Thor, J.; Andersson Gäre, B. The active use of a national quality registry can improve diabetes care— Results from a multi-centre quality improvement collaborative program. (Under review).
- Peterson, A.; Bojestig, M.; Gudbjörnsdottir, S.; Löfgren, U.B.; Schiöler, L.; Thor, J.; Andersson Gäre, B. Using a national quality register together with systematic improvement tools to improve diabetes care. In Proceedings of the Sixteenth International Scientific Symposium on Improving Quality and Value in Health Care, Orlando, FL, USA, 6 December 2010.
- Nationella Diabetesregistret. Nationella Diabetesregistret. Årsrapport. 2012 års Resultat.; Västra Götalandsregionen: Göteborg, Sweden, 2013. [Google Scholar]
- Spector, P.E. Summated Rating Scale Construction: Introduction; Sage Pubns.: California, CA, USA, 1992. [Google Scholar]
Appendix
A1. IRT Analysis
| Scale | N | No of Items | RMSEA | RMSEA (90% CI) |
|---|---|---|---|---|
| Type 1 diabetes | ||||
| Self-Management Skills | 1119 | 2 | 0.066 | (0.052, 0.079) |
| Self-Management Ability | 1110 | 2 | 0.064 | (0.050, 0.078) |
| Sense of Security | 1113 | 2 | 0.062 | (0.047, 0.075) |
| Social Activities | 1110 | 2 | 0.062 | (0.048, 0.076) |
| Work Activities | 756 | 3 | 0.452 | (0.444, 0.461) |
| Access | 1073 | 2 | 0.063 | (0.048, 0.076) |
| Service & Information | 1102 | 5 | 0.049 | (0.044, 0.053) |
| Involvement | 1098 | 2 | 0.077 | (0.063, 0.090) |
| Type 2 diabetes | ||||
| Self-Management Skills | 1763 | 2 | 0.102 | (0.091, 0.112) |
| Self-Management Ability | 1759 | 2 | 0.092 | (0.081, 0.103) |
| Sense of Security | 1765 | 2 | 0.055 | (0.043, 0.065) |
| Social Activities | 1760 | 2 | 0.079 | (0.068, 0.089) |
| Work Activities | 690 | 3 | 0.893 | (0.884, 0.902) |
| Access | 1728 | 2 | 0.064 | (0.053, 0.075) |
| Service & Information | 1726 | 5 | 0.056 | (0.052, 0.059) |
| Involvement | 1696 | 2 | 0.085 | (0.074, 0.095) |
A2. Validation of Scales
| Type 1 Diabetes | Type 2 Diabetes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Scale | Item | Di | Ei1 | Ei2 | Ei3 | Ei4 | Di | Ei1 | Ei2 | Ei3 | Ei4 |
| SM 1 | 1 | 4.617 | −2.654 | −2.045 | −1.173 | 0.153 | 4.248 | −2.536 | −1.799 | −0.736 | 0.385 |
| 2 | 4.617 | −2.612 | −2.029 | −1.126 | 0.257 | 4.248 | −2.398 | −1.780 | −0.756 | 0.390 | |
| SM 2 | 3 | 1.977 | −2.718 | −1.764 | −0.624 | 0.614 | 2.370 | −2.674 | −1.777 | −0.781 | 0.362 |
| 4 | 1.977 | −3.134 | −1.859 | −0.389 | 1.008 | 2.370 | −2.683 | −1.811 | −0.771 | 0.424 | |
| SS | 24 | 1.917 | −2.456 | −1.347 | −0.253 | 1.071 | 4.826 | −2.218 | −1.595 | −0.887 | −0.094 |
| 25 | 1.917 | −1.974 | −0.956 | 0.066 | 1.551 | 1.621 | −2.444 | −1.402 | −0.356 | 0.843 | |
| SA | 29 | 2.913 | −1.773 | −1.024 | −0.228 | 0.889 | 4.534 | −1.717 | −1.026 | −0.464 | 0.257 |
| 30 | 4.604 | −1.901 | −1.172 | −0.565 | 0.280 | 4.534 | −1.846 | −1.050 | −0.522 | 0.057 | |
| WA | 26 | 3.078 | −2.078 | −1.397 | −0.694 | 0.223 | 3.497 | −2.256 | −1.483 | −0.888 | −0.262 |
| 27 | 4.969 | −2.322 | −1.682 | −1.080 | −0.317 | 5.037 | −2.455 | −1.653 | −1.053 | −0.500 | |
| 28 | 3.783 | −2.272 | −1.462 | −0.891 | −0.108 | 5.006 | −2.473 | −1.564 | −1.012 | −0.372 | |
| A * | 5&6 | 3.045 | −1.691 | −0.974 | −0.041 | 0.789 | 3.794 | −1.664 | −1.025 | −0.333 | 0.421 |
| 7&8 | 3.045 | −1.649 | −1.000 | −0.096 | 1.009 | 3.794 | −1.703 | −1.139 | −0.337 | 0.508 | |
| SI * | 9&10 | 3.645 | −2.361 | −1.607 | −0.877 | −0.076 | 3.740 | −2.434 | −1.742 | −0.978 | −0.202 |
| 11&12 | 2.938 | −2.865 | −1.988 | −1.104 | −0.201 | 3.697 | −2.355 | −1.677 | −1.024 | −0.206 | |
| 13&14 | 3.390 | −2.318 | −1.693 | −0.819 | 0.075 | 3.070 | −2.207 | −1.613 | −0.861 | 0.089 | |
| 19&20 | 4.692 | −2.434 | −1.853 | −1.221 | −0.597 | 4.360 | −2.414 | −1.869 | −1.217 | −0.556 | |
| 21&22 | 4.910 | −2.169 | −1.686 | −1.046 | −0.226 | 4.927 | −2.210 | −1.750 | −1.035 | −0.252 | |
| I* | 15&16 | 4.617 | −2.445 | −1.795 | −1.036 | −0.142 | 4.597 | −1.773 | −1.254 | −0.568 | 0.267 |
| 17&18 | 4.617 | −2.354 | −1.698 | −0.989 | −0.027 | 4.597 | −1.634 | −1.174 | −0.529 | 0.292 | |
| Scale | Type 1 Diabetes | Type 2 Diabetes | ||||
|---|---|---|---|---|---|---|
| Correlation | Difference *, Mean (SD) | Correlation | Difference *, Mean (SD) | |||
| Self-Management Skills | 1.00 | −1.4 | (1.6) | 1.00 | 0.2 | (0.6) |
| Self-Management Ability | 1.00 | −0.5 | (0.5) | 0.96 | 0.1 | (6.2) |
| Sense of Security | 1.00 | −0.6 | (0.7) | 1.00 | −1.2 | (1.0) |
| Social Activities | 0.99 | 0.5 | (3.2) | 1.00 | 0.6 | (0.8) |
| Work Activities | 1.00 | −0.7 | (1.0) | 0.93 | 7.4 | (6.1) |
| Access | 0.97 | −1.6 | (6.1) | 1.00 | 0.6 | (1.0) |
| Service & Information | 0.99 | −0.7 | (3.4) | 0.99 | −0.3 | (2.9) |
| Involvement | 1.00 | 0.5 | (1.1) | 1.00 | 0.1 | (0.7) |
A3. Discussion of the Scales
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borg, S.; Palaszewski, B.; Gerdtham, U.-G.; Fredrik, Ö.; Roos, P.; Gudbjörnsdottir, S. Patient-Reported Outcome Measures and Risk Factors in a Quality Registry: A Basis for More Patient-Centered Diabetes Care in Sweden. Int. J. Environ. Res. Public Health 2014, 11, 12223-12246. https://doi.org/10.3390/ijerph111212223
Borg S, Palaszewski B, Gerdtham U-G, Fredrik Ö, Roos P, Gudbjörnsdottir S. Patient-Reported Outcome Measures and Risk Factors in a Quality Registry: A Basis for More Patient-Centered Diabetes Care in Sweden. International Journal of Environmental Research and Public Health. 2014; 11(12):12223-12246. https://doi.org/10.3390/ijerph111212223
Chicago/Turabian StyleBorg, Sixten, Bo Palaszewski, Ulf-G Gerdtham, Ödegaard Fredrik, Pontus Roos, and Soffia Gudbjörnsdottir. 2014. "Patient-Reported Outcome Measures and Risk Factors in a Quality Registry: A Basis for More Patient-Centered Diabetes Care in Sweden" International Journal of Environmental Research and Public Health 11, no. 12: 12223-12246. https://doi.org/10.3390/ijerph111212223
APA StyleBorg, S., Palaszewski, B., Gerdtham, U.-G., Fredrik, Ö., Roos, P., & Gudbjörnsdottir, S. (2014). Patient-Reported Outcome Measures and Risk Factors in a Quality Registry: A Basis for More Patient-Centered Diabetes Care in Sweden. International Journal of Environmental Research and Public Health, 11(12), 12223-12246. https://doi.org/10.3390/ijerph111212223




