Surveying Mercury Levels in Hair, Blood and Urine of under 7-Year Old Children from a Coastal City in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subject Selection
2.2. Sample Collection
2.3. Mercury Analysis
2.4. Statistical Analysis
3. Results
3.1. Distribution of Mercury Levels in Blood, Urine, and Hair
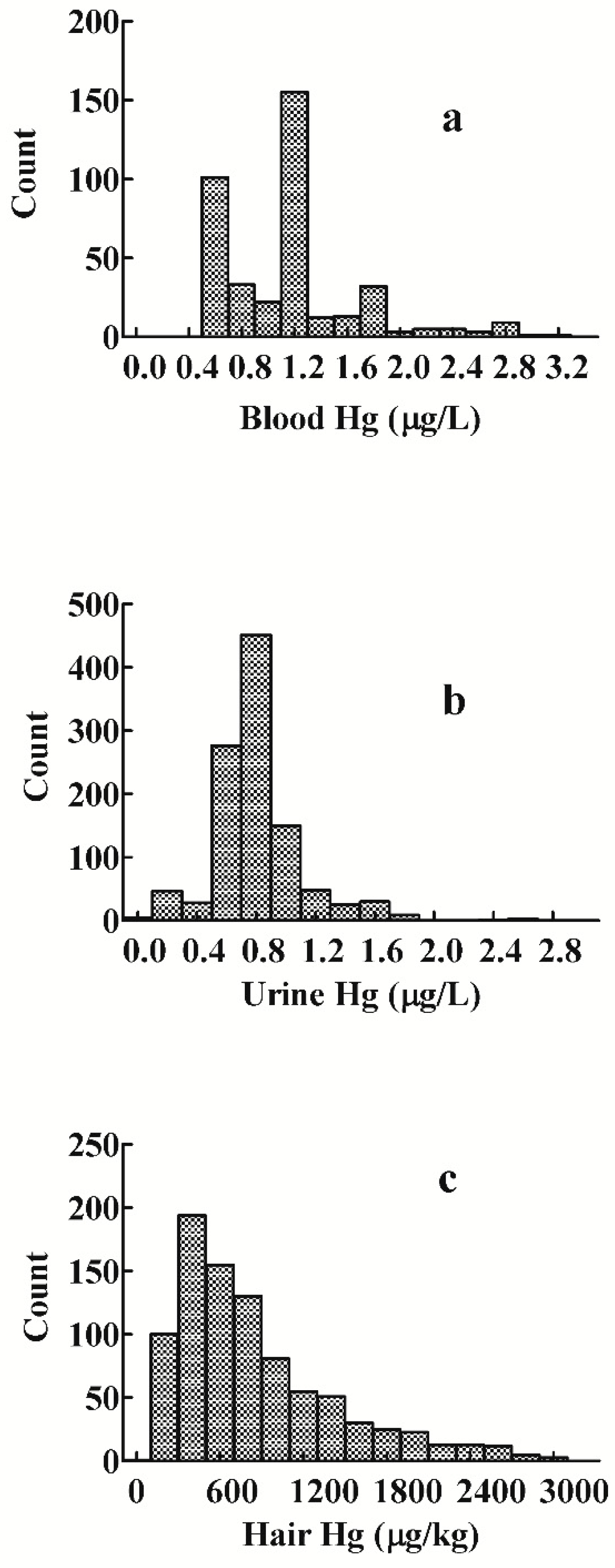
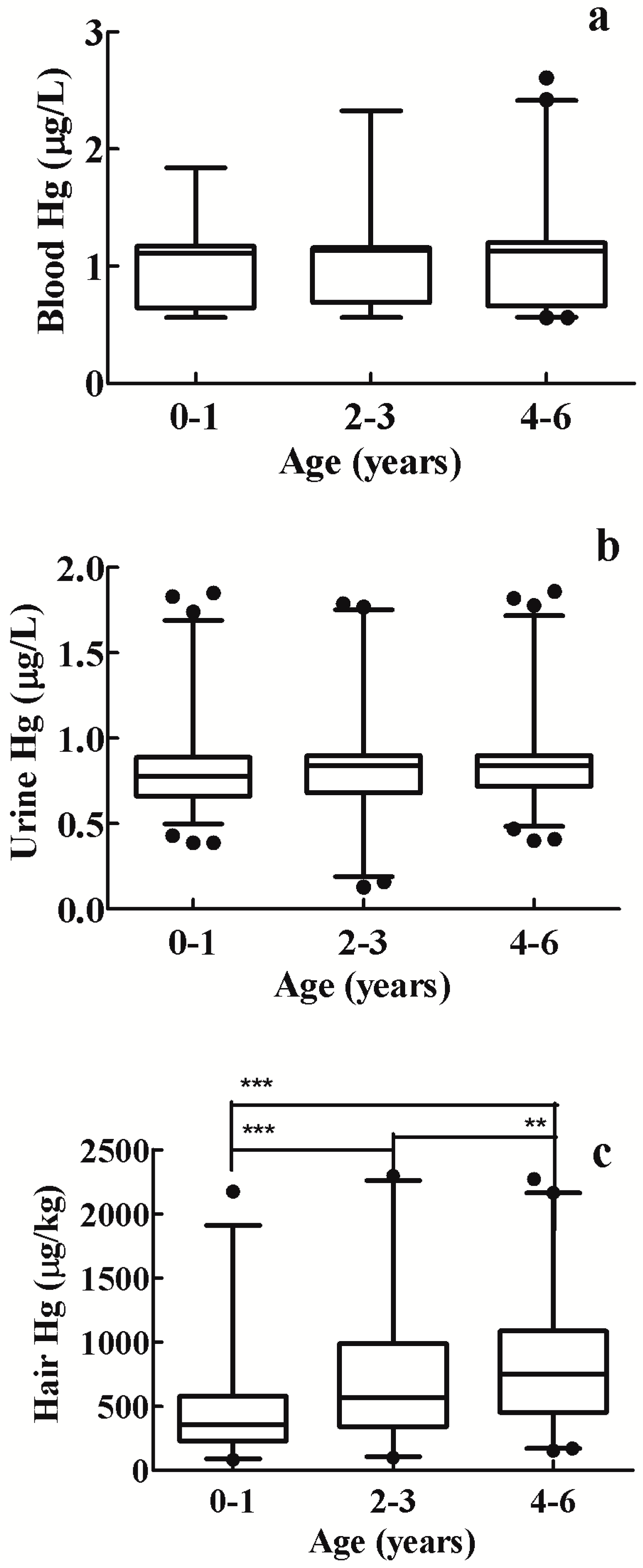
3.2. Blood Mercury Levels
3.3. Urine Mercury Levels
| Sample | n | Range | x ± SD | Median | GM a | CI-CM b | P95 c | CI P 95 d | RVs |
|---|---|---|---|---|---|---|---|---|---|
| Blood (μg/L) | 395 | 0.56–3.15 | 1.14 ± 0.51 | 1.13 | 1.05 | 1.00−1.09 | 2.28 | 1.84−2.64 | 2.30 |
| Urine (μg/L) | 1072 | 0.03–2.63 | 0.82 ± 0.30 | 0.83 | 0.75 | 0.73−0.77 | 1.42 | 1.31−1.51 | 1.50 |
| Hair (μg/kg) | 581 | 99.0–3280.0 | 748.2 ± 598.4 | 550.0 | 569.8 | 536.7−604.9 | 2060.0 | 1900.4−2310.2 | 2100.0 |
| Sample | n | Range | x ± SD | Median | GM a | CI-CM b | P95 c | CI P 95 d | RVs |
|---|---|---|---|---|---|---|---|---|---|
| Blood | |||||||||
| 0–1 years (μg/L) | 62 | 0.56–2.61 | 1.05 ± 0.42 | 1.11 | 0.97 | 0.88–1.08 | 1.77 | — | 1.80 |
| 2–3 years (μg/L) | 82 | 0.56–2.83 | 1.07 ± 0.39 | 1.14 | 1.00 | 0.93–1.08 | 1.75 | 1.39–2.70 | 1.80 |
| 4–6 years (μg/L) | 251 | 0.56–3.15 | 1.16 ± 0.53 | 1.14 | 1.06 | 1.00–1.12 | 2.30 | 2.00–2.75 | 2.30 |
| Urine | |||||||||
| 0–1 years (μg/L) | 425 | 0.03–2.63 | 0.79 ± 0.29 | 0.75 | 0.73 | 0.70–0.76 | 1.35 | 1.25–1.48 | 1.40 |
| 2–3 years (μg/L) | 252 | 0.10–1.79 | 0.82 ± 0.31 | 0.83 | 0.75 | 0.71–0.79 | 1.51 | 1.30–1.67 | 1.50 |
| 4–6 years (μg/L) | 395 | 0.13–2.55 | 0.84 ± 0.30 | 0.83 | 0.78 | 0.75–0.81 | 1.46 | 1.23–1.65 | 1.50 |
| Hair | |||||||||
| 0–1 years (μg/kg) | 195 | 116.0–3259.0 | 483.6 ± 390.2 | 363.0 | 388.0 | 354.9–424.2 | 1173.0 | 995.2–1523.7 | 1200.0 |
| 2–3 years (μg/kg) | 172 | 99.0–2932.0 | 809.2 ± 625.3 | 602.5 | 619.0 | 554.4–691.2 | 2195.0 | 1949.7–2643.3 | 2200.0 |
| 4–6 years (μg/kg) | 214 | 153.0–3280.0 | 939.4 ± 647.8 | 768.0 | 752.9 | 687.4–824.5 | 2466.0 | 2022.5–2725.7 | 2500.0 |
| Sample | n | Range | x ± SD | Median | GM a | CI-CM b | P95 c | CI P 95 d | RVs |
|---|---|---|---|---|---|---|---|---|---|
| Blood | |||||||||
| Boys (μg/L) | 214 | 0.56−3.15 | 1.16 ± 0.56 | 1.13 | 1.05 | 0.99−1.12 | 2.68 | 2.17−2.80 | 2.70 |
| Girls (μg/L) | 181 | 0.56−2.90 | 1.11 ± 0.44 | 1.14 | 1.04 | 0.98−1.10 | 1.84 | 1.74−2.27 | 1.90 |
| Urine | |||||||||
| Boys (μg/L) | 606 | 0.04−2.63 | 0.83 ± 0.31 | 0.83 | 0.77 | 0.74−0.80 | 1.50 | 1.39−1.63 | 1.50 |
| Girls (μg/L) | 466 | 0.03−2.55 | 0.79 ± 0.28 | 0.82 | 0.73 | 0.70−0.76 | 1.30 | 1.19−1.47 | 1.30 |
| Hair | |||||||||
| Boys (μg/kg) | 308 | 116.0−3272.0 | 746.6 ± 596.9 | 570.5 | 572.5 | 528.0−620.7 | 2165.0 | 1755.6−2573.3 | 2200.0 |
| Girls (μg/kg) | 273 | 99.0−3280.0 | 750.1 ± 601.2 | 531.0 | 566.8 | 518.5−619.5 | 2051.0 | 1762.7−2473.2 | 2100.0 |
3.4. Hair Mercury Levels
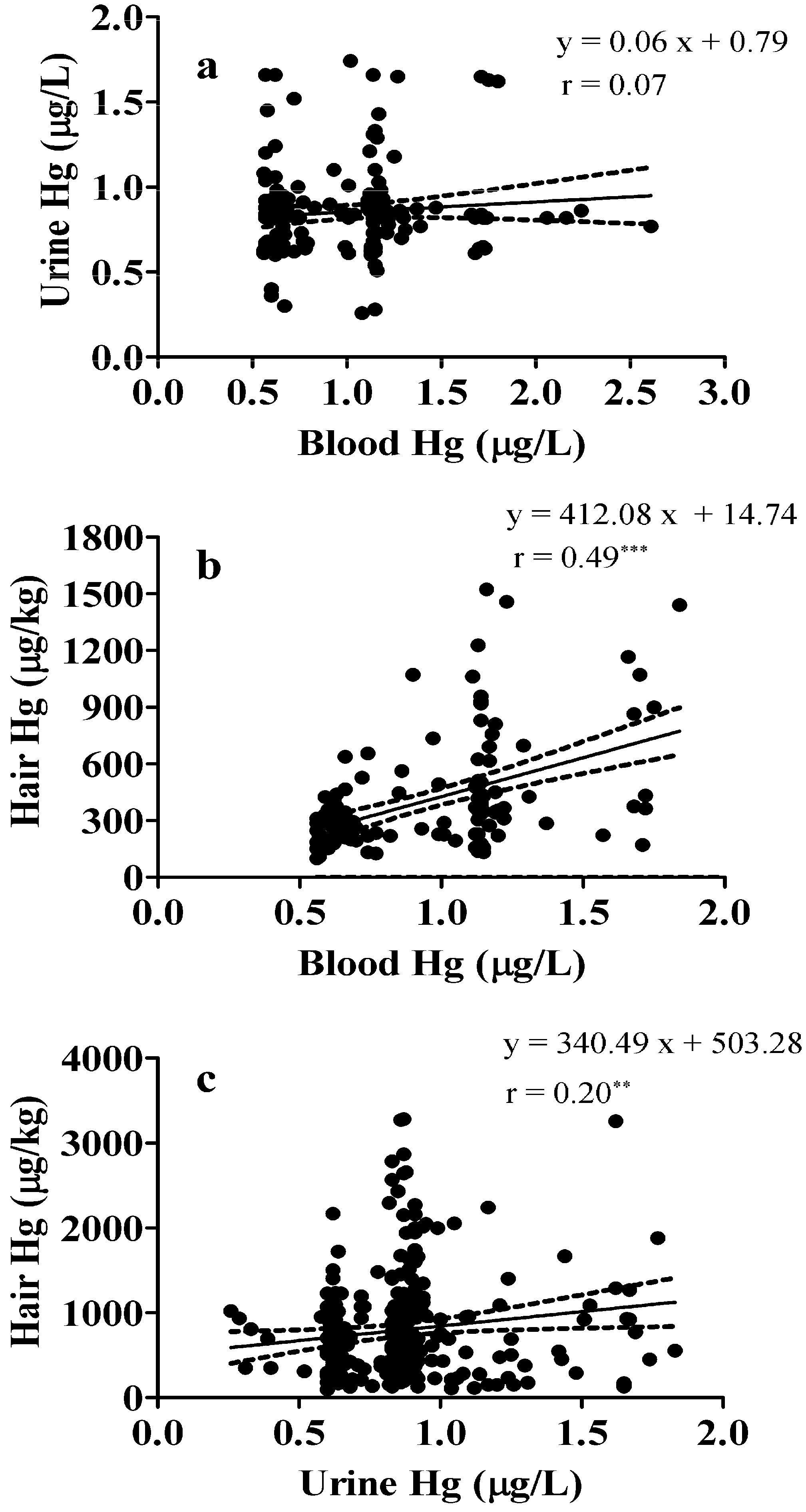
3.5. Correlation between Blood, Urine, and Hair Mercury Levels in Children
4. Discussion
5. Strengths and Limitations
6. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Pirrone, N.; Cinnirella, S.; Feng, X.; Finkelman, R.B.; Friedli, H.R.; Leaner, J.; Mason, R.; Mukherjee, A.B.; Stracher, G.; Streets, D.G.; et al. Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmos. Chem. Phys. 2010, 10, 5951–5964. [Google Scholar] [CrossRef]
- Stein, J.; Schettler, T.; Wallinga, D.; Valenti, M. In harm’s way: Toxic threats to child development. J. Dev. Behav. Pediatr. 2002, 23, S13–S22. [Google Scholar] [CrossRef] [PubMed]
- Stokstad, E. Environment. Uncertain science underlies new mercury standards. Science 2004, 303. [Google Scholar] [CrossRef]
- Angerer, J.; Ewers, U.; Wilhelm, M. Human biomonitoring: State of the art. Int. J. Hyg. Environ. Health 2007, 210, 201–228. [Google Scholar] [CrossRef] [PubMed]
- Ewers, U.; Krause, C.; Schulz, C.; Wilhelm, M. Reference values and human biological monitoring values for environmental toxins. Report on the work and recommendations of the Commission on Human Biological Monitoring of the German Federal Environmental Agency. Int. Arch. Occup. Environ. Health 1999, 72, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.; Ewers, U.; Schulz, C. Revised and new reference values for some trace elements in blood and urine for human biomonitoring in environmental medicine. Int. J. Hyg. Environ. Health 2004, 207, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Heudorf, U.; Butte, W.; Schulz, C.; Angerer, J. Reference values for metabolites of pyrethroid and organophosphorous insecticides in urine for human biomonitoring in environmental medicine. Int. J. Hyg. Environ. Health 2006, 209, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.; Ewers, U.; Schulz, C. Revised and new reference values for some persistent organic pollutants (POPs) in blood for human biomonitoring in environmental medicine. Int. J. Hyg. Environ. Health 2003, 206, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.C.; Jardim, W.; Dorea, J.G.; Fosberg, B.; Souza, J. Hair mercury speciation as a function of gender, age, and body mass index in inhabitants of the Negro river basin, Amazon, Brazil. Arch. Environ. Contam. Toxicol. 2001, 40, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Mahaffey, K.R.; Clickner, R.P.; Bodurow, C.C. Blood organic mercury and dietary mercury intake: National health and nutrition examination Survey, 1999 and 2000. Environ. Health Perspect. 2003, 112, 562–570. [Google Scholar] [CrossRef]
- McKelvey, W.; Jeffery, N.; Clark, N.; Kass, D.; Parsons, P.J. Population-based inorganic mercury biomonitoring and the identification of skin care products as a source of exposure in New York city. Environ. Health Perspect. 2010, 119, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.; Mason, H.J.; Ritchie, K.A.; White, M. Comparison of hair, nails and urine for biological monitoring of low level inorganic mercury exposure in dental workers. Biomarkers 2004, 9, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Chen, C.; Li, B.; Wang, J.; Gao, Y.; Zhao, Y.; Chai, Z. Scalp hair as a biomarker in environmental and occupational mercury exposed populations: Suitable or not? Environ. Res. 2008, 107, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.B.; Wang, R.Y.; Needham, L.L. Biologic monitoring of exposure to environmental chemicals throughout the life stages: Requirements and issues for consideration for the National Children’s Study. Environ. Health Perspect. 2005, 113, 1083–1091. [Google Scholar] [PubMed]
- Nuttall, K.L. Interpreting hair mercury levels in individual patients. Ann. Clin. Lab. Sci 2006, 36, 248–261. [Google Scholar] [PubMed]
- Carneiro, M.F.; Moresco, M.B.; Chagas, G.R.; de Oliveira Souza, V.C.; Rhoden, C.R.; Barbosa, F., Jr. Assessment of trace elements in scalp hair of a young urban population in Brazil. Biol. Trace Elem. Res. 2011, 143, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Maggi, C.; Berducci, M.T.; Bianchi, J.; Giani, M.; Campanella, L. Methylmercury determination in marine sediment and organisms by Direct Mercury Analyser. Anal. Chim. Acta 2009, 641, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Angerer, J.; Ewers, U.; Kolossa-Gehring, M. The German human biomonitoring commission. Int. J. Hyg. Environ. Health 2007, 210, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Hrubá, F.; Strömberg, U.; Černá, M.; Chen, C.; Harari, F.; Harari, R.; Horvat, M.; Koppová, K.; Kos, A.; Krsková, A.; et al. Blood cadmium, mercury, and lead in children: An international comparison of cities in six European countries, and China, Ecuador, and Morocco. Environ. Int. 2012, 41, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.A.; Teschke, K.; Rideout, K.; Copes, R. Trace element levels in adults from the west coast of Canada and associations with age, gender, diet, activities, and levels of other trace elements. Chemosphere 2007, 70, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Oken, E.; Radesky, J.S.; Wright, R.O.; Bellinger, D.C.; Amarasiriwardena, C.J.; Kleinman, K.P.; Hu, H.; Gillman, M.W. Maternal fish intake during pregnancy, blood mercury, and child cognition at age 3 years in a US cohort. Am. J. Epidemiol. 2008, 167, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Lu, L.; Zeng, G.; Zhang, J.; Shi, J.; Li, J. The Research of 0–35 Months Children Hair Mercury Level and its Influencing Factors. Chinese J. Public Health. 2013, 29, 25–27. [Google Scholar]
- Walkowiak, J.; Altmann, L.; Kramer, U.; Sveinsson, K.; Turfeld, M.; Weishoff-Houben, M.; Winneke, G. Cognitive and sensorimotor functions in 6-year-old children in relation to lead and mercury levels adjustment for intelligence and contrast sensitivity in computerized testing. Neurotoxicol. Teratol. 1998, 20, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Schulte, A.; Stoll, R.; Wittich, M.; Pieper, K.; Stachniss, V. Mercury concentrations in the urine of children with and without amalgam fillings. Schweiz Monatsschr. Zahnmed. 1994, 104, 1336–1340. [Google Scholar] [PubMed]
- Pesch, A.; Wilhelm, M.; Rostek, U.; Schmitz, N.; Weishoff-Houben, M.; Ranft, U.; Idel, H. Mercury concentrations in urine, scalp hair, and saliva in children from Germany. J. Expo. Anal. Environ. Epidemiol. 2002, 12, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Freire, C.; Ramos, R.; Lopez-Espinosa, M.J.; Dıez, S.; Vioque, J.; Ballester, F.; Fernández, M.F. Hair mercury levels, fish consumption, and cognitive development in preschool children from Granada, Spain. Environ. Res. 2010, 110, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Kang, Y.; Cheng, Z.; Wang, H.; Huang, M.; Wu, S.; Chen, K.; Wong, M.H. Hair mercury levels and food consumption in residents from the pearl river delta: South China. Food Chem. 2013, 136, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Phelps, R.W.; Clarkson, T.W.; Kershaw, T.G.; Wheatley, B. Interrelationships of blood and hair mercury concentrations in a North American population exposed to methylmercury. Arch. Environ. Health 1980, 35, 161–168. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Chen, X.; Yan, C.; Wu, X.; Zeng, G. Surveying Mercury Levels in Hair, Blood and Urine of under 7-Year Old Children from a Coastal City in China. Int. J. Environ. Res. Public Health 2014, 11, 12029-12041. https://doi.org/10.3390/ijerph111112029
Chen G, Chen X, Yan C, Wu X, Zeng G. Surveying Mercury Levels in Hair, Blood and Urine of under 7-Year Old Children from a Coastal City in China. International Journal of Environmental Research and Public Health. 2014; 11(11):12029-12041. https://doi.org/10.3390/ijerph111112029
Chicago/Turabian StyleChen, Guixia, Xiaoxin Chen, Chonghuai Yan, Xingdong Wu, and Guozhang Zeng. 2014. "Surveying Mercury Levels in Hair, Blood and Urine of under 7-Year Old Children from a Coastal City in China" International Journal of Environmental Research and Public Health 11, no. 11: 12029-12041. https://doi.org/10.3390/ijerph111112029
APA StyleChen, G., Chen, X., Yan, C., Wu, X., & Zeng, G. (2014). Surveying Mercury Levels in Hair, Blood and Urine of under 7-Year Old Children from a Coastal City in China. International Journal of Environmental Research and Public Health, 11(11), 12029-12041. https://doi.org/10.3390/ijerph111112029




