Microbial Anaerobic Digestion (Bio-Digesters) as an Approach to the Decontamination of Animal Wastes in Pollution Control and the Generation of Renewable Energy
Abstract
:1. Introduction
2. Environmental and Public Health Implications of Animal Manure
2.1. Sources of Contaminants in Animal Manure

2.2. Adverse Effects of Animal Wastes on the Environment and Humans
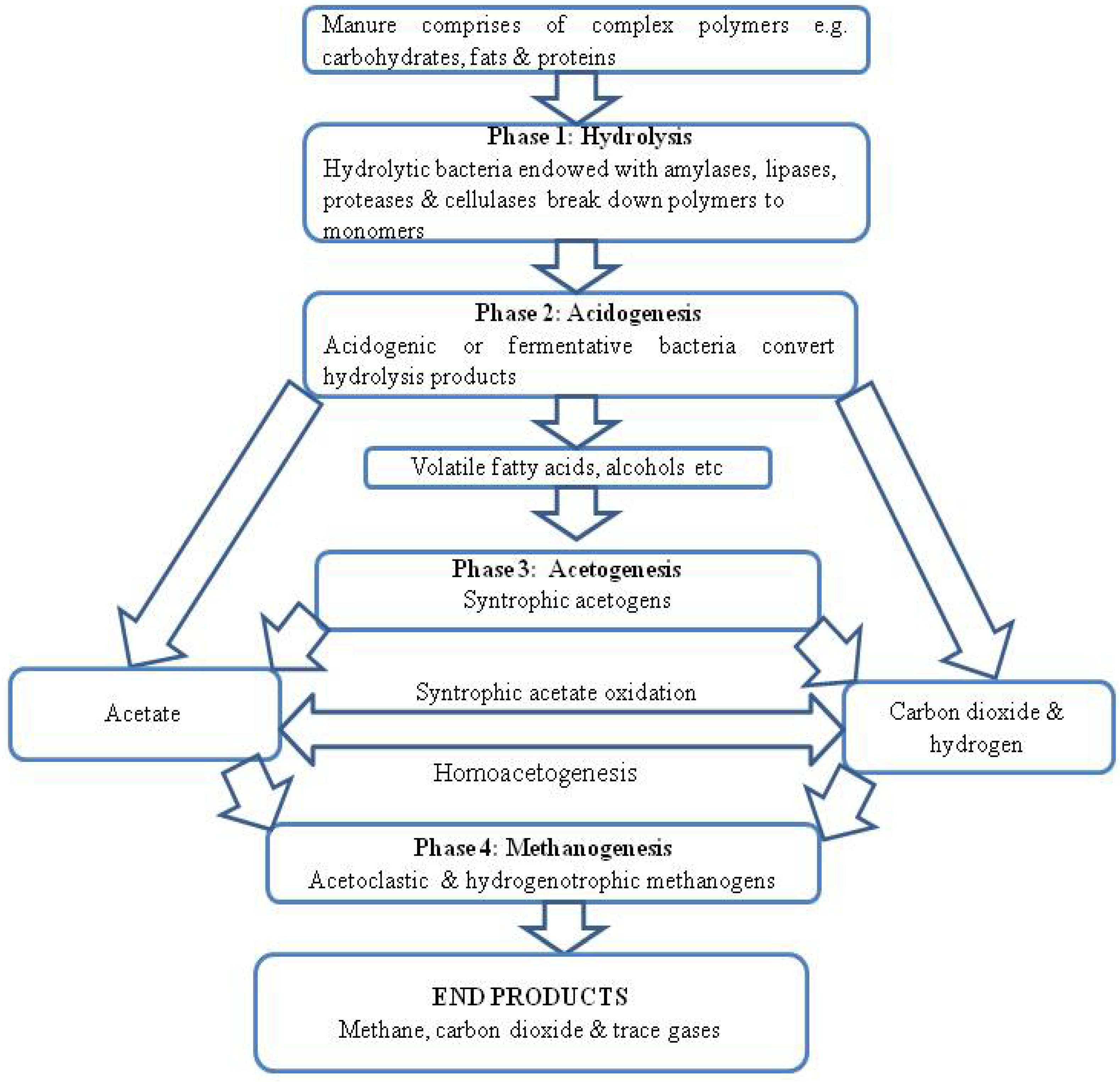
3. Anaerobic Digestion of Animal Wastes in Bio-Digesters
| Component | Symbol | Percentage |
|---|---|---|
| Methane | CH4 | 50–75 |
| Carbon dioxide | CO2 | 25–45 |
| Hydrogen | H2 | 1–2 |
| Ammonia | NH3 | <1 |
| Water vapor | H2O | 2–7 |
| Oxygen | O2 | <2 |
| Hydrogen sulphide | H2S | <1 |
3.1. Microbial Communities Involved in Anaerobic Digestion of Animal Manure and Methods of Their Identification
3.1.1. Acidogens
3.1.2. Syntrophic Acetogens
| Acetate-oxidizing bacteria | Microbial description | Hydrogenotrophic methanogens | References |
|---|---|---|---|
| AOR | Anaerobic, rod-shaped, gram positive, non-spore forming and thermophilic (60 °C) | Methanobacterium sp. strain THF | Lee and Zinder [55] |
| Clostridium ultunense | Anaerobic, spore-forming, rod-shaped, gram negative and mesophilic (37 °C) | Methanoculleus sp. strain MAB1 | Schnürer et al. [56] |
| Thermacetogenium phaeum | Anaerobic, rod-shaped, gram negative but with gram positive cell wall structure and thermophilic (between 55 and 58 °C) | Methanothermobacter thermoautotrophicus TM | Hattori et al. [57] |
| Thermotoga lettingae | Anaerobic, rod-shaped, non-spore forming, mobile, gram negative and thermophilic (65 °C) | Methanothermobacter thermoautotrophicus or Thermodesulfovibrio yellowstonii | Balk et al. [58] |
| Syntrophaceticus schinkii | Anaerobic, spore-forming, variable cell shape, gram variable and mesophilic (between 25 and 40 °C) | Methanoculleus sp. strain MAB1 | Westerholm et al. [59] |
3.1.3. Methanogens (Archaea)
3.2. Techniques for Identifying Microorganisms Involved in the Anaerobic Digestion Process
3.3. Types of Bio-Digesters for Treating Animal Manure
3.4. Factors Influencing Anaerobic Digestion of Animal Manure
3.4.1. Temperature
3.4.2. pH and Alkalinity
3.4.3. Ammonia Concentration
3.4.4. Hydraulic Retention Time and Organic Loading Rate
3.4.5. Substrate Characteristics and Heavy Metals
3.4.6. Mixing
4. Conclusions
Acknowledgements
Conflicts of Interest
References
- Federal Energy Management Program, Biomass Energy-Focus on Wood Waste. In Biomass and Alternative Methane Fuels; BAMF Fact Sheet: Oak Ridge, TN, USA, 2004.
- Wilkie, A.C. Biomethane from Biomass, Biowaste and Biofuels. In Bionergy; Wall, J.D., Harwood, C.S., Deamin, A.L., Eds.; ASM Press: Washingston, DC, USA, 2008; pp. 195–215. [Google Scholar]
- Uzodinma, E.O.; Ofoefule, A.U.; Eze, J.I.; Mbaeyi, I.; Onwuka, N.D. Effect of some organic wastes on the biogas yield from carbonated soft drink sludge. Sci. Res. Essays 2008, 3, 401–405. [Google Scholar]
- Mukumba, P.; Makaka, G.; Mamphweli, S.; Simon, M.; Meyer, E. An insight into the status of biogas digesters technologies in South Africa with reference to the Eastern Cape Province. Fort Hare Pap. 2012, 19, 5–29. [Google Scholar]
- Karakashev, D.; Batstone, D.J.; Angelidaki, I. Influence of environmental conditions on methanogenic compositions in anaerobic biogas reactors. Appl. Environ. Microbiol. 2005, 71, 331–338. [Google Scholar] [CrossRef]
- Kröber, M.; Bekel, T.; Diaz, N.N.; Goesmann, A.; Jaenicke, S.; Krause, L.; Miller, D.; Runte, K.J.; Viehöver, P.; Pühler, A.; et al. Phylognetic characterization of a biogas plant microbial community integrating clone library 16S rDNA sequences and metagenome sequence data obtained by 454-pyrosequencing. J. Biotechnol. 2009, 142, 38–49. [Google Scholar] [CrossRef]
- Li, J.; Jha, A.K.; He, J.; Ban, Q.; Chang, S.; Wang, P. Assessment of the effects of dry anaerobic co-digestion of cow dung with waste water sludge on biogas yield and biodegradability. Int. J. Phys. Sci. 2011, 6, 3679–3688. [Google Scholar]
- Anunputtikul, W.; Rodtong, S. Laboratory Scale Experiments for Biogas Production from Cassava Tubers. In Proceedings of the Joint International Conference on “Sustainable Energy and Environment (SEE)”, Hua Hin, Thailand, 1–3 December 2004; 3-017(0). pp. 238–243.
- Sakar, S.; Yetilmezsoy, K.; Kocak, E. Anaerobic digestion technology in poultry and livestock waste treatment. Waste Manag. Res. 2009, 27, 3–18. [Google Scholar] [CrossRef]
- St-Pierre, B.; Wright, A.D.G. Metagenomic analysis of methanogen populations in three full- scale mesophilic anaerobic manure digesters operated on dairy farms in Vermont, USA. Bioresour. Technol. 2013, 138, 277–284. [Google Scholar] [CrossRef]
- Wilkie, A.C. Anaerobic Digestion: Holistic Bioprocessing of Animal Manure. In Proceedings of the Animal Residuals Management Conference, Alexandria, VA, USA, 14–18 October 2000; pp. 1–12.
- Brown, V.J. Biogas a bright idea for Africa. Environ. Health Perspect. 2006, 114, A300–A303. [Google Scholar] [CrossRef]
- Liu, F.H.; Wang, S.B.; Zhang, J.S.; Zhang, J.; Yan, X.; Zhou, H.K.; Zhao, G.P.; Zhou, Z.H. The structure of the bacterial and archaeal community in a biogas digester as revealed by denaturing gradient gel electrophoresis and 16S rDNA sequencing analysis. J. Appl. Microbiol. 2009, 106, 952–966. [Google Scholar] [CrossRef]
- Mauky, E. Biogas Use. Technologies and Trends in Germany; DBFZ, Federal Ministry of Economics and Technology, Eclareon: Berlin, Germany, 2009; pp. 1–28. [Google Scholar]
- Rechberger, P. Biogas Markets and Opportunities—A European Review; Anaerobic Digestion in Ireland, Tullamore, AEBIOM: Brussels, Belgium, 2009. [Google Scholar]
- Jenkins, S.R.; Armstrong, C.W.; Monti, M.M. Health Effects of Biosolids Applied to Land: Available Scientific Evidence. Virginia Department of Health, 2007. Available online: http://www.vdh.virginia.gov/epidemiology/DEE/documents/biosolids.pdf (accessed on 6 July 2013).
- Burkholder, J.; Libra, B.; Weyer, P.; Heathcote, S.; Kolpin, D.; Thorne, P.S.; Wichman, M. Impacts of waste from concentrated animal feeding operations on water quality. Environ. Health Perspect. 2007, 115, 308–312. [Google Scholar]
- Carbone, S.R.; da Silva, F.M.; Tavares, C.R.G.; Dias Filho, B.P. Bacterial population of a two-phase anaerobic digestion process treating effluent of cassava starch factory. Environ. Technol. 2002, 23, 591–597. [Google Scholar] [CrossRef]
- Litchfield, J.H. Salmonella Food Poisoning. In Safety of Food, 2nd ed.; Graham, C.W., Ed.; AVI Publishing: Westport, CT, USA, 1980; pp. 120–122. [Google Scholar]
- Eriksson, O.; Reich, M.C.; Frostell, B.; Bjorklund, A.; Assefa, G.; Sundqvist, J.-O.; Granath, J.; Baky, A.; Thyselius, L. Municipal solid waste management from a systems perspective. J. Clean. Prod. 2005, 13, 241–252. [Google Scholar] [CrossRef]
- Willey, J.M.; Sherwood, L.M.; Woolverton, C. Microbial Interactions. In Prescott’s Microbiology, 8th ed.; McGraw-Hill Companies Inc.: New York, USA, 2011; pp. 713–728. [Google Scholar]
- Nyachoti, C.M.; Omogbenigun, F.O.; Rademacher, M.; Blank, G. Performance responses and indicators of gastrointestinal health in early-weaned pigs fed low-protein amino acid-supplemented diets. J. Anim. Sci. 2006, 84, 125–134. [Google Scholar]
- Seppälä, M.; Pyykkönen, V.; Väisänen, A.; Rintala, J. Biomethane production from maize and liquid cow manure-effect of the share of maize, post methanation potential and digestate characteristics. Fuel 2013, 107, 209–216. [Google Scholar] [CrossRef]
- Nwanta, J.A.; Onunkwo, J.; Ezenduka, E. Analysis of Nsukka metropolitan abattoir solid waste and its bacterial contents in south eastern Nigeria: Public health implication. Arch. Environ. Occup. Health. 2010, 65, 21–26. [Google Scholar] [CrossRef]
- Ribaudo, M.; Gollehon, N.; Ailley, M.; Kaplan, J.; Johansson, R.; Agapoff, J.; Christenan, L.; Breneman, V.; Peters, M. Manure Management for Water Quality: Costs to Animal Feeding Operations of Applying Manure Nutrients to Land. In Agricultural Economic Report; United States Department of Agriculture: Washington, DC, USA, 2003; No. AER-824; p. 97. [Google Scholar]
- Health Care Canada, Guidelines for Canadian Drinking Water Quality: Technical Guideline Document-Bacterial Waterborne Pathogens-Current and Emerging Organisms of Concern. Water Quality and Health Bureau, Healthy Environment and Consumer Safety Branch, Health Canada: Ottawa, ON, Canada, 2006; pp. 1–34.
- Rapala, J.; Lahti, K.; Rasanen, L.A.; Esala, A.L.; Niemela, S.I.; Sivonen, K. Endotoxins associated with cyanobacteria and their removal during drinking water treatment. Water Res. 2002, 36, 2627–2635. [Google Scholar] [CrossRef]
- Wilkie, A.C. Anaerobic Digestion of Dairy Manure: Design and Process Considerations. In Dairy Manure Management: Treatment, Handling and Community Relations; Natural Resource, Agriculture, and Engineering Service, Cornell University: Ithaca, NY, USA, 2005; NRAES-176; pp. 301–312. [Google Scholar]
- Garcia, M.L.; Angenent, L.T. Interactions between temperature and ammonia in mesophilic digesters for animal waste treatment. Water Res. 2009, 43, 2373–2382. [Google Scholar] [CrossRef]
- Rico, C.; Rico, J.L.; Muňoz, N.; Gòmez, B.; Tejero, I. Effect of mixing on biogas production during mesophilic anaerobic digestion of screened dairy manure in a pilot plant. Eng. Life Sci. 2011, 11, 476–481. [Google Scholar] [CrossRef]
- Lutge, B.; Standish, B. Assessing the potential for electricity generation from animal waste biogas on South African farms. Agrekon: Agric. Econ. Res. Policy Pract. S. Afr. 2013, 52, 1–24. [Google Scholar]
- Burke, D.A. Dairy Waste Anaerobic Digestion Handbook: Options for Recovering Beneficial Products from Animal Manure; Environmental Energy Company: Olympia, WA, USA, 2001; pp. 1–51. Available online: http://www.makingenergy.com (accessed on 6 July 2013).
- Tucker, M.F. Farm digesters for small dairies in Vermont. BioCycle 2008, 49, 44. [Google Scholar]
- Goodrich, P.R.P.E. Anaerobic Digester Systems for Mid-Sized Dairy Farms; The Minnesota Project: St. Paul, MN, USA, 2005; pp. 1–46. [Google Scholar]
- Lozano, C.J.S.; Mendoza, M.V.; de Arango, M.C.; Monroy, E.F.C. Microbiological characterization and specific methanogenic activity of anaerobe sludges used in urban solid waste treatment. Waste Manag. 2009, 29, 704–711. [Google Scholar] [CrossRef]
- Song, H.; Clarke, W.P.; Blackall, L.L. Concurrent microscopic observations and activity measurements of cellulose hydrolyzing and methanogenic populations during the batch anaerobic digestion of crystalline cellulose. Biotechnol. Bioeng. 2005, 91, 369–378. [Google Scholar] [CrossRef]
- Franke-Whittle, I.H.; Goberna, M.; Pfister, V.; Insam, H. Design and development of the anaerochip microarray for investigation of methanogenic communities. J. Microbiol. Methods 2009, 79, 279–288. [Google Scholar]
- De Graaf, D.; Fendler, R. Biogas Production in Germany; Federal Environment Agency. Dessau Rosslau, Baltic Sea Region Programme. Dessau Rosslau, Baltic Sea Region Programme: Dessau-Rosslau, Germany, 2010; pp. 1–24. [Google Scholar]
- Cha, G.-C.; Chung, H.-K.; Kim, D.-J. Characteristics of temperature change on the substrate degradation and bacterial population in one-phase and two-phase anaerobic digestion. Environ. Eng. Res. 2001, 6, 99–108. [Google Scholar]
- Demirel, B.; Scherer, P. The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: A review. Rev. Environ. Sci. Biotechnol. 2008, 7, 173–190. [Google Scholar] [CrossRef]
- Brioukhanov, A.L.; Netrusov, A.I.; Eggen, R.I.L. The catalase and superoxide dismutase genes are transcriptionally up-regulated upon oxidative stress in the strictly anaerobic archaeon Methanosarcina barkeri. Microbiology 2006, 152, 1671–1677. [Google Scholar] [CrossRef]
- Keiner, A.; Leisinger, T. Oxygen sensitivity of methanogenic bacteria. Syst. Appl. Microbiol. 1983, 4, 305–312. [Google Scholar] [CrossRef]
- Fetzer, S.; Bak, F.; Conrad, R. Sensitivity of methanogenic bacteria from paddy soil to oxygen and desiccation. FEMS Microbiol. Ecol. 1993, 12, 107–115. [Google Scholar] [CrossRef]
- Anderson, K.L.; Apolinario, E.E.; Sowers, K.R. Desiccation as a long-term survival mechanism for the archaeon Methanosarcina barkeri. Appl. Environ. Microbiol. 2012, 78, 1473–1479. [Google Scholar] [CrossRef]
- Barber, R.D.; Ferry, J.G. Methanogenesis. Encyclopedia for Life; Nature Publishing Group: New York, NY, USA, 2001; pp. 1–8. Available online: http://www.els.net (accessed on 5 April 2013 ).
- McInerney, M.J.; Sieber, J.R.; Gunsalus, R.P. Syntrophy in anaerobic global carbon cycles. Curr. Opin. Biotechnol. 2009, 20, 623–632. [Google Scholar] [CrossRef]
- Blumer-Schuette, S.E.; Kataeva, I.; Westpheling, J.; Adams, M.W.W.; Kelly, R.M. Extremely thermophilic microorganisms for biomass conversion: status and prospects. Curr. Opin. Biotechnol. 2008, 19, 210–217. [Google Scholar] [CrossRef]
- Wirth, R.; Kovács, E.; Maròti, G.; Bagi, Z.; Rakhely, G.; Kovács, K.L. Characterization of a biogas—Producing microbial community by short-read next generation DNA sequencing. Biotechnol. Biofuels 2012, 5, 41. [Google Scholar] [CrossRef]
- Burrell, P.C.; O’Sullivan, C.; Song, H.; Clarke, W.P.; Black-all, L.L. The identification, detection and spatial resolution of Clostridium populations responsible for cellulose degradation in a methanogenic landfill leachate bioreactor. Appl. Environ. Microbiol. 2004, 70, 2414–2419. [Google Scholar] [CrossRef]
- Li, A.; Chu, Y.; Wang, X.; Ren, L.; Yu, J.; Liu, X.; Yan, J.; Zhang, L.; Wu, S.; Li, S. A pyrosequencing-based metagenomic study of methane-producing microbial community in solid-state biogas reactor. Biotechnol. Biofuels 2013, 6, 3. [Google Scholar] [CrossRef]
- McInerney, M.J.; Struchtemeyer, C.G.; Sieber, J.; Mouttaki, H.; Stams, A.J.M.; Schnink, B.; Rohlin, L.; Gunsalus, R.P. Physiology, Ecology, Phylogeny, and Genomics of Microorganisms Capable of Syntrophic Metabolism. Ann. N. Y. Acad. Sci. 2008, 1125, 58–72. [Google Scholar]
- Hori, T.; Sasaki, D.; Haruta, S.; Shigematsu, T.; Ueno, Y.; Ishii, M.; Igarashi, Y. Detection of active, potentially acetate-oxidizing syntrophs in an anaerobic digester by flux measurement and formyltetrahydrofolate synthetase expression profiling. Microbiology 2011, 157, 1980–1989. [Google Scholar]
- Siriwongrungson, V.; Zeng, R.J.; Angelidaki, I. Homoacetogenesis as the alternative pathway for H2 sink during thermophilic anaerobic degradation of butyrate under suppressed methanogenesis. Water Res. 2007, 41, 4202–4210. [Google Scholar]
- Hattori, S.; Galushko, A.S.; Kamagata, Y.; Schink, B. Operation of the CO dehydrogenase/acetyl coenzyme A pathway in both acetate oxidation and formation by the syntrophically acetate oxidizing bacterium Thermacetogenium phaeum. J. Bacteriol. 2005, 187, 3471–3476. [Google Scholar] [CrossRef]
- Lee, M.J.; Zinder, S.H. Isolation and characterization of a thermophilic bacterium which oxidizes acetate in syntrophic association with a methanogen and which grows acetogenically on H2-CO2. Appl. Environ. Microbiol. 1988, 52, 124–129. [Google Scholar]
- Schnürer, A.; Schink, B.; Svensson, B.H. Clostridium ultunense sp. nov., a mesophilic bacterium oxidizing acetate in syntrophic association with a hydrogenotrophic methanogen bacterium. Int. J. Syst. Bacteriol. 1996, 46, 1145–1152. [Google Scholar]
- Hattori, S.; Kamagata, Y.; Hanada, S.; Shuon, H. Thermacetogenium phaeum gen. nov., sp. nov., a strictly anaerobic thermophilic, syntrophic acetate-oxidizing bacterium. Int. J. Syst. Evol. Microbiol. 2000, 50, 1601–1609. [Google Scholar]
- Balk, M.; Weijma, J.; Stams, A.J.M. Thermotoga lettingae sp. nov., a novel thermophilic, methanol-degrading bacterium isolated from a thermophilic anaerobic reactor. Int. J. Syst. Evol. Microbiol. 2002, 52, 1361–1368. [Google Scholar]
- Westerholm, M.; Roos, S.; Schnürer, A. Syntrophaceticus schinkii gen. nov., sp. nov., an anaerobic syntrophic acetate-oxidizing bacterium isolated from a mesophilic anaerobic filter. FEMS Microbiol. Lett. 2010, 309, 100–104. [Google Scholar]
- Zhu, W.; Reich, C.I.; Olsen, G.J.; Giometti, C.S.; Yates, J.R. Shotgun proteomics of Methanococcus jannaschii and insights into methanogenesis. J. Proteome Res. 2004, 3, 538–548. [Google Scholar] [CrossRef]
- Attwood, G.T.; Kelly, W.J.; Altermann, E.H.; Leahy, S.C. Analysis of the Methanobrevibacter ruminantium draft genome: Understanding methanogen biology to inhibit their action in the rumen. Aust. J. Exp. Agric. 2007, 48, 83–88. [Google Scholar]
- Ver Eecke, H.C.; Butterfield, D.A.; Huber, J.A.; Lilley, M.D.; Olson, E.J.; Roe, K.K.; Evans, L.J.; Merkel, A.Y.; Cantin, H.V.; Holden, J.F. Hydrogen limited growth of hyperthermophilic methanogens at deep-sea hydrothermal vent. Proc. Natl. Acad. Sci. USA 2012, 109, 13674–13679. [Google Scholar] [CrossRef]
- Brune, A. Methanogenesis in the Digestive Tracts of Insects. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.W., Ed.; Springer-Verlag: Berlin/Herdelberg, Germany, 2010; pp. 707–728. [Google Scholar]
- Westerholm, M.; Levén, L.; Schnürer, A. Bioaugmentation of syntrophic acetate-oxidizing culture in biogas reactors exposed to increasing levels of ammonia. Appl. Environ. Microbiol. 2012, 78, 7619–7625. [Google Scholar] [CrossRef]
- De Macario, E.C. Taxonomy of Methanogens. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Springer: New York, NY, USA, 2008. [Google Scholar]
- Batstone, D.J.; Keller, J.; Angelidaki, I.; Kalyuzhnyi, S.V.; Pavlostathis, S.G.; Rozzi, A.; Sanders, W.T.M.; Siegrist, H.; Vavilin, V.A. The IWA anaerobic digestion model No.1 (ADM1). Water Sci. Technol. 2002, 45, 65–73. [Google Scholar]
- Krakat, N.; Westphal, A.; Schmidt, S.; Scherer, P. Anaerobic digestion of renewable biomass: Thermophilic temperature governs methanogen population dynamics. Appl. Environ. Microbiol. 2010, 76, 1842–1850. [Google Scholar] [CrossRef]
- Krakat, N.; Schmidt, S.; Scherer, P. Mesophilic fermentation of renewable biomass: Does hydraulic retention time regulate methanogen diversity. Appl. Environ. Microbiol 2010, 76, 6322–6326. [Google Scholar] [CrossRef]
- Klocke, M.; Mähnert, P.; Mundt, K.; Souidi, K.; Linke, B. Microbial community analysis of a biogas-producing completely stirred tank reactor fed continuously with fodder beet silage as mono-substrate. Syst. Appl. Microbiol. 2007, 30, 139–151. [Google Scholar] [CrossRef]
- Klocke, M.; Nettmann, E.; Bergmann, I.; Mundt, K.; Souidi, K.; Mumme, J.; Linke, B. Characterization of the methanogenic archaea within two-phase biogas reactor systems operated with plant biomass. Syst. Appl. Microbiol. 2008, 31, 190–205. [Google Scholar] [CrossRef]
- Amani, T.; Nosrati, M.; Sreekrishnan, T.R. Anaerobic digestion from the viewpoint of microbiological, chemical, and operational aspects: A review. Environ. Rev. 2010, 18, 255–278. [Google Scholar] [CrossRef]
- Solera, R.; Romero, L.I.; Sales, D. Determination of the microbial population in thermophilic anaerobic reactor: Comparative analysis by different counting methods. Anaerobe 2001, 7, 79–86. [Google Scholar] [CrossRef]
- Ziganshin, A.M.; Schmidt, T.; Scholwin, F.; II’inskaya, O.N.; Harms, H.; Kleinsteuber, S. Bacteria and Archaea involved in anaerobic digestion of distillers grains with solubles. Appl. Microbiol. Biotechnol. 2011, 89, 2039–2052. [Google Scholar] [CrossRef]
- Rivière, D.; Desvignes, V.; Pelletier, E.; Chaussonnerie, S.; Guermazi, S.; Weissenbach, J.; Li, T.; Camacho, P.; Sghir, A. Towards the definition of a core of microorganisms involved in anaerobic digestion of sludge. Int. Soc. Microb. Ecol. 2009, 3, 700–714. [Google Scholar]
- Scully, C.; Collins, G.; O’Flaherty, V. Assessment of anaerobic wastewater treatment failure using terminal restriction fragment length polymorphism analysis. J. Appl. Microbiol. 2005, 99, 1463–1471. [Google Scholar] [CrossRef]
- Kataoka, N.; Tokiwa, Y.; Takeda, K. Improved technique for identification and enumeration of methanogenic bacterial colonies on roll tubes by epifluorescence microscopy. Appl. Environ. Microbiol. 1991, 57, 3671–3673. [Google Scholar]
- Singh, L.S.; Mazumder, P.B. Differential approaches for studying methanogens: Methods, analysis and prospects. Assam Univ. J. Sci. Technol. 2010, 6, 123–128. [Google Scholar]
- Schlüter, A.; Bekel, T.; Diaz, N.N.; Dondrup, M.; Eichenlaub, R.; Gartemann, K-H.; Krahn, I.; Krause, L.; Krömeke, H.; Kruse, O.; et al. The metagenome of a biogas-producing microbial community of a production-scale biogas plant fermenter analyzed by the 454-pyrosequencing technology. J. Biotechnol. 2008, 136, 77–90. [Google Scholar]
- Lee, C.; Kim, J.; Shin, S.G.; Hwang, S. Monitoring bacterial and archaeal community shifts in a mesophilic anaerobic batch reactor treating a high-strength organic wastewater. FEMS Microbiol. Ecol. 2008, 65, 544–554. [Google Scholar] [CrossRef]
- Cirne, D.G.; Lehtomäki, A.; Björnsson, L.; Blackall, L.L. Hydrolysis and microbial community analyses in two-stage anaerobic digestion of energy crops. J. Appl. Microbiol. 2006, 103, 516–527. [Google Scholar]
- Jaenicke, S.; Ander, C.; Bekel, T.; Bisdorf, R.; Dröge, M.; Gartemann, K.-H.; Jüneman, S.; Kaiser, O.; Krause, L.; Tille, F.; et al. Comparative and joint analyses of two-metagenomic dataset from a biogas fermenter obtained by 454-pyrosequencing. PLoS One 2011, 6, e14519. [Google Scholar] [CrossRef]
- Li, M.; Cao, H.; Hong, Y.-G.; Gu, J.-D. Seasonal dynamics of anammox bacteria in estuarial sediment Mai Po nature reserve revealed by analyzing the 16S rRNA and hydrazine oxidoreductase (hzo) genes. Microbes Environ. 2011, 26, 15–22. [Google Scholar] [CrossRef]
- Ozgun, D.; Basak, S.; Cinar, O. Current Molecular Biologic Techniques for Anaerobic Ammonium Oxidizing (Anammox) Bacteria. In Proceedings of the Sixteenth International Water Technology Conference, IWTC, Istanbul, Turkey, 7–10 May 2012; pp. 1–15.
- Harhangi, H.R.; Roy, M.L.; Alen, T.V.; Hu, B.-L.; Groen, J.; Kartal, B.; Tringe, S.G.; Quan, Z.-X.; Jetten, M.S.M.; den Camp, H.J.M.O. Hydrazine synthase, a unique phylomarker with which to study the presence and biodiversity of anammox bacteria. Appl. Environ. Microbiol. 2012, 78, 752–758. [Google Scholar] [CrossRef]
- Zhou, M.; McAllister, T.A.; Guan, L.L. Molecular identification of rumen methanogen: Technologies, advances and prospects. Anim. Feed Sci. Technol. 2011, 166–167, 76–86. [Google Scholar] [CrossRef]
- Lutton, P.E.; Wayne, J.M.; Sharp, R.J.; Riley, P.W. The mcrA gene as an alternative to 16S rRNA in the phylogenetic analysis of methanogen population in landfills. Microbiology 2002, 148, 3521–3530. [Google Scholar]
- Denman, S.E.; Tomkins, N.W.; McSweeney, C.S. Quantitation and diversity analysis of ruminal methanogenic populations in response to the antimethanogenic compound bromochloromethane. FEMS Microbiol. Ecol. 2006, 62, 313–322. [Google Scholar]
- Jiang, B.; Song, K.; Ren, J.; Deng, M.; Sun, F.; Zhang, X. Comparison of metagenomic samples using sequence signatures. BMC Genomics 2012, 13, 730. [Google Scholar] [CrossRef]
- Mashhadi, Z. Identification and Characterization of the Enzymes Involved in Biosynthesis of FAD and Tetrahydromethanopterin in Methanococcus jannaschii. Ph.D. Thesis, Virginia Polytechnic Institute and State University (Virginia Tech), Blacksburg, VA , USA, 30 June 2010; pp. 1–136. [Google Scholar]
- Leahy, S.C.; Kelly, W.J.; Altermann, E.; Ronimus, R.S.; Yeoman, C.J.; Pacheco, D.M.; Li, D.; Kong, Z.; McTavish, S.; Sang, C.; et al. The genome sequence of the rumen methanogen Methanobrevibacter ruminantium reveals new possibilities for controlling ruminant methane emission. PLoS One 2010, 5, e8926. [Google Scholar] [CrossRef]
- Handelsman, J. Metagenomics: Application of genomics to uncultured microorganisms. Microbiol. Mol. Biol. Rev. 2004, 68, 669–685. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Dupont, C.L. Microbial metagenomics: Beyond the genome. Annu. Rev. Mar. Sci. 2004, 3, 347–371. [Google Scholar] [CrossRef]
- Ilaboya, I.R.; Assekhame, F.F.; Ezugwu, M.O.; Erameh, A.A.; Omofuma, F.E. Studies on biogas generation from agricultural wastes; analysis of the effects of alkaline on gas generation. World Appl. Sci. J. 2010, 9, 537–545. [Google Scholar]
- Umaňa, O.; Nikolaeva, S.; Sanchez, E.; Borja, R.; Raposo, F. Treatment of screened dairy manure by upflow anaerobic fixed bed reactors packed with waste tyre rubber and a combination of waste tyre rubber and Zeolite: Effect of the hydraulic retention time. Bioresour. Technol. 2008, 99, 7412–7417. [Google Scholar] [CrossRef]
- Balsam, J.; Ryan, D. Anaerobic Digestion of Animal Wastes: Factors to Consider; ATTRA: Butte, MT, USA, 2006; pp. 1–10. [Google Scholar]
- Cioabla, A.E.; Lonel, L.; Dumitrel, G.-A.; Popescu, F. Comparative study on factors affecting anaerobic digestion of agricultural vegetal residues. Biotechnol. Biofuels 2012, 5, 39. [Google Scholar] [CrossRef]
- Choorit, W.; Wisarnwan, P. Effect of temperature on the anaerobic digestion of palm oil mill effluent. Electron. J. Biotechnol. 2007, 10, 376–385. [Google Scholar]
- Saleh, M.M.A.; Mahmood, U.F. Anaerobic Digestion Technology for Industrial Waste Water Treatment. In Proceedings of the Eighth International Water Technology Conference, IWTC, Alexandria, Egypt, 26–28 March 2004; pp. 817–833.
- Rittmann, B.E.; McCarty, P.L. Environmental Biotechnology: Principles and Applications; McGraw-Hill Book Co.: New York, NY, USA, 2001; p. 768. [Google Scholar]
- El-Mashad, H.M.; Zeeman, G.; van Loon, W.K.P.; Bot, G.P.A.; Lettinga, G. Effect of temperature and temperature fluctuation on thermophilic anaerobic digestion of cattle manure. Bioresour. Technol. 2004, 95, 191–201. [Google Scholar] [CrossRef]
- Campos, E.; Palatsi, J.; Flotats, X. Co-Digestion of Pig Slurry and Organic Wastes from Food Industry. In Proceedings of the 2th International Symposium on Anaerobic Digestion of Solid Waste, Barcelona, Junio, Spain, 15–18 June 1999; pp. 192–195.
- Gerardi, M.H. The Microbiology of Anaerobic Digesters; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2003; pp. 91–118. [Google Scholar]
- Molinuevo-Salces, B.; García-González, M.C.; González-Fernández, C.; Cuetos, M.J.; Morán, A.; Gòmez, X. Anaerobic co-digestion of livestock wastes with vegetable processing wastes: A statistical analysis. Bioresour. Technol. 2010, 101, 9479–9485. [Google Scholar] [CrossRef]
- Veeken, A.; Kalyuzhnyi, S.; Scharff, H.; Hamelers, B. Effect of pH and VFA on hydrolysis of organic solid waste. J. Environ. Eng. 2000, 126, 1076–1081. [Google Scholar] [CrossRef]
- El Hadj, T.B.; Astals, S.; Galí, A.; Mace, S.; Mata-Álvarez, J. Ammonia influence in anaerobic digestion of OFMSM. Water Sci. Technol. 2009, 59, 1153–1158. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process. A review. bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Strik, D.P.B.T.B.; Domnanovich, A.M.; Holubar, P. A pH-based control of ammonia in biogas during anaerobic digestion of artificial pig manure and maize silage. Process Biochem. 2006, 41, 1235–1238. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ahring, B.K. Thermophilic anaerobic digestion of livestock waste: The effect of ammonia. Appl. Microbiol. Biotechnol. 1993, 38, 560–564. [Google Scholar]
- Bolzonella, D.; Pavan, P.; Battistoni, P.; Cecchi, F. Mesophilic anaerobic digestion of waste activated sludge: Influence of solid retention time in the wastewater treatment process. Process Biochem. 2005, 40, 1453–1460. [Google Scholar] [CrossRef]
- Karim, K.; Hoffmann, R.; Klasson, K.T.; Al-Dahhan, M.H. Anaerobic digestion of animal waste: Effects of mode of mixing. Water Res. 2009, 39, 3597–3606. [Google Scholar]
- Babaee, A.; Shayegan, J. Effects of Organic Loading Rates (OLR) on Production of Methane from Anaerobic Digestion of Vegetable Waste. In Proceedings of the World Renewable Energy Congress, Linköping, Sweden, 8–13 May 2011; pp. 411–417.
- Rincòn, B.; Travieso, L.; Sanchez, E.; Martín, M.L.A.; Martín, A.; Raposo, F.; Borja, R. The effect of organic loading rate on the anaerobic digestion of two-phase olive mill solid residue derived from fruits with low ripening index. J. Chem. Technol. Biotechnol. 2007, 82, 259–266. [Google Scholar] [CrossRef]
- Rincòn, B.; Borja, R.; González, J.M.; Portillo, M.C.; Sáiz-Jiménez, C. Influence of organic loading rate and hydraulic retention time on the performance, stability, and microbial communities of one-stage anaerobic digestion of two-phase olive mill solid residue. Biochem. Eng. J. 2008, 40, 253–261. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Wastewater Engineering: Treatment and Reuse, 4th ed.; Metcalf and Eddy, Inc., Tata Mcgraw-Hill Publishing Company Ltd.: New Delhi, India, 2003. [Google Scholar]
- Zhang, R.; El-Mashad, H.M.; Hartman, K.; Wang, F.; Liu, G.; Choate, C.; Gamble, P. Characterization of food waste as feedstock for anaerobic digestion. Bioresour. Technol. 2007, 98, 929–935. [Google Scholar] [CrossRef]
- Matseh, I. Effect of Ni and Co as trace elements on digestion performance and biogas produced from the fermentation of palm oil mill effluent. Int. J. Waste Resour. 2012, 2, 16–19. [Google Scholar]
- Gustavsson, J. Cobalt and Nickel Bioavailability for Biogas Formation. Ph.D. Thesis, Department of Thematic studies, University of Linköping, Linköping, Sweden, 19 January 2012; pp. 1–64. [Google Scholar]
- Pobeheim, H.; Munk, B.; Johansson, J.; Guebitz, G.M. Influence of trace elements on methane formation froma synthetic model substrate for maize silage. Bioresour. Technol. 2010, 101, 836–839. [Google Scholar] [CrossRef]
- Facchin, V.; Cavinato, C.; Fatone, F.; Pavan, P.; Cecchi, F.; Bolzonella, D. Effect of trace element supplementation on the mesophilic anaerobic digestion of food wastes in batch trials. The influence of inoculum origin. Biochem. Eng. J. 2013, 70, 71–77. [Google Scholar] [CrossRef]
- Rojas, C.; Fang, S.; Uhlenhut, F.; Borchert, A.; Stein, I.; Schlaak, M. Stirring and biomass starter influences the anaerobic digestion of different substrates for biogas production. Eng. Life Sci. 2010, 10, 339–347. [Google Scholar] [CrossRef]
- Ghanimeh, S.; El Fadel, M.; Saikaly, P. Mixing effect on thermophilic anaerobic digestion of source-sorted organic fraction of municipal solid waste. Bioresour. Technol. 2012, 117, 63–71. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Manyi-Loh, C.E.; Mamphweli, S.N.; Meyer, E.L.; Okoh, A.I.; Makaka, G.; Simon, M. Microbial Anaerobic Digestion (Bio-Digesters) as an Approach to the Decontamination of Animal Wastes in Pollution Control and the Generation of Renewable Energy. Int. J. Environ. Res. Public Health 2013, 10, 4390-4417. https://doi.org/10.3390/ijerph10094390
Manyi-Loh CE, Mamphweli SN, Meyer EL, Okoh AI, Makaka G, Simon M. Microbial Anaerobic Digestion (Bio-Digesters) as an Approach to the Decontamination of Animal Wastes in Pollution Control and the Generation of Renewable Energy. International Journal of Environmental Research and Public Health. 2013; 10(9):4390-4417. https://doi.org/10.3390/ijerph10094390
Chicago/Turabian StyleManyi-Loh, Christy E., Sampson N. Mamphweli, Edson L. Meyer, Anthony I. Okoh, Golden Makaka, and Michael Simon. 2013. "Microbial Anaerobic Digestion (Bio-Digesters) as an Approach to the Decontamination of Animal Wastes in Pollution Control and the Generation of Renewable Energy" International Journal of Environmental Research and Public Health 10, no. 9: 4390-4417. https://doi.org/10.3390/ijerph10094390
APA StyleManyi-Loh, C. E., Mamphweli, S. N., Meyer, E. L., Okoh, A. I., Makaka, G., & Simon, M. (2013). Microbial Anaerobic Digestion (Bio-Digesters) as an Approach to the Decontamination of Animal Wastes in Pollution Control and the Generation of Renewable Energy. International Journal of Environmental Research and Public Health, 10(9), 4390-4417. https://doi.org/10.3390/ijerph10094390






