Indicators for Healthy Ageing — A Debate
Abstract
:1. Introduction
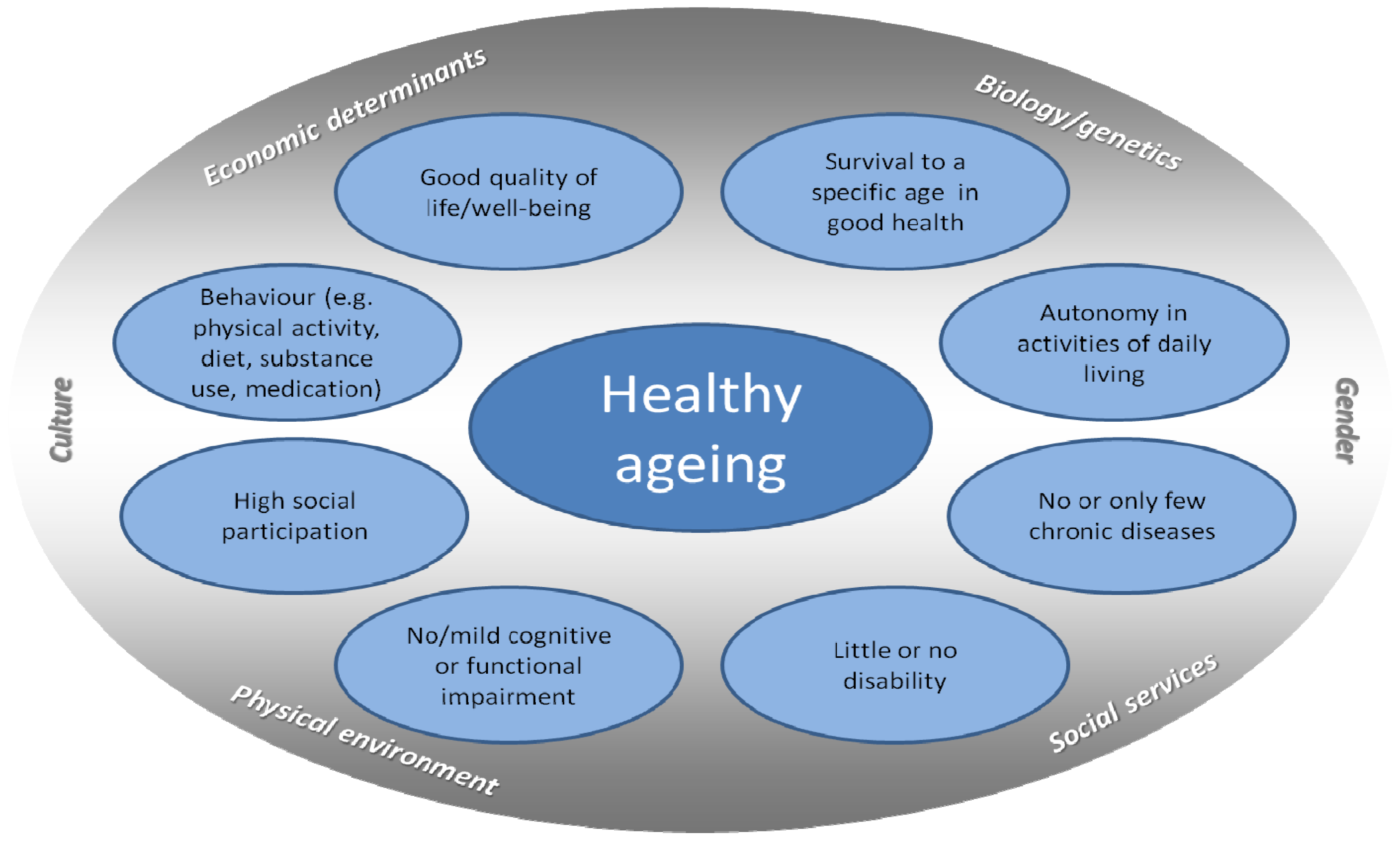
- Prevalence of chronic diseases and multimorbidity;
- Development of healthy life expectancy at the transition to oldest-age;
- Physical activity in old age;
- Assessment of cognitive capability in old age;
- Functioning and disability in old age.
2. Results
2.1. Prevalence of Chronic Diseases and Multimorbidity
2.2. The Development of Healthy Life Expectancy at the Transition to Oldest-Age
2.3. Physical Activity in Old Age
2.4. The Assessment of Cognitive Capability in Old Age
2.5. Functioning and Disability in Old Age
3. Conclusions
Acknowledgments
Conflicts of Interest
References
- The Greying of the Baby Boomers. A Century-Long View of Ageing in European Populations. Available online: http://ec.europa.eu/digital-agenda/futurium/en/content/greying-baby-boomers-century-long-view-ageing-european-populations (accessed on 22 November 2013).
- Ageing Characterises the Demographic Perspectives of the European Societies. Available online: http://epp.eurostat.ec.europa.eu/portal/page/portal/product_details/publication?p_product_code=KS-SF-08-072 (accessed on 25 November 2013).
- Rowe, J.W.; Kahn, R.L. Human aging: Usual and successful. Science 1987, 237, 143–149. [Google Scholar]
- Lowry, K.A.; Vallejo, A.N.; Studenski, S.A. Successful aging as a continuum of functional independence: Lessons from physical disability models of aging. Aging Dis. 2012, 3, 5–15. [Google Scholar]
- Active Aging: A Policy Framework. Available online: http://www.who.int/ageing/publications/active_ageing/en/ (accessed on 25 November 2013).
- Peel, N.; Bartlett, H.; McClure, R. Healthy ageing: How is it defined and measured? Australa. J. Ageing 2004, 23, 115–119. [Google Scholar] [CrossRef]
- Fernandez-Ballesteros, R.; Robine, J.M.; Walker, A.; Kalache, A. Active aging: A global goal. Curr. Gerontol. Geriatr. Res. 2013, 2013. [Google Scholar] [CrossRef]
- McLaughlin, S.J.; Jette, A.M.; Connell, C.M. An examination of healthy aging across a conceptual continuum: Prevalence estimates, demographic patterns, and validity. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 783–789. [Google Scholar] [CrossRef]
- Crimmins, E.M.; Beltrán-Sánchez, H. Mortality and morbidity trends: Is there compression of morbidity? J. Gerontol. B Psychol. Sci. Soc. Sci. 2011, 66B, 75–86. [Google Scholar] [CrossRef]
- Fries, J.F.; Bruce, B.; Chakravarty, E. Compression of morbidity 1980–2011: A focused review of paradigms and progress. J. Aging Res. 2011, 2011. [Google Scholar] [CrossRef]
- Parker, M.G.; Thorslund, M. Health trends in the elderly population: Getting better and getting worse. Gerontologist 2007, 47, 150–158. [Google Scholar] [CrossRef]
- Robine, J.-M.; Michel, J.-P. Looking forward to a general theory on population aging. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, M590–M597. [Google Scholar] [CrossRef]
- Marengoni, A.; Angleman, S.; Melis, R.; Mangialasche, F.; Karp, A.; Garmen, A.; Meinow, B.; Fratiglioni, L. Aging with multimorbidity: A systematic review of the literature. Ageing Res. Rev. 2011, 10, 430–439. [Google Scholar] [CrossRef]
- Fortin, M.; Stewart, M.; Poitras, M.-E.; Almirall, J.; Maddocks, H. A systematic review of prevalence studies on multimorbidity: Toward a more uniform methodology. Ann. Fam. Med. 2012, 10, 142–151. [Google Scholar] [CrossRef]
- Gershon, R.C.; Cella, D.; Fox, N.A.; Havlik, R.J.; Hendrie, H.C.; Wagster, M.V. Assessment of neurological and behavioural function: The NIH toolbox. Lancet Neurol. 2010, 9, 138–139. [Google Scholar] [CrossRef]
- Victorson, D.; Manly, J.; Wallner-Allen, K.; Fox, N.; Purnell, C.; Hendrie, H.; Havlik, R.; Harniss, M.; Magasi, S.; Correia, H.; et al. Using the NIH toolbox in special populations: Considerations for assessment of pediatric, geriatric, culturally diverse, non-english-speaking, and disabled individuals. Neurology 2013, 80, S13–S19. [Google Scholar]
- Kuh, D.; New Dynamics of Ageing Preparatory Network. A life course approach to healthy aging, frailty, and capability. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 717–721. [Google Scholar] [CrossRef]
- Rodriguez-Manas, L.; Feart, C.; Mann, G.; Vina, J.; Chatterji, S.; Chodzko-Zajko, W.; Gonzalez-Colaco Harmand, M.; Bergman, H.; Carcaillon, L.; Nicholson, C.; et al. Searching for an operational definition of frailty: A delphi method based consensus statement. The frailty operative definition-consensus conference project. J. Gerontol. Series A Biol. Sci. Med. Sci. 2013, 68, 62–67. [Google Scholar] [CrossRef]
- European Innovation Partnership on Active and Healthy Ageing. Available online: http://ec.europa.eu/research/innovation-union/index_en.cfm?section=active-healthy-ageing&pg=home (accessed on 22 Feberuary 2013).
- Boersch-Supan, A.; Juerges, H.E. The Survey of Health, Ageing and Retirement in Europe—Methodology; Mannheim Research Institute for the Economics of Aging (MEA): Mannheim, Germany, 2005. [Google Scholar]
- Healthy Ageing across the Life Course. Available online: http://www.halcyon.ac.uk/ (0222) (accessed on 22 November 2013).
- Nelson, M.E.; Rejeski, W.J.; Blair, S.N.; Duncan, P.W.; Judge, J.O.; King, A.C.; Macera, C.A.; Castaneda-Sceppa, C. Physical activity and public health in older adults: Recommendation from the American college of sports medicine and the American heart association. Med. Sci. Sports Exerc. 2007, 39. [Google Scholar] [CrossRef]
- Fuchs, J.; Busch, M.; Lange, C.; Scheidt-Nave, C. Prevalence and patterns of morbidity among adults in Germany. Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz 2012, 55, 576–586. [Google Scholar] [CrossRef]
- Europe in Figures—Eurostat Yearbook 2012. Available online: http://epp.eurostat.ec.europa.eu/portal/page/portal/product_details/publication?p_product_code=KS-CD-12-001 (accessed on 22 November 2013).
- Nusselder, W.J. Compression of Morbidity. In Determining Health Expectancies; Robine, J.-M., Jagger, C., Mathers, C.D., Crimmins, E.M., Suzman, R.M., Eds.; John Wiley & Sons Inc: Chichester, UK, 2003. [Google Scholar]
- Fries, J.F. The compression of morbidity. Milbank Q. 2005, 83, 801–823. [Google Scholar] [CrossRef]
- Mergenthaler, A. Die entwicklung der gesunden lebenserwartung im alter. Ein kohortenvergleich auf der grundlage des deutschen alterssurveys. Bevölkerungsforschung Aktuell 2011, 32, 2–7. [Google Scholar]
- Sullivan, D.F. A single index of morbidity and mortality. HSMHA Health Rep. 1971, 86, 347–354. [Google Scholar] [CrossRef]
- West, B.; Welch, K.B.; Galecki, A.T. Linear Mixed Models. A Practical Guide Using Statistical Software; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Imai, K.; Soneji, S. On the estimation of disability-free life expectancy: Sullivan’s method and its extension. J. Am. Stat. Assoc. 2007, 102, 1199–1211. [Google Scholar] [CrossRef]
- Barendregt, J.J. Disability-Adjusted Life Years (DALYS) and Disability-Adjusted Life Expectancy (Dale). In Determining Health Expectancies; Robine, J.-M., Jagger, C., Mathers, C.D., Crimmins, E.M., Suzman, R.M., Eds.; John Wiley & Sons Inc: Chichester, UK, 2003. [Google Scholar]
- Doblhammer, G.; Kytir, J. Compression or expansion of morbidity? Trends in healthy-life expectancy in the elderly austrian population between 1978 and 1998. Soc. Sci. Med. 2001, 52, 385–391. [Google Scholar] [CrossRef]
- Chodzko-Zajko, W.J.; Proctor, D.N.; Singh, M.A.F.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef]
- Patel, K.V.; Coppin, A.K.; Manini, T.M.; Lauretani, F.; Bandinelli, S.; Ferrucci, L.; Guralnik, J.M. Midlife physical activity and mobility in older age—the inchianti study. Am. J. Prev. Med. 2006, 31, 217–224. [Google Scholar] [CrossRef]
- Wu, S.C.; Leu, S.Y.; Li, C.Y. Incidence of and predictors for chronic disability in activities of daily living among older people in taiwan. J. Am. Geriatr. Soc. 1999, 47, 1082–1086. [Google Scholar]
- Kaplan, M.S.; Newsom, J.T.; McFarland, B.H.; Lu, L. Demographic and psychosocial correlates of physical activity in late life. Am. J. Prev. Med. 2001, 21, 306–312. [Google Scholar] [CrossRef]
- Pratt, M.; Macera, C.A.; Wang, G.J. Higher direct medical costs associated with physical inactivity. Phys. Sports Med. 2000, 28, 63–70. [Google Scholar]
- Thiem, U.; Theile, G.; Junius-Walker, U.; Holt, S.; Thürmann, P.; Hinrichs, T.; Platen, P.; Diederichs, C.; Berger, K.; Hodek, J.M.; et al. Prerequisites for a new health care model for elderly people with multimorbidity. Zeitschrift fur Gerontologie und Geriatrie 2011, 44, 115–120. [Google Scholar] [CrossRef]
- Moschny, A.; Platen, P.; Klaaßen-Mielke, R.; Trampisch, U.; Hinrichs, T. Physical activity patterns in older men and women in German: A cross-sectional study. BMC Public Health 2011, 11. [Google Scholar] [CrossRef]
- Moschny, A.; Platen, P.; Klaaßen-Mielke, R.; Trampisch, U.; Hinrichs, T. Barriers to physical activity in older adults in Germany: A cross-sectional study. Int. J. Behav. Nutr. Phys. Act. 2011, 8. [Google Scholar] [CrossRef]
- Hinrichs, T.; Moschny, A.; Klaaßen-Mielke, R.; Trampisch, U.; Thiem, U.; Platen, P. General practitioner advice on physical activity: Analyses in a cohort of older primary health care patients (getABI). BMC Fam. Pract. 2011, 12. [Google Scholar] [CrossRef]
- GetABI Study Group. GetABI: German epidemiological trial on ankle brachial index for elderly patients in family practice to detect peripheral arterial disease, significant marker for high mortality. J. Vasc. Dis. 2002, 31, 241–248. [Google Scholar]
- Trampisch, U.; Platen, P.; Burghaus, I.; Moschny, A.; Wilm, S.; Thiem, U.; Hinrichs, T. Reliability of the PRISCUS-PAQ: Questionnaire to assess physical activity of persons aged 70 years and older. Zeitschrift fur Gerontologie und Geriatrie 2010, 43, 399–406. [Google Scholar] [CrossRef]
- Trampisch, U.S.; Platen, P.; Moschny, A.; Wilm, S.; Thiem, U.; Hinrichs, T. Measurement of physical activity in older adults: Correlation between the PRISCUS-PAQ and accelerometry. Zeitschrift fur Gerontologie und Geriatrie 2012, 45, 212–217. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- Sano, M. Neuropsychological testing in the diagnosis of dementia. J. Geriatr. Psychiatry Neurol. 2006, 19, 155–159. [Google Scholar] [CrossRef]
- Collie, A.; Maruff, P.; Darby, D.G.; McStephen, M. The effects of practice on the cognitive test performance of neurologically normal individuals assessed at brief test-retest intervals. J. Int. Neuropsychol. Soc. 2003, 9, 419–428. [Google Scholar]
- Chelune, G.J.; Naugle, R.I.; Lüders, H.; Sedlak, J.; Awad, I.A. Individual change after epilepsy surgery: Practice effects and base-rate information. Neuropsychology 1993, 7, 41–52. [Google Scholar] [CrossRef]
- Stein, J.; Luppa, M.; Brähler, E.; König, H.-H.; Riedel-Heller, S.G. The assessment of changes in cognitive functioning: Reliable change indices for neuropsychological instruments in the elderly—A systematic review. Dement. Geriatr. Cogn. Disord. 2010, 29, 275–286. [Google Scholar] [CrossRef]
- Anstey, K.; Christensen, H. Education, activity, health, blood pressure and apolipoprotein E as predictors of cognitive change in old age: A review. Gerontology 2000, 46, 163–177. [Google Scholar] [CrossRef]
- Stern, Y. Cognitive reserve. Neuropsychologia 2009, 47, 2015–2028. [Google Scholar] [CrossRef]
- Valenzuela, M.; Sachdev, P. Can cognitive exercise prevent the onset of dementia? Systematic review of randomized clinical trials with longitudinal follow-up. Am. J. Geriatr. Psychiatry 2009, 17, 179–187. [Google Scholar] [CrossRef]
- Stein, J.; Luppa, M.; Luck, T.; Maier, W.; Wagner, M.; Daerr, M.; van den Bussche, H.; Zimmermann, T.; Köhler, M.; Bickel, H.; et al. The assessment of changes in cognitive functioning: Age-, education-, and gender-specific reliable change indices for older adults tested on the cerad-np battery: Results of the German study on ageing, cognition, and dementia in primary care patients (AgeCoDe). Am. J. Geriatr. Psychiatry 2012, 20. [Google Scholar] [CrossRef]
- Stein, J.; Luppa, M.; Maier, W.; Tebarth, F.; Heser, K.; Scherer, M.; Zimmermann, T.; Eisele, M.; Bickel, H.; Mosch, E.; et al. The assessment of changes in cognitive functioning in the elderly: Age- and education-specific reliable change indices for the SIDAM. Dement. Geriatr. Cogn. Disord. 2012, 33, 73–83. [Google Scholar] [CrossRef]
- Stein, J.; Luppa, M.; Maier, W.; Wagner, M.; Wolfsgruber, S.; Scherer, M.; Kohler, M.; Eisele, M.; Weyerer, S.; Werle, J.; et al. Assessing cognitive changes in the elderly: Reliable change indices for the mini-mental state examination. Acta Psychiatr. Scand. 2012, 126, 208–218. [Google Scholar] [CrossRef]
- International Classification of Functioning, Disability and Health (ICF). Available online: http://www.who.int/classifications/icf/en/ (accessed on 22 November 2013).
- Peters, A.; Doring, A.; Ladwig, K.H.; Meisinger, C.; Linkohr, B.; Autenrieth, C.; Baumeister, S.E.; Behr, J.; Bergner, A.; Bickel, H.; et al. Multimorbidity and successful aging: The population-based KORA-age study. Zeitschrift fur Gerontologie und Geriatrie 2011, 44, 41–54. [Google Scholar] [CrossRef]
- Strobl, R.; Muller, M.; Emeny, R.; Peters, A.; Grill, E. Distribution and determinants of functioning and disability in aged adults—results from the German KORA-age study. BMC Public Health 2013, 13. [Google Scholar] [CrossRef]
- Fries, J.F.; Spitz, P.W.; Young, D.Y. The dimensions of health outcomes: The health assessment questionnaire, disability and pain scales. J. Rheumatol. 1982, 9, 789–793. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fuchs, J.; Scheidt-Nave, C.; Hinrichs, T.; Mergenthaler, A.; Stein, J.; Riedel-Heller, S.G.; Grill, E. Indicators for Healthy Ageing — A Debate. Int. J. Environ. Res. Public Health 2013, 10, 6630-6644. https://doi.org/10.3390/ijerph10126630
Fuchs J, Scheidt-Nave C, Hinrichs T, Mergenthaler A, Stein J, Riedel-Heller SG, Grill E. Indicators for Healthy Ageing — A Debate. International Journal of Environmental Research and Public Health. 2013; 10(12):6630-6644. https://doi.org/10.3390/ijerph10126630
Chicago/Turabian StyleFuchs, Judith, Christa Scheidt-Nave, Timo Hinrichs, Andreas Mergenthaler, Janine Stein, Steffi G. Riedel-Heller, and Eva Grill. 2013. "Indicators for Healthy Ageing — A Debate" International Journal of Environmental Research and Public Health 10, no. 12: 6630-6644. https://doi.org/10.3390/ijerph10126630
APA StyleFuchs, J., Scheidt-Nave, C., Hinrichs, T., Mergenthaler, A., Stein, J., Riedel-Heller, S. G., & Grill, E. (2013). Indicators for Healthy Ageing — A Debate. International Journal of Environmental Research and Public Health, 10(12), 6630-6644. https://doi.org/10.3390/ijerph10126630



