Comparison of the Cytotoxic Potential of Cigarette Smoke and Electronic Cigarette Vapour Extract on Cultured Myocardial Cells
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
| Samples | Nicotine concentration (mg/mL) | Main ingredients a | Distributor/Manufacturer |
|---|---|---|---|
| City | 6 | 30% VG/60% PG | Alter Ego/El Greco |
| Americano | 9 | 30% VG/60% PG | Alter Ego/El Greco |
| Tribeca | 12 | VG/PG * | Alter Ego/Halo |
| Classic | 18 | 30% VG/60% PG | Alter Ego/El Greco |
| Cinnamon & Cookies | 6 | 50% VG/50% PG | Atmos Lab |
| RY69 | 6 | 50% VG/50% PG | Atmos Lab |
| Green apple | 12 | 50% VG/50% PG | Atmos Lab |
| Bebeka | 18 | 50% VG/50% PG | Atmos Lab |
| Base b | 0 | 50% VG/50% PG | Flavourart |
| MaxBlend | 9 | 85% VG | Flavourart |
| RY4 | 9 | 85% VG | Flavourart |
| Virginia | 18 | 85% VG | Flavourart |
| El Toro Cigarrillos (1) c | 12 | VG/PG * | House Of Liquid |
| El Toro Cigarrillos (2) c | 12 | VG/PG * | House Of Liquid |
| Silverberry | 12 | VG/PG * | House Of Liquid |
| El Toro Guevara c | 18 | VG/PG * | House Of Liquid |
| El Toro Puros c | 24 | VG/PG * | House Of Liquid |
| Golden Margy | 6 | 20% VG/80% PG | Nobacco |
| Golden Virginia | 8 | 90% VG/10% PG | Nobacco |
| American Tobacco | 11 | 90% VG/10% PG | Nobacco |
| Tobacco Echo | 18 | 20% VG/80% PG | Nobacco |

2.2. Cell Cultures
2.3. Production of Extracts
2.4. Treatment and Exposure
2.5. MTT Assay
2.6. Quality Check of Assay
2.7. Statistical Analysis
3. Results
3.1. Cell Viability from Exposure to CS and EC Vapor Generated at Low Voltage
| Dilutions | ||||||
|---|---|---|---|---|---|---|
| Samples-nicotine (mg/mL) | 100% a | 50% b | 25% c | 12.5% d | 6.25% e | p * |
| Base-0 | 105.1 ± 1.2 | 103.5 ± 1.9 | 101.3 ± 4.2 | 100.7 ± 3.4 | 100.4 ± 2.3 | 0.251 |
| Golden Margy-6 | 89.2 ± 0.2 | 93.0 ± 2.2 | 92.1 ± 1.3 | 95.3 ± 3.6 | 93.0 ± 6.3 | 0.361 |
| RY69-6 | 98.9 ± 4.6 | 101.2 ± 5.4 | 96.0 ± 13.0 | 100.5 ± 2.7 | 100.2 ± 9.2 | 0.932 |
| City-6 | 93.6 ± 2.5 | 89.4 ± 4.2 | 94.6 ± 2.3 | 93.3 ± 2.3 | 93.8 ± 2.8 | 0.282 |
| Cinnamon Cookies-6 | 64.8 ± 2.5 | 100.8 ± 2.0 | 97.2 ± 2.9 | 99.3 ± 1.7 | 99.2 ± 3.8 | <0.001 |
| Golden Virginia-8 | 86.6 ± 1.8 | 89.1 ± 1.0 | 94.2 ± 3.0 | 95.5 ± 0.7 | 97.1 ± 1.4 | <0.001 |
| RY4-9 | 73.8 ± 3.7 | 106.6 ± 1.1 | 104.4 ± 1.9 | 103.6 ± 4.0 | 100.7 ± 0.8 | <0.001 |
| MaxBlend-9 | 104.4 ± 1.6 | 102.4 ± 2.0 | 102.4 ± 2.8 | 101.2 ± 7.6 | 102.7 ± 2.0 | 0.901 |
| Americano-9 | 85.0 ± 2.0 | 98.3 ± 1.7 | 90.9 ± 4.4 | 94.7 ± 3.5 | 94.1 ± 5.9 | 0.017 |
| American Tobacco-11 | 109.0 ± 1.6 | 106.8 ± 0.5 | 104.9 ± 1.0 | 101.3 ± 3.1 | 103.6 ± 2.5 | 0.007 |
| Tribeca-12 | 110.8 ± 2.8 | 103.9 ± 5.5 | 106.6 ± 7.9 | 102.4 ± 5.1 | 101.7 ± 3.0 | 0.268 |
| Green apple-12 | 106.6 ± 2.0 | 106.8 ± 2.0 | 105.2 ± 3.3 | 103.6 ± 4.5 | 99.2 ± 2.5 | 0.060 |
| El Toro Cigarrillos-12(1) f | 39.1 ± 1.2 | 52.5 ± 1.8 | 81.0 ± 2.0 | 92.6 ± 0.4 | 99.2 ± 1.0 | <0.001 |
| El Toro Cigarrillos-12(2) f | 22.3 ± 4.0 | 66.9 ± 6.2 | 104.1 ± 5.8 | 109.9 ± 6.0 | 112.0 ± 8.8 | <0.001 |
| Silverberry-12 | 108.2 ± 8.5 | 107.2 ± 2.7 | 106.0 ± 1.7 | 103.2 ± 0.7 | 100.3 ± 2.0 | 0.200 |
| Virginia-18 | 82.1 ± 0.8 | 95.8 ± 8.6 | 95.1 ± 3.0 | 90.6 ± 7.0 | 93.3 ± 8.5 | 0.136 |
| Classic-18 | 95.0 ± 5.1 | 104.0 ± 9.1 | 101.1 ± 12.9 | 107.3 ± 8.3 | 89.7 ± 6.4 | 0.176 |
| Tobacco echo-18 | 96.1 ± 5.0 | 96.4 ± 7.7 | 101.7 ± 3.1 | 102.7 ± 4.7 | 96.3 ± 7.3 | 0.479 |
| Bebeka-18 | 75.7 ± 8.6 | 87.5 ± 2.2 | 90.8 ± 1.6 | 95.9 ± 1.9 | 99.0 ± 2.3 | <0.001 |
| El Toro Guevara-18 f | 84.5 ± 3.0 | 91.0 ± 3.5 | 94.6 ± 1.3 | 98.8 ± 2.0 | 102.5 ± 1.7 | <0.001 |
| El Toro Puros-24 f | 2.2 ± 0.6 | 7.4 ± 3.9 | 84.5 ± 6.5 | 115.3 ± 11.7 | 111.9 ± 7.4 | <0.001 |
| CS g | 3.9 ± 0.2 | 5.2 ± 0.8 | 3.1 ± 0.2 | 38.2 ± 0.6 | 76.9 ± 2.0 | <0.001 |
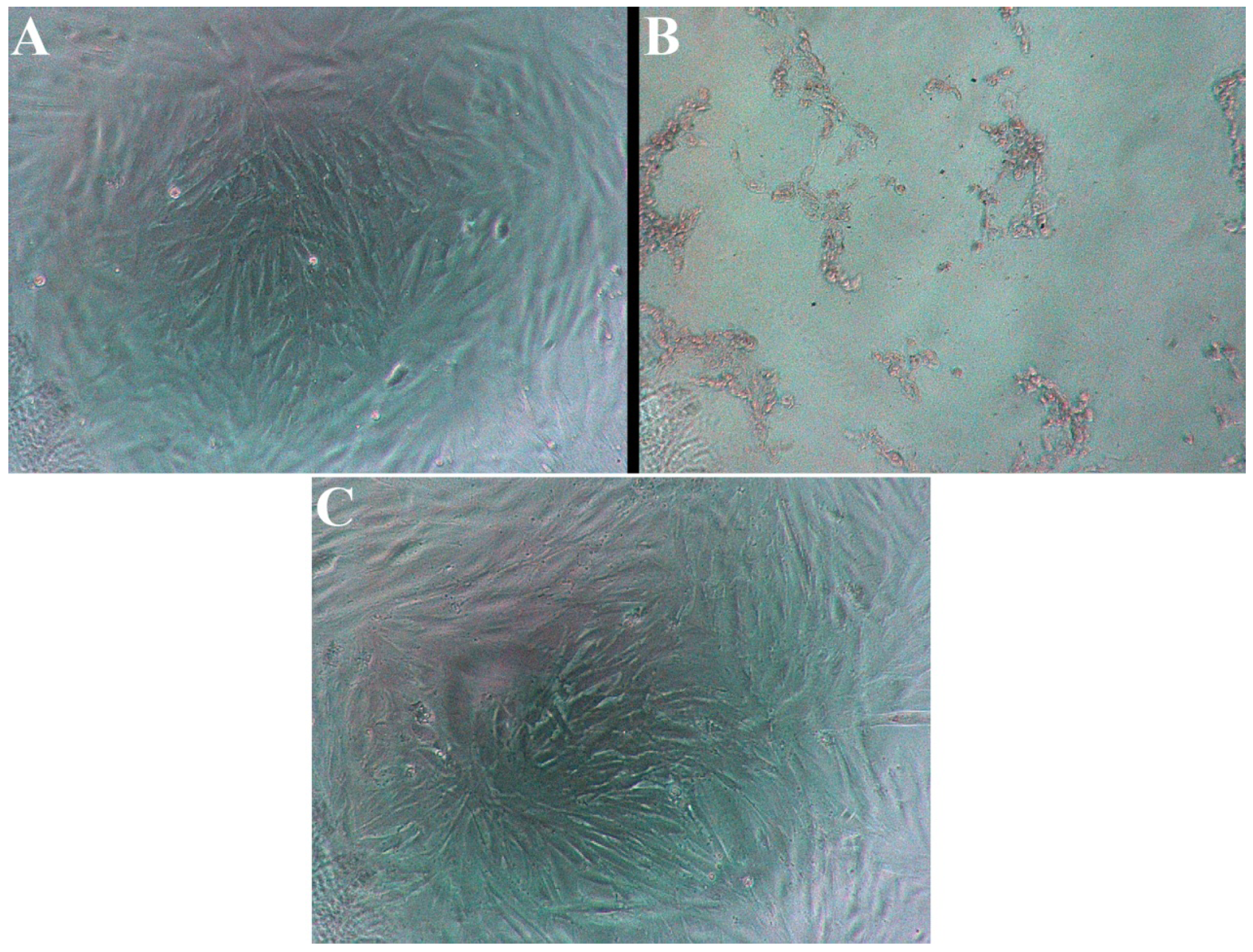
3.2. Cell Viability from Exposure to EC Vapor Generated at High Voltage
| Samples-nicotine (mg/mL) | Voltage | Dilutions | |||||
|---|---|---|---|---|---|---|---|
| 100% a | 50% b | 25% c | 12.5% d | 6.25% e | |||
| Golden Margy-6 | 3.7 | 89.2 ± 0.2 | 93.0 ± 2.2 | 92.1 ± 1.3 | 95.3 ± 3.6 | 93.0 ± 6.3 | |
| 4.5 | 82.2 ± 1.2 | 83.4 ± 3.6 | 81.6 ± 1.3 | 83.5 ± 2.4 | 85.0 ± 3.1 | ||
| MaxBlend-9 | 3.7 | 104.4 ± 1.6 | 102.4 ± 2.0 | 102.4 ± 2.8 | 101.2 ± 7.6 | 102.7 ± 2.0 | |
| 4.5 | 95.4 ± 2.0 | 97.7 ± 1.8 | 100.6 ± 2.1 | 96.4 ± 2.5 | 97.7 ± 5.5 | ||
| Tribeca-12 | 3.7 | 110.8 ± 2.8 | 103.9 ± 5.5 | 106.6 ± 7.9 | 102.4 ± 5.1 | 101.7 ± 3.0 | |
| 4.5 | 92.7 ± 2.7 | 103.6 ± 2.6 | 101.6 ± 3.0 | 97.7 ± 1.7 | 89.3 ± 4.7 | ||
| Green apple-12 | 3.7 | 106.6 ± 2.0 | 106.8 ± 2.0 | 105.2 ± 3.3 | 103.6 ± 4.5 | 99.2 ± 2.5 | |
| 4.5 | 72.9 ± 3.5 | 101.3 ± 10.0 | 105.7 ± 3.3 | 104.9 ± 0.9 | 96.2 ± 0.7 | ||
| p value * | 0.069 | 0.080 | 0.175 | 0.156 | 0.039 | ||
3.3. Nicotine Effects on Myocardial Cell Viability
| Viability according to nicotine concentration (mg/mL) | |||
|---|---|---|---|
| Extract concentrations | 6–11 (n = 9) | 12–24 (n = 11) | p * |
| 100% | 89.5 ± 14.1% | 74.8 ± 37.1% | 0.247 |
| 50% | 98.6 ± 6.7% | 83.6 ± 30.6% | 0.141 |
| 25% | 97.4 ± 5.2% | 97.3 ± 8.9% | 0.981 |
| 12.5% | 98.3 ± 3.7% | 102.0 ± 7.3% | 0.181 |
| 6.25% | 98.1 ± 3.7% | 100.5 ± 6.8% | 0.357 |
3.4. IC50 and NOAEL for EC and CS
| Dilutions | |||
|---|---|---|---|
| Samples-nicotine (mg/mL) | IC50 | NOAEL | |
| Base-0 | >100% | 100% | |
| Golden Margy-6 | >100% | 100% | |
| Golden Margy-6 * | >100% | 100% | |
| RY69-6 | >100% | 100% | |
| City-6 | >100% | 100% | |
| Cinnamon Cookies-6 | >100% | 50% | |
| Golden Virginia-8 | >100% | 25% | |
| RY4-9 | >100% | 50% | |
| MaxBlend-9 | >100% | 100% | |
| MaxBlend-9 * | >100% | 100% | |
| Americano-9 | >100% | 100% | |
| American Tobacco-11 | >100% | 100% | |
| Tribeca-12 | >100% | 100% | |
| Tribeca-12 * | >100% | 12.5% | |
| Green apple-12 | >100% | 100% | |
| Green apple-12 * | >100% | 50% | |
| El Toro Cigarrillos-12(1) a | 52% | 6.25% | |
| El Toro Cigarrillos-12(2) a | 69% | 25% | |
| Silverberry | >100% | 100% | |
| Virginia-18 | >100% | 100% | |
| Classic-18 | >100% | 100% | |
| Tobacco echo-18 | >100% | 100% | |
| Bebeka-18 | >100% | 50% | |
| El Toro Guevara-18 a | >100% | 12.5% | |
| El Toro Puros-24 a | 36% | 12.5% | |
| CS b | 11% | 6.25% | |
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ockene, I.S.; Miller, N.H. Cigarette smoking, cardiovascular disease, and stroke: A statement for healthcare professionals from the American Heart Association. Circulation 1997, 96, 3243–3247. [Google Scholar] [CrossRef]
- Rigotti, N.A.; Pipe, A.L.; Benowitz, N.L.; Arteaga, C.; Garza, D.; Tonstad, S. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: A randomized trial. Circulation 2009, 121, 221–229. [Google Scholar]
- Moore, D.; Aveyard, P.; Connock, M.; Wang, D.; Fry-Smith, A.; Barton, P. Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: Systematic review and meta-analysis. BMJ 2009, 338. [Google Scholar] [CrossRef]
- Rodu, B. The scientific foundation for tobacco harm reduction, 2006–2011. Harm Reduct. J. 2011, 8. [Google Scholar] [CrossRef]
- King, B.A.; Alam, S.; Promoff, G.; Arrazola, R.; Dube, S.R. Awareness and ever use of electronic cigarettes among U.S. adults, 2010–2011. Nicotine Tob. Res. 2013. [Google Scholar] [CrossRef]
- Cobb, N.K.; Byron, M.J.; Abrams, D.B.; Shields, P.G. Novel nicotine delivery systems and public health: The rise of the “e-cigarette”. Am. J. Public Health 2010, 100, 2340–2342. [Google Scholar] [CrossRef]
- Chen, I.L. FDA summary of adverse events on electronic cigarettes. Nicotine Tob. Res. 2013, 15, 615–616. [Google Scholar]
- WHO Statement. Questions and Answers on Electronic Cigarettes or Electronic Nicotine Delivery Systems (ENDS). 2013. Available online: http://www.who.int/tobacco/communications/statements/eletronic_cigarettes/en/index.html (accessed on 14 October 2013).
- Pryor, W.A.; Stone, K. Oxidants in cigarette smoke: Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrie. Ann. NY Acad. Sci. 1993, 686, 12–28. [Google Scholar] [CrossRef]
- Yamada, S.; Zhang, X.Q.; Kadono, T.; Matsuoka, N.; Rollins, D.; Badger, T.; Rodesch, C.K.; Barry, W.H. Direct toxic effects of aqueous extract of cigarette smoke on cardiac myocytes at clinically relevant concentrations. Toxicol. Appl. Pharmacol. 2009, 236, 71–77. [Google Scholar]
- Romagna, G.; Allifranchini, E.; Bocchietto, E.; Todeschi, S.; Esposito, M.; Farsalinos, K.E. Cytotoxicity evaluation of electronic cigarette vapor extract on cultured mammalian fibroblasts (ClearStream-LIFE): Comparison with tobacco cigarette smoke extract. Inhal. Toxicol. 2013, 25, 354–361. [Google Scholar]
- Farsalinos, K.E.; Romagna, G.; Tsiapras, D.; Kyrzopoulos, S.; Voudris, V. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: Implications for research protocol standards definition and for public health authorities’ regulation. Int. J. Environ. Res. Public Health 2013, 10, 2500–2514. [Google Scholar] [CrossRef]
- ISO 10993:5 Standard. Biological Evaluation of Medical Devices—Part 5: Tests for in vitro Cytotoxicity. 2009. Available online: http://www.iso.org/iso/home/store/catalogue_tc/catalogue_detail.htm?csnumber=36406 (accessed on 14 October 2013).
- Turner, N.A.; Xia, F.; Azhar, G.; Zhang, X.; Liu, L.; Wei, J.Y. Oxidative stress induces DNA fragmentation and caspase activation via the c-Jun NH2-terminal kinase pathway in H9c2 cardiac muscle cells. J. Mol. Cell. Cardiol. 1998, 30, 1789–1801. [Google Scholar] [CrossRef]
- Das, A.; Dey, N.; Ghosh, A.; Das, S.; Chattopadhyay, D.J.; Chatterjee, I.B. Molecular and cellular mechanisms of cigarette smoke-induced myocardial injury: Prevention by vitamin C. PLoS One 2012, 7. [Google Scholar] [CrossRef]
- Niu, J.; Wang, K.; Kolattukudy, P.E. Cerium oxide nanoparticles inhibit oxidative stress and nuclear factor-κB activation in H9c2 cardiomyocytes exposed to cigarette smoke extract. J. Pharmacol. Exp. Ther. 2011, 338, 53–61. [Google Scholar] [CrossRef]
- Zordoky, B.N.; El-Kadi, A.O. 2,3,7,8-Tetrachlorodibenzo-p-dioxin and beta-naphthoflavone induce cellular hypertrophy in H9c2 cells by an aryl hydrocarbon receptor-dependant mechanism. Toxicol. Vitro 2010, 24, 863–871. [Google Scholar] [CrossRef]
- ISO 3308: Routine Analytical Cigarette-Smoking Machine—Definitions and Standard Conditions; International Organization for Standardization (ISO): Geneva, Switzerland, 2000.
- Mossman, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Method. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Dawkins, L.; Turner, J.; Roberts, A.; Soar, K. “Vaping” profiles and preferences: An online survey of electronic cigarette users. Addiction 2013, 108, 1115–1125. [Google Scholar] [CrossRef]
- arela-Carver, A.; Parker, H.; Kleinert, C.; Rimoldi, O. Adverse effects of cigarette smoke and induction of oxidative stress in cardiomyocytes and vascular endothelium. Curr. Pharm. Des. 2010, 16, 2551–2558. [Google Scholar] [CrossRef]
- Bernhard, D.; Wang, X.L. Smoking, oxidative stress and cardiovascular diseases—Do anti-oxidative therapies fail? Curr. Med. Chem. 2007, 14, 1703–1712. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Shi, J.; Larson, D.F.; Watson, R.R. Side-stream cigarette smoke induces dose-response in systemic inflammatory cytokine production and oxidative stress. Exp. Biol. Med. 2002, 227, 823–829. [Google Scholar]
- Armani, C.; Landini, L., Jr; Leone, A. Molecular and biochemical changes of the cardiovascular system due to smoking exposure. Curr. Pharm. Des. 2009, 15, 1038–1053. [Google Scholar] [CrossRef]
- Izzotti, A.; D’Agostini, F.; Balansky, R.; Degan, P.; Pennisi, T.M.; Steele, V.E.; De Flora, S. Exposure of mice to cigarette smoke and/or light causes DNA alterations in heart and aorta. Mutat. Res. 2008, 644, 38–42. [Google Scholar] [CrossRef]
- Gvozdjáková, A.; Bada, V.; Sány, L.; Kucharská, J.; Krutý, F.; Bozek, P.; Trstanský, L.; Gvozdják, J. Smoke cardiomyopathy: Disturbance of oxidative processes in myocardial mitochondria. Cardiovasc. Res. 1984, 18, 229–232. [Google Scholar] [CrossRef]
- Taylor, A.E.; Johnson, D.C.; Kazemi, H. Environmental tobacco smoke and cardiovascular disease. A position paper from the Council on Cardiopulmonary and Critical Care, American Heart Association. Circulation 1992, 86, 699–702. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Y.; Asahi, M.; Otsu, K. Acrolein, an environmental toxin, induces cardiomyocyte apoptosis via elevated intracellular calcium and free radicals. Cell. Biochem. Biophys. 2011, 61, 131–136. [Google Scholar] [CrossRef]
- Aberle, N.S.; Privratsky, J.R.; Burd, L.; Ren, J. Combined acetaldehyde and nicotine exposure depresses cardiac contraction in ventricular myocytes: Prevention by folic acid. Neurotoxicol. Teratol. 2003, 25, 731–736. [Google Scholar] [CrossRef]
- Szende, B.; Tyihák, E. Effect of formaldehyde on cell proliferation and death. Cell. Biol. Int. 2010, 34, 1273–1282. [Google Scholar] [CrossRef]
- Limaye, D.A.; Shaikh, Z.A. Cytotoxicity of cadmium and characteristics of its transport in cardiomyocytes. Toxicol. Appl. Pharmacol. 1999, 154, 59–66. [Google Scholar] [CrossRef]
- Antal, M.J.; Mok, W.S.L.; Roy, J.C.; T-Raissi, A. Pyrolytic sources of hydrocarbons from biomass. J. Anal. Appl. Pyrol. 1985, 8, 291–303. [Google Scholar] [CrossRef]
- Stein, Y.S.; Antal, M.J.; Jones, M.J. A study of the gas-phase pyrolysis of glycerol. J. Anal. Appl. Pyrol. 1983, 4, 283–296. [Google Scholar] [CrossRef]
- Goniewicz, M.L.; Knysak, J.; Gawron, M.; Kosmider, L.; Sobczak, A.; Kurek, J.; Prokopowicz, A.; Jablonska-Czapla, M.; Rosik-Dulewska, C.; Havel, C.; Jacob, P.; Benowitz, N. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control 2013. [Google Scholar] [CrossRef]
- Lauterbach, J.; Laugesen, M. Comparison of Toxicant Levels in Mainstream Aerosols Generated by Ruyan® Electronic Nicotine Delivery Systems (ENDS) and Conventional Cigarette Products. Proceedings of Society of Toxicology Conference, San Francisco, CA, USA, 11–15 March, 2012; Available online: http://www.healthnz.co.nz/News2012SOTposter1861.pdf (accessed on 6 September 2013).
- Suzuki, J.; Bayna, E.; dalle Moll, E.; Lew, W.Y. Nicotine inhibits cardiac apoptosis induced by lipopolysaccharide in rats. J. Amer. Coll. Cardiol. 2003, 41, 482–488. [Google Scholar]
- Laytragoon-Lewin, N.; Bahram, F.; Rutqvist, L.E.; Turesson, I.; Lewin, F. Direct effects of pure nicotine, cigarette smoke extract, Swedish-type smokeless tobacco (snus) extract and ethanol on human normal endothelial cells and fibroblasts. Anticancer Res. 2011, 31, 1527–1534. [Google Scholar]
- Argentin, G.; Cicchetti, R. Genotoxic and antiapoptotic effect of nicotine on human gingival fibroblasts. Toxicol. Sci. 2004, 79, 75–81. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, C.H.; Kim, M.S.; Kim, J.Y.; Jung, K.J.; Chung, J.H.; An, W.G.; Lee, J.W.; Yu, B.P.; Chung, H.Y. Suppression of age-related inflammatory NF-kappaB activation by cinnamaldehyde. Biogerontology 2007, 8, 545–554. [Google Scholar] [CrossRef]
- Allen, C.M.; Blozis, G.G. Oral mucosal reactions to cinnamon-flavored chewing gum. J. Amer. Dent. Assn. 1988, 116, 664–667. [Google Scholar]
- Friedman, M.; Kozukue, N.; Harden, L.A. Cinnamaldehyde content in foods determined by gas chromatography-mass spectrometry. J. Agr. Food Chem. 2000, 48, 5702–5709. [Google Scholar] [CrossRef]
- Ulker, Z.; Alpsoy, L.; Mihmanli, A. Assessment of cytotoxic and apoptotic effects of benzaldehyde using different assays. Hum. Exp. Toxicol. 2013, 32, 858–864. [Google Scholar] [CrossRef]
- McDaniel, P.A.; Solomon, G.; Malone, R.E. The tobacco industry and pesticide regulations: Case studies from tobacco industry archives. Environ. Health Perspect. 2005, 113, 1659–1665. [Google Scholar] [CrossRef]
- Bahl, V.; Lin, S.; Xu, N.; Davis, B.; Wang, Y.H.; Talbot, P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod. Toxicol. 2012, 34, 529–537. [Google Scholar]
- Cahn, Z.; Siegel, M. Electronic cigarettes as a harm reduction strategy for tobacco control: A step forward of a repeat of past mistakes? J. Public Health Policy 2011, 32, 16–31. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Farsalinos, K.E.; Romagna, G.; Allifranchini, E.; Ripamonti, E.; Bocchietto, E.; Todeschi, S.; Tsiapras, D.; Kyrzopoulos, S.; Voudris, V. Comparison of the Cytotoxic Potential of Cigarette Smoke and Electronic Cigarette Vapour Extract on Cultured Myocardial Cells. Int. J. Environ. Res. Public Health 2013, 10, 5146-5162. https://doi.org/10.3390/ijerph10105146
Farsalinos KE, Romagna G, Allifranchini E, Ripamonti E, Bocchietto E, Todeschi S, Tsiapras D, Kyrzopoulos S, Voudris V. Comparison of the Cytotoxic Potential of Cigarette Smoke and Electronic Cigarette Vapour Extract on Cultured Myocardial Cells. International Journal of Environmental Research and Public Health. 2013; 10(10):5146-5162. https://doi.org/10.3390/ijerph10105146
Chicago/Turabian StyleFarsalinos, Konstantinos E., Giorgio Romagna, Elena Allifranchini, Emiliano Ripamonti, Elena Bocchietto, Stefano Todeschi, Dimitris Tsiapras, Stamatis Kyrzopoulos, and Vassilis Voudris. 2013. "Comparison of the Cytotoxic Potential of Cigarette Smoke and Electronic Cigarette Vapour Extract on Cultured Myocardial Cells" International Journal of Environmental Research and Public Health 10, no. 10: 5146-5162. https://doi.org/10.3390/ijerph10105146
APA StyleFarsalinos, K. E., Romagna, G., Allifranchini, E., Ripamonti, E., Bocchietto, E., Todeschi, S., Tsiapras, D., Kyrzopoulos, S., & Voudris, V. (2013). Comparison of the Cytotoxic Potential of Cigarette Smoke and Electronic Cigarette Vapour Extract on Cultured Myocardial Cells. International Journal of Environmental Research and Public Health, 10(10), 5146-5162. https://doi.org/10.3390/ijerph10105146






