Arsenic Contaminated Groundwater and Its Treatment Options in Bangladesh
Abstract
:1. Introduction
2. Arsenic in Water: Occurrence and Speciation
3. Contamination Level of As and Vulnerable Areas
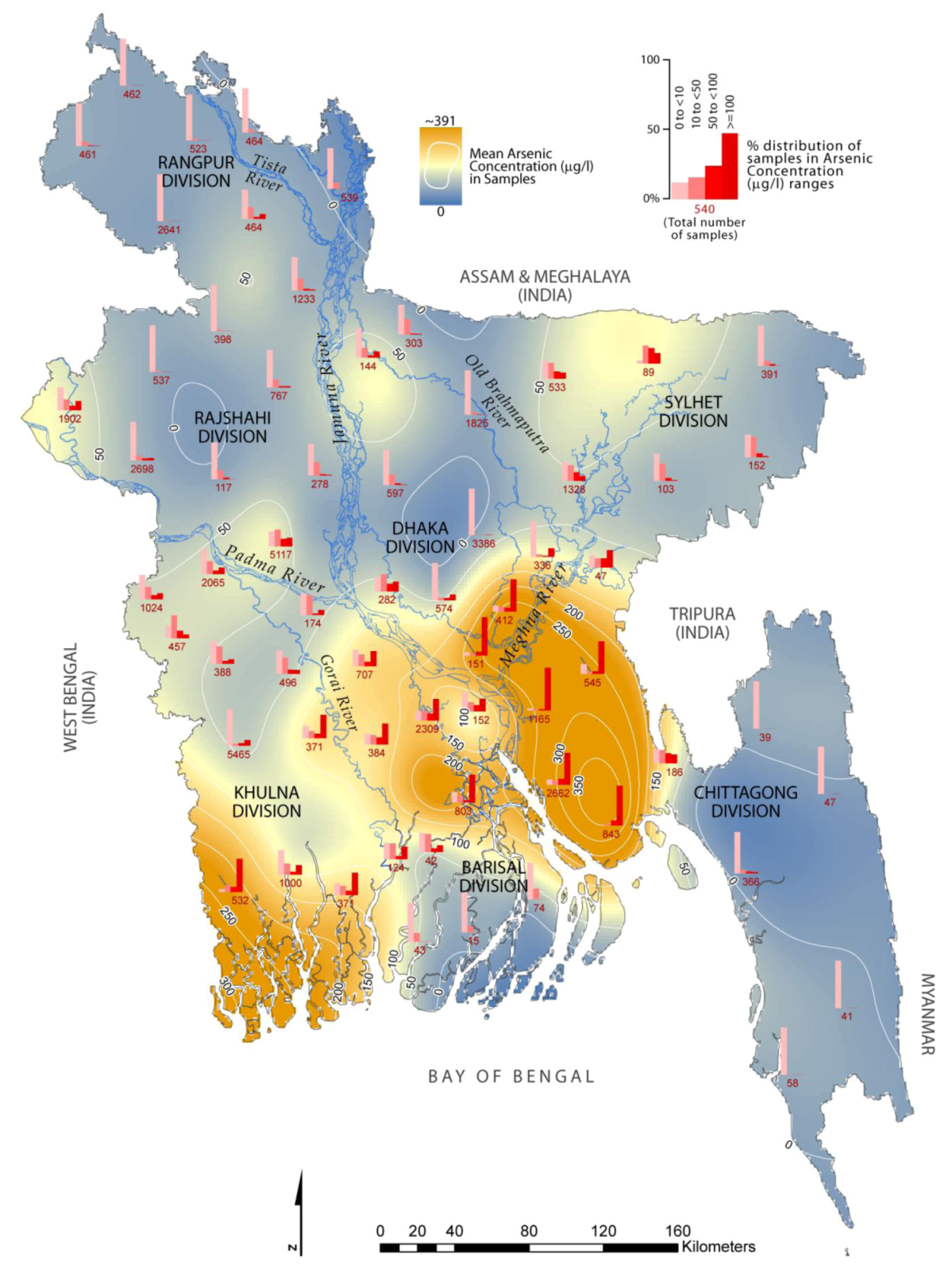
| Division | District (number of sampled wells in parenthesis) | Severely affected with As concentration ≥100 µg/L (% samples) |
|---|---|---|
| Khulna | Bagherhat (371), Narail (371), Satkhira (532) | Satkhira (~70%) |
| Barishal | Barisal (803) | Barisal (~59%) |
| Sylhet | Sunamganj (89) | * |
| Dhaka | Gopalganj (384), Madaripur (2,309), Munshiganj (151), Narayanganj (412) | Munshiganj (~80%) |
| Narayanganj (~68%) | ||
| Chittagong | Brahmanbaria (47), Chandpur (1,165), Comilla (545), Lakshmipur (2,662), Noakhali (843) | Chandpur (~90%) |
| Lakshmipur (~70%) | ||
| Noakhali (~85%) | ||
| Comilla (~69%) |
4. Sources, Distribution and Mobility of As
| Aqueous parameters | Measured range+ (Mean value ± SD)++ | |
|---|---|---|
| Shallow aquifer (10–69 m), N = 89 | Deep aquifer (70–260 m), N = 34 | |
| pH | 6.4–7.9 (7.1 ± 0.2), n = 88 | 6.5–7.3 (7.0 ± 0.2), n = 34 |
| EC (µS/cm) | 410–3,650 (1,003.7 ± 637.7), n = 60 | 317–3,410 (960.8 ± 638.7), n = 28 |
| ORP (mV) | +95 to −2 (30.1 ± 28.4), n = 27 | 24–90 (46.2 ± 22.0), n = 9 |
| DO (mg/L) | <0.1–2.1 (0.6 ± 0.8), n = 39 | <0.1, n = 9 |
| Na+ (mg/L) | 8–480 (48.4 ± 71.5), n = 72 | 7.9–280 (62.3 ± 61.2), n = 28 |
| K+ (mg/L) | 2.4–20 (7.2 ± 3.4), n = 72 | 3.2–26.1 (7.7 ± 5.2), n = 28 |
| NH4+ (mg/L) | 0.7–19.9 (7.9 ± 5), n = 56 | 0.1–10.3 (2.8 ± 2.9), n = 15 |
| Ca2+ (mg/L) | 12–174.1 (82.4 ± 41.5), n = 83 | 7–211 (59.2 ± 41.3), n = 32 |
| Mg2+ (mg/L) | 11–105.7 (29.1 ± 17.0), n = 72 | 14–110 (30.6 ± 19.7), n = 28 |
| HCO3− (mg/L) | 220–931.4 (494.5 ± 134.2), n = 72 | 184–697 (359.7 ± 137.4), n = 28 |
| Cl− (mg/L) | 1.9–695 (46.3 ± 91.3), n = 72 | 1.5–797 (97.1 ± 161.8), n = 28 |
| NO3− (mg/L) | <0.03–5.9 (1.2 ± 1.7), n = 72 | <0.03–7.1 (2.0 ± 2.3), n = 29 |
| SO42− (mg/L) | <0.01–34 (3.4 ± 6.4), n = 72 | <0.01–46 (8.1 ± 10.8), n = 29 |
| PO43− (mg/L) | 0.46–15 (4.9 ± 3.2), n = 60 | 0.05–5.5 (1.5 ± 1.6), n = 29 |
| As (µg/L) | 22–1,000 (373.7 ± 244.1), n = 89 | 0.2–170 (48.0 ± 52.6), n = 34 |
| Fe (mg/L) | 0.06–22.2 (7.2 ± 4.8), n = 89 | 0.01–17.5 (3.0 ± 4.2), n = 34 |
| Mn (mg/L) | 0.02–2 (0.7 ± 0.5), n = 43 | 0.06–2.9 (0.6 ± 0.8), n = 22 |
| DOC (mg/L) | 0.64–15 (4.3 ± 3.0), n = 72 | 0.2–12 (2.5 ± 2.6), n = 28 |
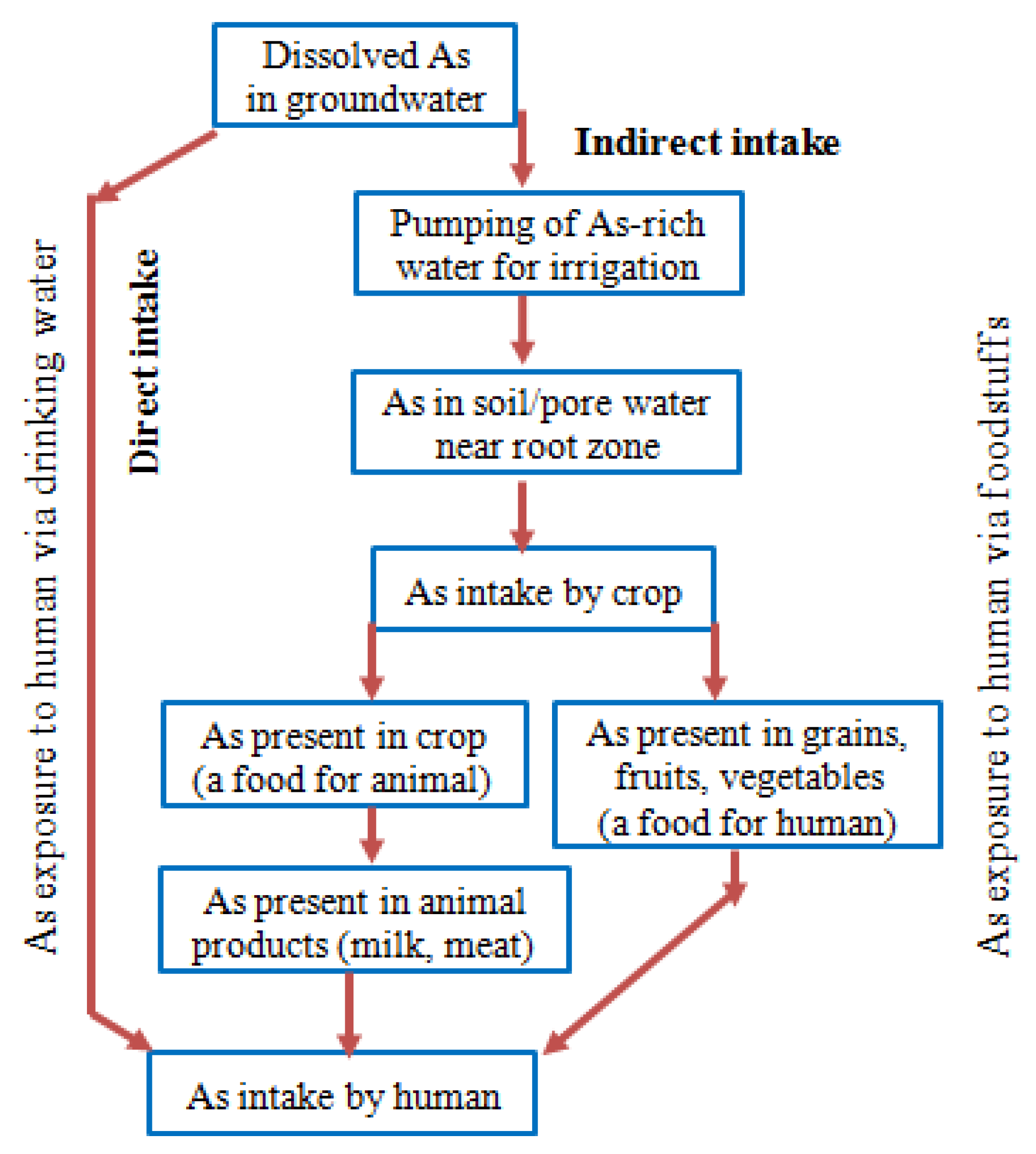
5. Exposure Pathways and Health Effects of Arsenic
| Foodstuffs | Total As (μg/kg) | Reference |
|---|---|---|
| Mean (Range) | ||
| Vegetables | ||
| Bangladesh a | (70–3,990) | [59] |
| Bangladesh | 54.5 (<5–540) | [53] |
| Europe | (<5–87) | [53] |
| UK (Food Standards Agency) | 2 for green vegetables | [60] |
| 4.9 for other vegetables | ||
| Rice | ||
| Australia | 30 (20–40) | [61] |
| Bangladesh | 500 (30–1,840) | [62] |
| China | 140 (20–460) | [57] |
| West Bengal (India) | 140 (20–400) | [63] |
| USA | 250 (30–660) | [57] |
| Bangladesh a | 496 (58–1,830) | [62] |
| Chinaa | 930 | [64] |
| West Bengal (India) a | 250 (140–480) | [63] |
| 330 (180–430) | [65] | |
| Fish and shrimp | ||
| Bangladesh b | (214–266) | [53] |
| Bangladesh | (97–1318) | [53] |
| Other foods | ||
| Bangladesh (Betel leaf) | 45.9 (44.9–46.9) | [53] |
6. Treatment Processes for Remediation of As Contaminated Water
6.1. Co-Precipitation, Coagulation, and Filtration
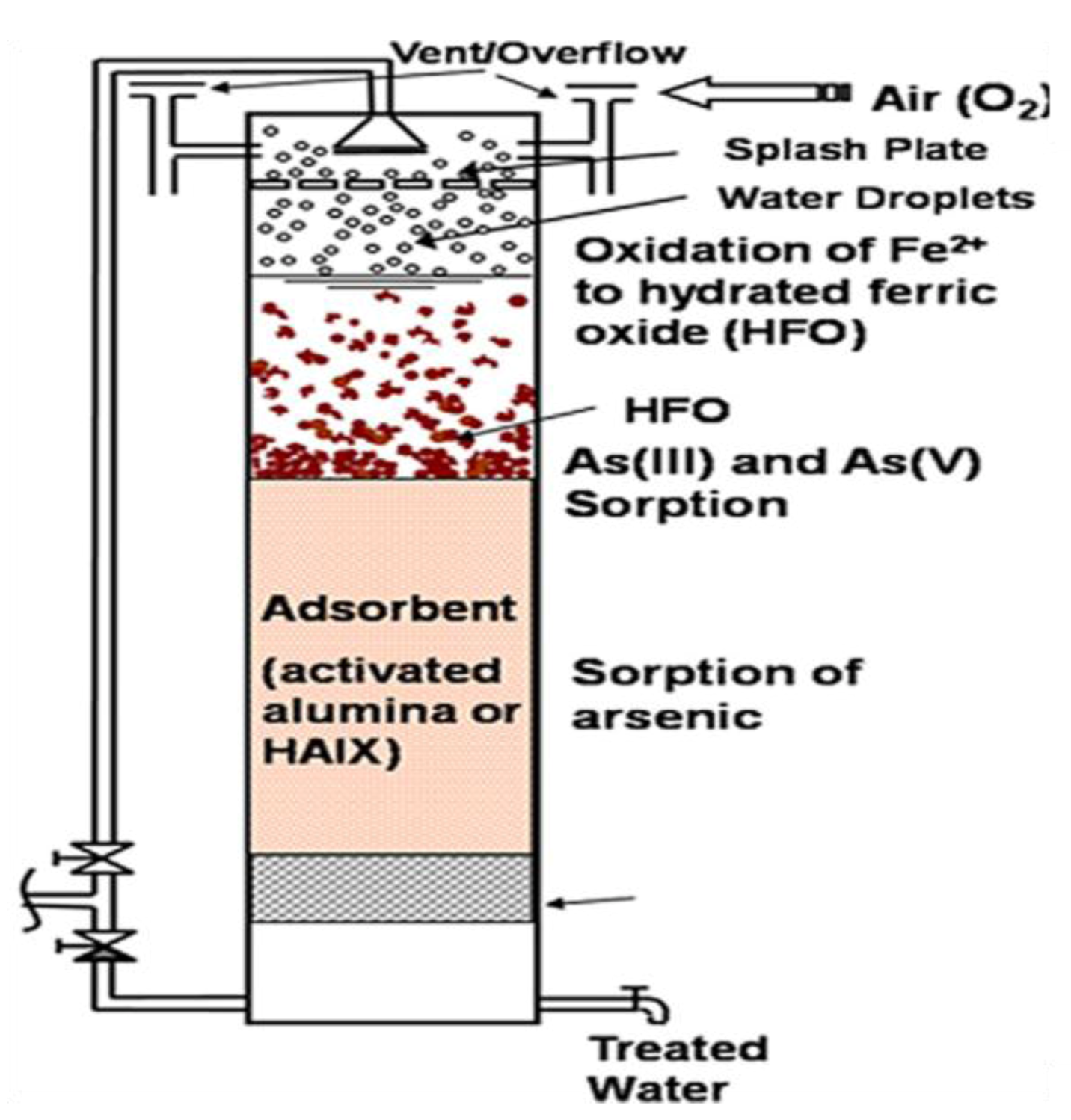
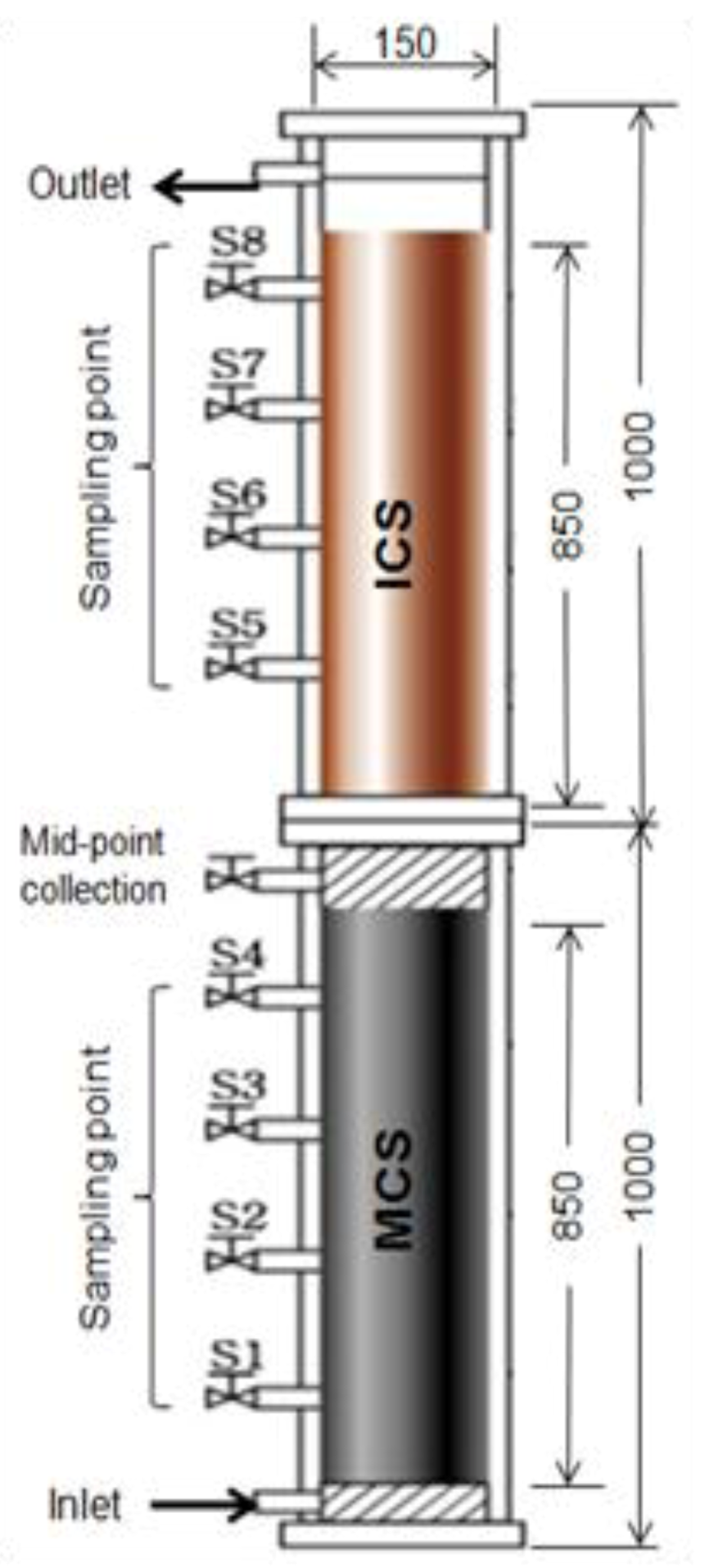
6.2. Precipitation and Filtration Unit at Household Scale

6.3. Arsenic Removal by Adsorption Based Technologies
6.3.1. New Development on the As Removal by Iron-doped Activated Carbon (AC)

6.3.2. Advances in As Removal by Activated Alumina (AA)
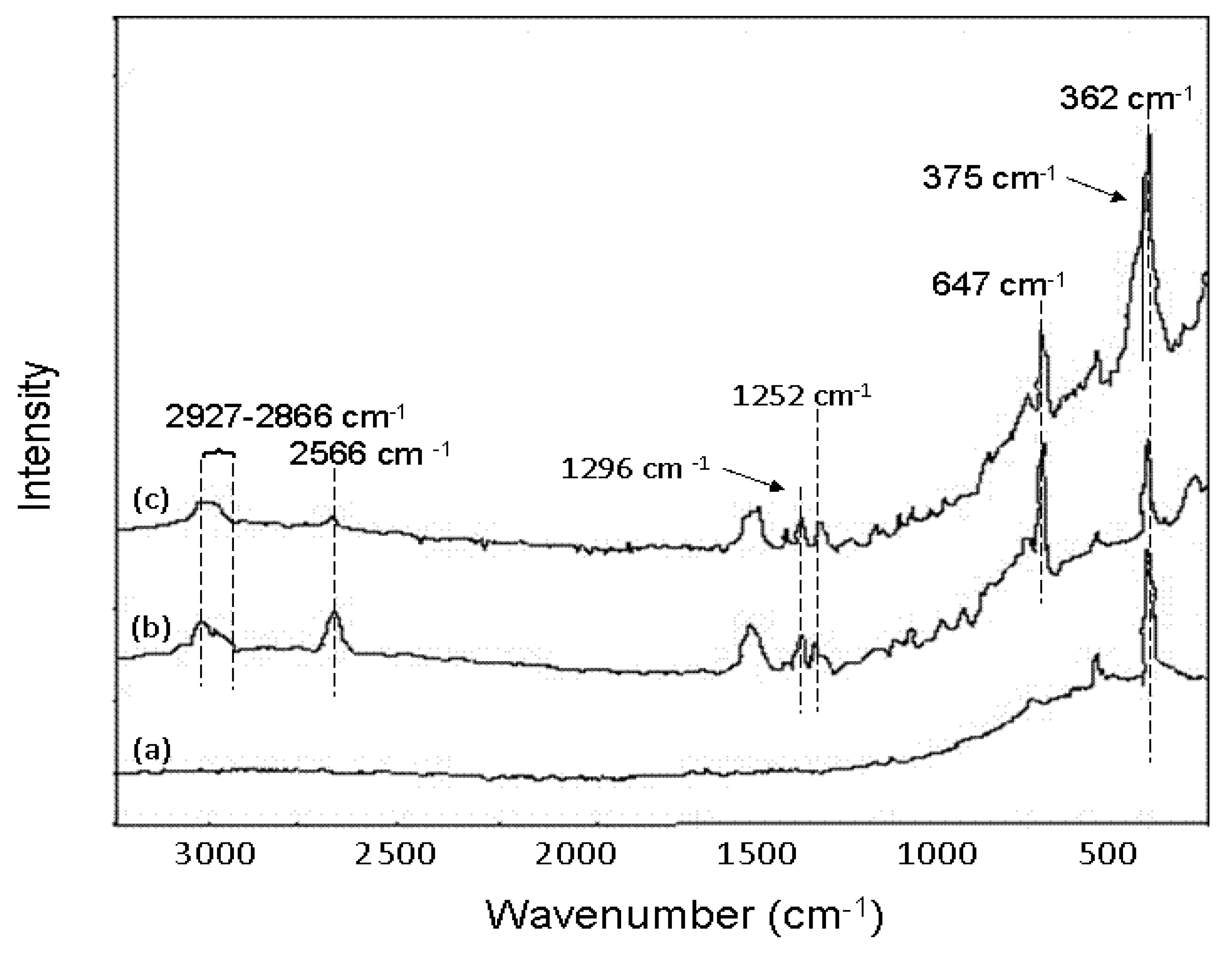
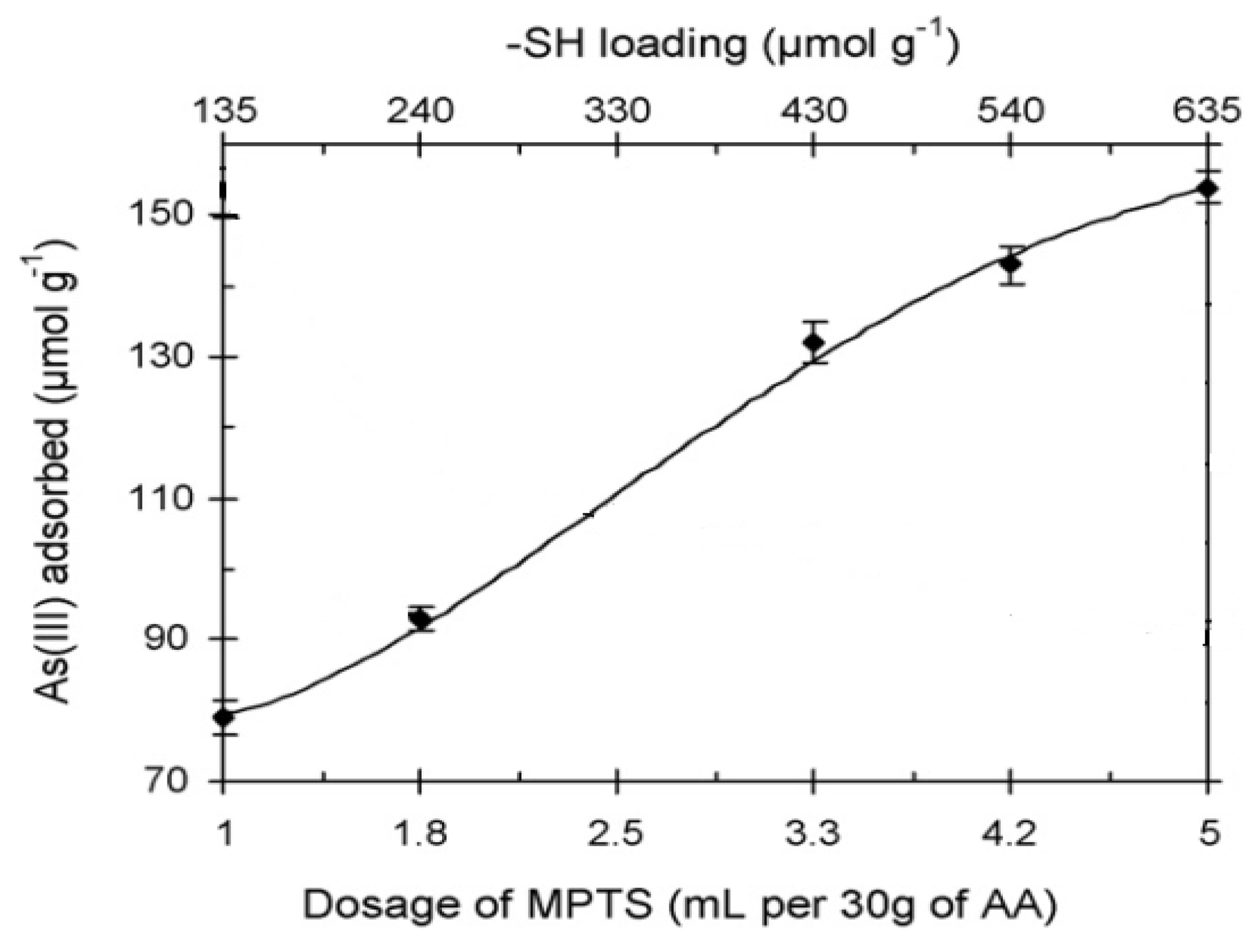
6.3.3. As Removal by Layered Double Hydroxide (LDH)
6.3.4. As Removal by Natural and Modified Zeolites and Clays
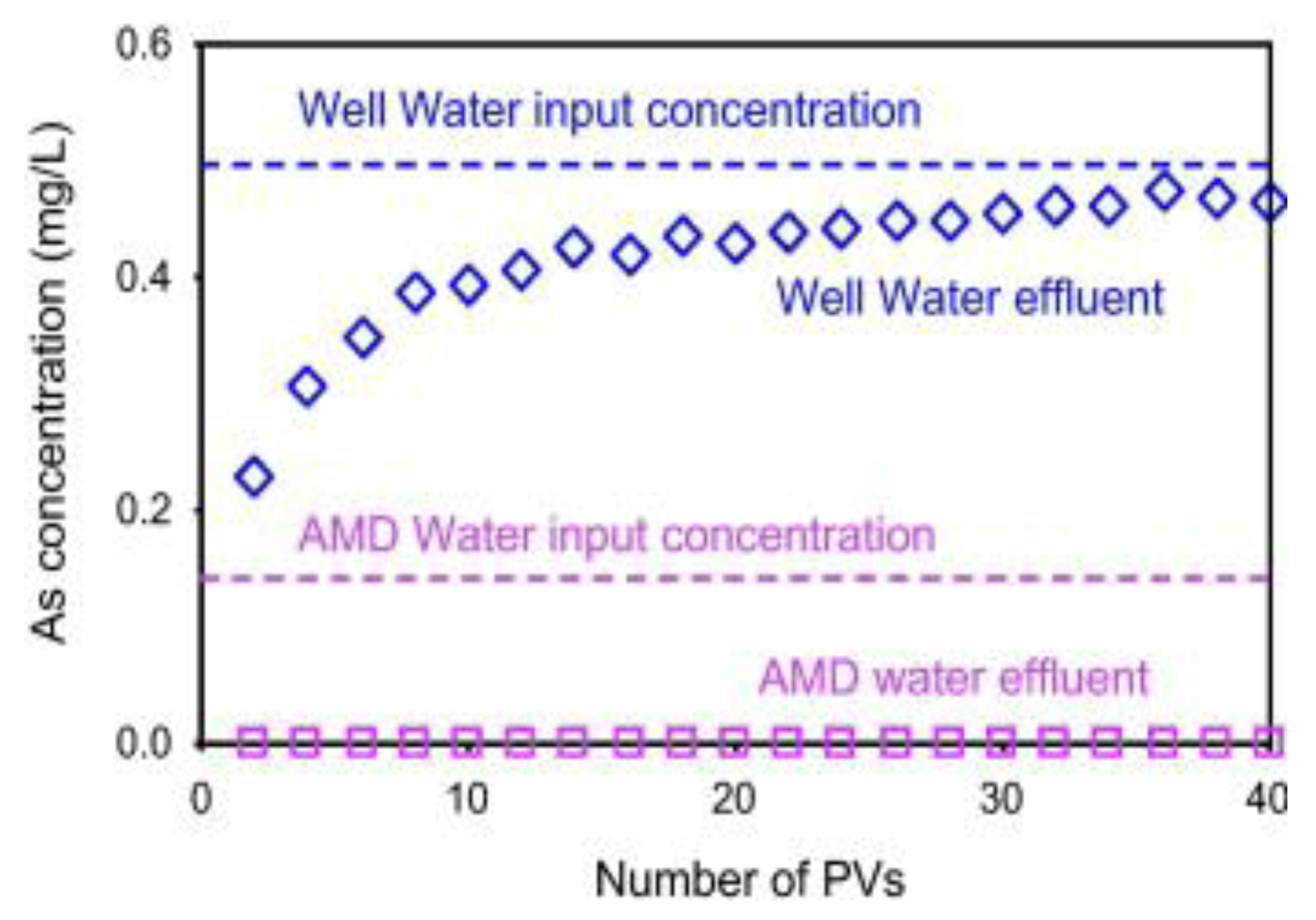
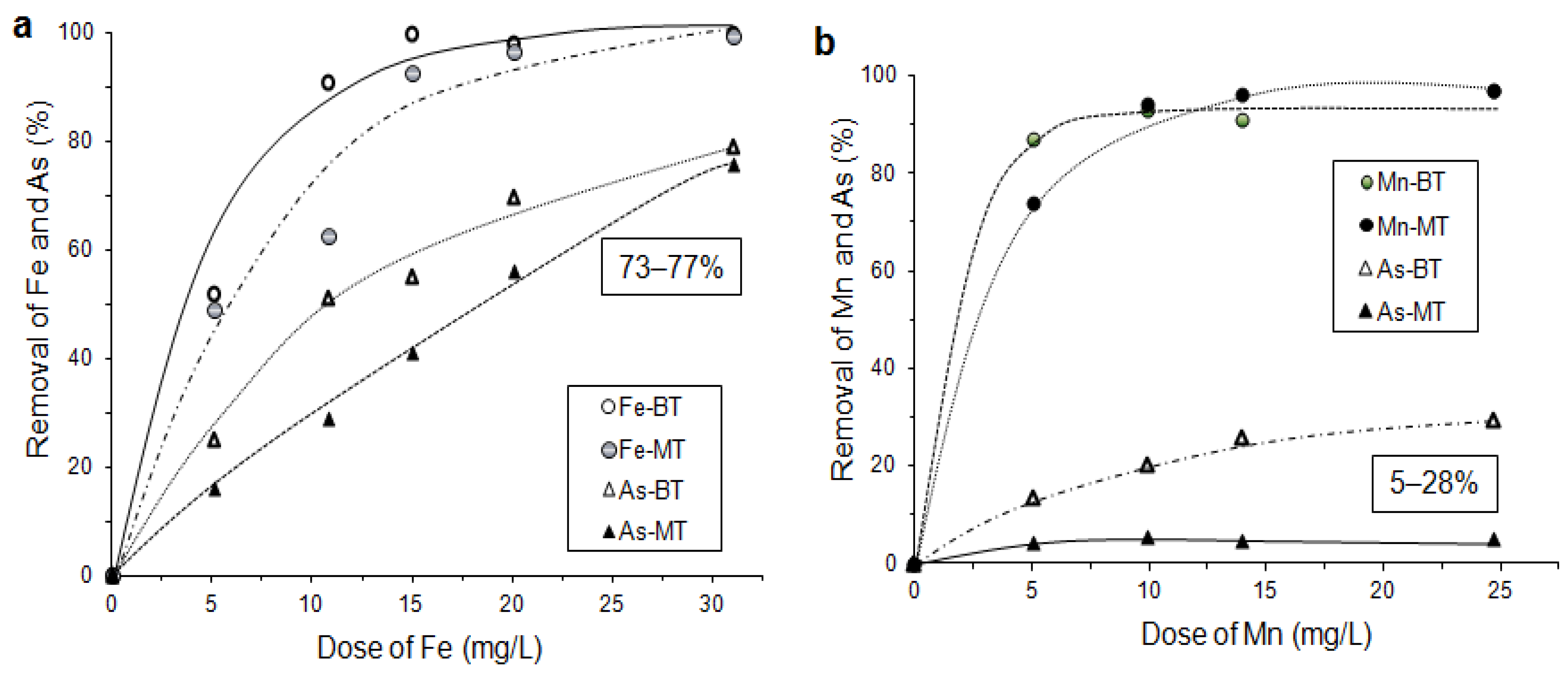
6.3.5. As Sorption by Laterite and Limonite with Oxidation
7. Discussion
| Treatment Process As(V) | Removal Efficiency * | As concentration in raw water | Ref. |
|---|---|---|---|
| Oxidation and Filtration | |||
| Aeration and filtration | >90% | 300 µg As(III)/L | This review |
| Fe2O3 filter | >95% | 100-400 µg As(III)/L | This review |
| As(III) oxidation by (OCl−) and Fe precipitation | >98% | 300 µg As(III)/L | This review |
| Co-precipitation | |||
| Enhanced lime softening | 90% | [27] | |
| Enhanced coagulation/filtration | |||
| With alum | <90% | [27] | |
| With ferric chloride | 95% | [27] | |
| Adsorption | |||
| Iron doped activated carbon | >95% | 311 µg As/L | This review |
| Hybrid activated alumina | >95% | 2–20 mg As/L | This review |
| Iron based sorbents | Up to 98% | [27] | |
| Layered double hydroxide (LDH) | Up to 96% | 300 µg As(V)/L | This review |
| Modified zeolites | up to 99% | 100–400 µg As/L | This review |
| Modified clays | Up to 80% | 0.15 µM As | This review |
| Laterite and limonite | Up to 95% | 500 µg As/L | This review |
8. Concluding Remarks
References
- Jain, C.K.; Singh, R.D. Technological options for the removal of arsenic with special reference to South East Asia. J. Environ. Manage. 2012, 107, 1–18. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Lacasa, E.; Canizares, P.; Saez, C.; Fernandez, F.J.; Rodrigo, M.A. Removal of arsenic by iron and aluminium electrochemically assisted coagulation. Separ. Purific. Technol. 2011, 79, 15–19. [Google Scholar] [CrossRef]
- Wang, S.L.; Liu, C.H.; Wang, M.K.; Chuang, Y.H.; Chiang, P.N. Arsenate adsorption by Mg/Al-NO3 layered double hydroxides with varying the Mg/Al ratio. Appl. Clay Sci. 2009, 43, 79–85. [Google Scholar] [CrossRef]
- Xu, Y.; Dai, Y.; Zhou, J.; Xu, Z.P.; Qian, G.; Lu, G.Q.M. Removal efficiency of arsenate and phosphate from aqueous solution using layered double hydroxide materials: Intercalation vs. precipitation. J. Mater. Chem. 2010, 20, 4684–4691. [Google Scholar] [CrossRef]
- Dadwhal, M.; Sahimi, M.; Tsotsis, T.T. Adsorption isotherms of arsenic on conditioned layered double hydroxides in the presence of various competing ions. Ind. Eng. Chem. Res. 2011, 50, 2220–2226. [Google Scholar] [CrossRef]
- Chetia, M.; Goswamee, R.L.; Banerjee, S.; Chatterjee, S.; Singh, L.; Srivastava, R.B.; Sarma, H.P. Arsenic removal from water using calcined Mg-Al layered double hydroxide. Clean Techn. Environ. Policy 2011. [Google Scholar] [CrossRef]
- Caussy, D.; Priest, N.D. Introduction to arsenic contamination and health risk assessment with special reference to Bangladesh. Rev. Environ. Contamin. Toxicol. 2008, 197, 1–15. [Google Scholar] [CrossRef]
- Argos, M.; Kalra, T.; Rathouz, P.J.; Chen, Y.; Pierce, B.; Parvez, F.; Islam, T.; Ahmed, A.; Rakibuz-Zaman, M.; Hasan, R.; Sarwar, G.; Slavkovich, V.; Geen, A.V.; Graziano, J.; Ahsan, H. Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): A prospective cohort study. Lancet 2010, 376, 252–258. [Google Scholar]
- PRB (Population Reference Bureau). World Population Data Sheet, 2010. Available online: www.prb.org/pdf10/10wpds_eng.pdf (accessed on 15 May 2012).
- Chaudhuri, A. Dealing with arsenic contamination in Bangladesh. MIT Undergraduate Res. J. (MURJ) 2004, 10, 25–30. [Google Scholar]
- Hossain, M.F. Arsenic contamination in Bangladesh—An overview. Agri. Eco. Environ. 2006, 113, 1–16. [Google Scholar] [CrossRef]
- Cheng, Z.; van Geen, A.; Jiang, C.; Meng, X.; Seddique, A.; Ahmed, K.M. Performance of a household-level arsenic removal system during 4-month deployments in Bangladesh. Environ. Sci. Technol. 2004, 38, 3442–3448. [Google Scholar] [CrossRef]
- Ahmed, K.M. Section 5: Management of the Groundwater Arsenic Disaster in Bangladesh. In Natural Arsenic in Groundwater: Occurrence, Remediation and Management; Bundschuh, J., Bhattacharya, P., Chandrasekharam, D., Eds.; Taylor & Francis Group: London, UK, 2005; pp. 283–286. [Google Scholar]
- Fazal, M.A.; Kawachi, T.; Ichion, E. Extent and severity of groundwater arsenic contamination in Bangladesh. Water Int. 2001, 26, 370–379. [Google Scholar] [CrossRef]
- Swartz, C.H.; Blute, N.K.; Badruzzaman, B.; Ali, A.; Brabander, D.; Jay, J.; Besancon, J.; Islam, S.; Hemond, H.F.; Harvey, C.F. Mobility of arsenic in a Bangladesh aquifer: Inferences from geochemical profiles, leaching data, and mineralogical characterization. Geochimica et Cosmochimica Acta 2004, 68, 4539–4557. [Google Scholar] [CrossRef]
- Dhar, R.K.; Biswas, B.K.; Samanta, G.; Mandal, B.K.; Roychowdhury, T.; Chanda, C.R.; Basu, G.; Chakraborti, D.; Roy, S.; Kabir, S.; Zafar, A.; Faruk, I.; Islam, K.S.; Choudhury, M.; Arif, A.I. Groundwater Arsenic Contamination and Sufferings of People in Bangladesh may be the Biggest Arsenic Calamity in the World. In International Conference on Arsenic Pollution Groundwater in Bangladesh: Cause, Effects and Remedies; DCH: Dhaka, Bangladesh, 1998; pp. 86–87. [Google Scholar]
- BGS-DPHE, Arsenic Contamination of Groundwater in Bangladesh, BGS Technical Report WC/00/19, 2001; British Geological Survey: Keyworth, UK, 2001.
- Chakraborti, D.; Rahman, M.M.; Das, B.; Murrill, M.; Dey, S.; Mukherjee, S.C.; Dhar, R.K.; Biswas, B.K.; Chowdhury, U.K.; Roy, S.; Sorif, S.; Selim, M.; Rahman, M.; Quamruzzaman, Q. Status of groundwater arsenic contamination in Bangladesh: A 14-year study report. Water Res. 2010, 44, 5789–5802. [Google Scholar]
- Chakraborti, D.; Sengupta, M.K.; Rahman, M.M.; Ahamed, S.; Chowdhury, U.K.; Hossain, M.A.; Mukherjee, S.C.; Pati, S.; Saha, K.C.; Dutta, R.N.; Zaman, Q.Q. Groundwater arsenic contamination and its health effects in the Ganga-Meghna-Brahmaputra Plain. J. Environ. Monitor. 2004, 6, 74–83. [Google Scholar] [CrossRef]
- Sarkar, S.; Greenleaf, J.E.; Gupta, A.; Ghosh, D.; Blaney, L.M.; Bandyopadhyay, P.; Biswas, R.K.; Dutta, A.K.; Sengupta, A.K. Evolution of community-based arsenic removal systems in remote villages in West Bengal, India: Assessment of decade-long operation. Water Res. 2010, 44, 5813–5822. [Google Scholar] [CrossRef]
- van Geen, A.; Zheng, Y.; Versteeg, R.; Stute, M.; Horneman, A.; Dhar, R.; Steckler, M.; Gelman, A.; Small, C.; Ahsan, H.; Graziano, J.H.; Hussain, I.; Ahmed, K.M. Spatial variability of arsenic in 6,000 tube wells in a 25 km2 area of Bangladesh. Water Resour. Res. 2003, 39, 1140. [Google Scholar] [CrossRef]
- Stollenwerk, K.G.; Breit, G.N.; Welch, A.H.; Yount, J.C.; Whitney, J.W.; Foster, A.L.; Uddin, M.N.; Majumder, R.K.; Ahmed, N. Arsenic attenuation by oxidized aquifer sediments in Bangladesh. Sci. Total Environ. 2007, 379, 133–150. [Google Scholar] [CrossRef]
- Islam, S. Natural Sediment may Shield Groundwater from Arsenic. Agriculture & Environment: Water News 2011, Science and Development Network. Available online: www.scidev.net/en/agriculture-and-environment/water/news/natural-sediment-may-shield-groundwater-from-arsenic.html (accessed on 13 October 2011).
- Radloff, K.A.; Zheng, Y.; Michael, H.A.; Stute, M.; Bostick, B.C.; Mihajlov, I.; Bounds, M.; Huq, M.R.; Choudhury, I.; Rahman, M.W.; Schlosser, P.; Ahmed, K.M.; van Geen, A. Arsenic migration to deep groundwater in Bangladesh influenced by adsorption and water demand. Nat. Geosci. 2011. [Google Scholar] [CrossRef]
- Visoottiviseth, P.; Ahmed, F. Technology for remediation and disposal of arsenic. Rev. Env. Contamin. 2008, 197, 77–128. [Google Scholar]
- Technologies and Costs for Removal of Arsenic from Drinking Water; Ground Water and Drinking Water Office, United States Environmental Protection Agency: Washington, DC, USA, 2000. Available online: www.epa.gov/ogwdw/arsenic/pdfs/treatments_and_costs.pdf (accessed on 19 June 2012).
- Berg, M.; Luzi, S.; Trang, P.T.K.; Viet, P.H.; Giger, W.; Stuben, D. Arsenic removal from groundwater by household sand filters: Comparative field study, model calculations, and health benefits. Environ. Sci. Technol. 2006, 40, 5567–5573. [Google Scholar] [CrossRef]
- Ahmed, M.F. An Overview of Arsenic Removal Technologies in Bangladesh and India; BUET-UNU International Workshop on Technologies for Arsenic Removal from Drinking Water: Dhaka, Bangladesh, 2001. [Google Scholar]
- Jiang, J.Q. Removing arsenic from groundwater for the developing world—A review. Water Sci. Technol. 2001, 44, 89–98. [Google Scholar]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behavior and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Akter, A.; Ali, M.H. Arsenic contamination in groundwater and its proposed remedial measures. Int. J. Environ. Sci. Tech. 2011, 8, 433–443. [Google Scholar]
- Nickson, R.T.; McArthur, J.M.; Ravenscroft, P.; Burgess, W.G.; Ahmed, K.M. Mechanism of arsenic release to groundwater, Bangladesh and West Bengal. Appl. Geochem. 2000, 15, 403–413. [Google Scholar] [CrossRef]
- Singh, A.K. Chemistry of arsenic in groundwater of Ganges-Brahmaputra river basin. Current Science 2006, 91, 599–606. [Google Scholar]
- Harvey, C.F.; Swartz, C.H.; Badruzzaman, A.B.M.; Keon-Blute, N.; Yu, W.; Ali, M.A.; Jay, J.; Beckie, R.; Niedan, V.; Brabander, D.; Oates, P.M.; Ashfaque, K.N.; Islam, S.; Hemond, H.F.; Ahmed, M.F. Groundwater arsenic contamination on the Ganges Delta: Biogeochemistry, hydrology, human perturbations, and human suffering on a large scale. C. R. Geoscience 2005, 337, 285–296. [Google Scholar] [CrossRef]
- Ahmed, K.M.; Bhattacharya, P.; Hasan, M.A.; Akhter, S.H.; Alam, S.M.M.; Bhuyian, M.A.H.; Imam, M.B.; Khan, A.A.; Sracek, O. Arsenic enrichment in groundwater of the alluvial aquifers in Bangladesh: An overview. Appl. Geochem. 2004, 19, 181–200. [Google Scholar]
- Bibi, M.H.; Ahmed, F.; Ishiga, H. Geochemical study of arsenic concentrations in groundwater of the Meghna River Delta, Bangladesh. J. Geochem. Explor. 2008, 97, 43–58. [Google Scholar] [CrossRef]
- BWDB (Bangladesh Water Development Board), Report on Deep Aquifer Characterization and Mapping Project, Phase-I; Ground Water Hydrology Division-I: Dhaka, Bangladesh, 2004.
- McArthur, J.M.; Banerjee, D.M.; Hudson-Edwards, K.A.; Mishra, R.; Purohit, R.; Ravenscroft, P.; Cronin, A.; Howarth, R.J.; Chatterjee, A.; Talukder, T.; Lowry, D.; Houghton, S.; Chadha, D.K. Natural organic matter in sedimentary basins and its relation to arsenic in anoxic ground water: The example of West Bengal and its worldwide implications. Appl. Geochem. 2004, 19, 1255–1293. [Google Scholar] [CrossRef]
- Zheng, Y.; Stute, M.; van Geen, A.; Gavrieli, I.; Dhara, R.; Simpson, H.J.; Schlosser, P.; Ahmed, K.M. Redox control of arsenic mobilization in Bangladesh groundwater. Appl. Geochem. 2004, 19, 201–214. [Google Scholar] [CrossRef]
- Maity, J.P.; Nath, B.; Chen, C.Y.; Bhattacharya, P.; Sracek, O.; Bundschuh, J.; Kar, S.; Thunvik, R.; Chatterjee, D.; Ahmed, K.M.; Jacks, G.; Mukherjee, A.B.; Jean, J.S. Arsenic-enriched groundwaters of India, Bangladesh and Taiwan—Comparison of hydrochemical characteristics and mobility constraints. J. Environ. Sci. Health A 2011, 46, 1163–1176. [Google Scholar]
- Anawar, H.M.; Akai, J.; Mihaljevič, M.; Sikder, A.M.; Ahmed, G.; Tareq, S.M.; Rahman, M.M. Arsenic contamination in groundwater of Bangladesh: Perspectives on geochemical, microbial and anthropogenic issues. Water 2011, 3, 1050–1076. [Google Scholar]
- Halim, M.A.; Majumder, R.K.; Nessa, S.A.; Oda, K.; Hiroshiro, Y.; Saha, B.B.; Hassain, S.M.; Latif, S.A.; Islam, M.A.; Jinno, K. Groundwater contamination with arsenic in Sherajdikhan, Bangladesh: Geochemical and hydrological implications. Environ. Geol. 2009, 58, 73–84. [Google Scholar] [CrossRef]
- Anawar, H.M.; Akai, J.; Komaki, K.; Terao, H.; Yoshioka, T.; Ishizuka, T.; Safiullah, S.; Kato, K. Geochemical occurrence of arsenic in groundwater of Bangladesh: Sources and mobilization processes. J. Geochem. Explor. 2003, 77, 109–131. [Google Scholar]
- Uddin, M.S.; Kurosawa, K. Effect of chemical nitrogen fertilizer application on the release of arsenic from sediment to groundwater in Bangladesh. Pro. Environ. Sci. 2011, 4, 294–302. [Google Scholar] [CrossRef]
- McArthur, J.M. Arsenic poisoning in the Ganges delta. Nature 1999, 401, 545–547. [Google Scholar]
- Vershinin, A.V.; Rozanov, A. The platinum electrode as an indicator of redox environment in marine sediments. Marine Chem. 1983, 14, 1–15. [Google Scholar] [CrossRef]
- Kurosawa, K.; Mochizuki, T.; Ho, T.T.L.; Nguyen, T.H.; Ho, H.N.; Hirata, M.; Egashira, K. Occurrences of arsenic in the surface and groundwater of farm villages near Hanoi, Vietnam, and factor relating to it. Jap. J. Tropi. Agri. 2005, 49, 98–106. [Google Scholar]
- Stroud, J.L.; Norton, G.J.; Islam, M.R.; Dasgupta, T.; White, R.P.; Price, A.H.; Meharg, A.A.; McGrath, S.P.; Zaho, F.-J. The dynamics of arsenic in four paddy fields in the Bengal delta. Environ. Pollut. 2011, 159, 947–953. [Google Scholar]
- Marchiset-Ferlay, N.; Savanovitch, C.; Sauvant-Rochat, M.-P. What is the best biomarker to assess arsenic exposure via drinking water? Environ. Inter. 2012, 39, 150–171. [Google Scholar]
- Sharma, P.; Goel, G.; Agarwal, A.; Tabe, K. Fate of arsenic in groundwater in south-east Asia: A threat to mankind and agriculture production. 2012; under preparation. [Google Scholar]
- Rahman, M.A.; Hasegawa, H. High levels of inorganic arsenic in rice in areas where arsenic-contaminated water is used for irrigation and cooking. Sci. Total Environ. 2011, 409, 4645–4655. [Google Scholar] [CrossRef]
- Al Rmalli, S.W.; Haris, P.I.; Harrington, C.F.; Ayub, M. A survey of arsenic in foodstuffs on sale in the United Kingdom and imported from Bangladesh. Sci. Tot. Environ. 2005, 337, 23–30. [Google Scholar]
- Ali, M.A.; Badruzzaman, A.B.M.; Jalil, M.A.; Hossain, M.D.; Ahmed, M.F.; Masud, A.A.; Kamruzzaman, M.; Rahman, M.A. Fate of Arsenic Extracted with Groundwater. In Fate of Arsenic in the Environment; Ahmed, M.F., Ed.; ITN International Training Network: Dhaka, Bangladesh, 2003; pp. 7–20. [Google Scholar]
- Su, Y.-H.; McGrath, S.P.; Zhao, F.-J. Rice is more efficient in arsenite uptake and translocation than wheat and barley. Plant Soil 2010, 328, 27–34. [Google Scholar] [CrossRef]
- Mondal, D.; Polya, D.A. Rice is a major exposure route for arsenic in Chakdaha block, Nadia district, West Bengal, India: A probabilistic risk assessmet. Appl. Geochem. 2008, 23, 2987–2998. [Google Scholar] [CrossRef]
- Meharg, A.A.; Williams, P.N.; Adomako, E.; Lawgali, Y.Y.; Deacon, C.; Villada, A. Geographical variation in total and inorganic arsenic content of polished (white) rice. Environ. Sci. Technol. 2009, 43, 1612–1617. [Google Scholar]
- Misbahuddin, M. Consumption of arsenic through cooked rice. Lancet 2003, 361, 435–436. [Google Scholar] [CrossRef]
- Das, H.K.; Mitra, A.K.; Sengupta, P.K.; Hossain, A.; Islam, F.; Rabbani, G.H. Arsenic concentrations in rice, vegetables, and fish in Bangladesh: A preliminary study. Environ. Inter. 2004, 30, 383–387. [Google Scholar] [CrossRef]
- Statement on Arsenic in Food: Results of the 1999 Total Diet Study; Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment, The Food Standards Agency: London, UK, 2003. Available online: http://cot.food.gov.uk/pdfs/ArsenicStatement.PDF (accessed on 30 May 2012).
- Williams, P.N.; Islam, M.R.; Adomako, E.E.; Raab, A.; Hossain, S.A.; Zhu, Y.G. Increase in rice grain arsenic for regions of Bangladesh irrigating paddies with elevated arsenic in groundwaters. Environ. Sci. Technol. 2006, 40, 4903–4908. [Google Scholar]
- Meharg, A.A.; Mazibur, M.D. Arsenic contamination of Bangladesh paddy field soils: Implications for rice contribution to arsenic consumption. Environ. Sci. Technol. 2003, 37, 229–234. [Google Scholar] [CrossRef]
- Pal, A.; Chowdhury, U.K.; Mondal, D.; Das, B.; Nayak, B.; Ghosh, A. Arsenic burden from cooked rice in the populations of arsenic affected and nonaffected areas and Kolkata city in West-Bengal, India. Environ. Sci. Technol. 2009, 43, 3349–3355. [Google Scholar] [CrossRef]
- Liu, H.; Probst, A.; Liao, B. Metal contamination of soils and crops affected by the Chenzhou lead/zinc mine spill (Hunan, China). Sci. Total Environ. 2005, 339, 153–166. [Google Scholar] [CrossRef]
- Roychowdhury, T.; Uchino, T.; Tokunaga, H.; Ando, M. Survey of arsenic in food composites from an arsenic-affected area of West Bengal, India. Food Chem. Toxicol. 2002, 40, 1611–1621. [Google Scholar]
- Yunus, M.; Sohel, N.; Hore, S.K.; Rahman, M. Arsenic exposure and adverse health effects: A review of recent findings from arsenic and health studies in Matlab, Bangladesh. Kaohsiung J. Medical Sci. 2011, 27, 371–376. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; Gunten, U.V.; Wehrli, B. Global water pollution and human health. Annual Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar]
- Li, Z.; Jean, J.S.; Jiang, W.T.; Chang, P.H.; Chen, C.J.; Liao, L. Removal of arsenic from water using Fe-exchanged natural zeolite. J. Hazard. Mater. 2011, 187, 318–323. [Google Scholar] [CrossRef]
- McClintock, T.R.; Chen, Y.; Bundschuh, J.; Oliver, J.T.; Navoni, J.; Olmos, V.; Lepori, E.V.; Ahsan, H.; Parvez, F. Arsenic exposure in Latin America: Biomarkers, risk assessments and related health effects. Sci. Total Environ. 2012, 429, 76–91. [Google Scholar] [CrossRef]
- Ahsan, H.; Chen, Y.; Parvez, F.; Zablotska, L.; Argos, M.; Hussain, I.; Momotaj, H.; Levy, D.; Cheng, Z.; Slavkovich, V.; van Geen, A.; Howe, G.R.; Graziano, J.H. Arsenic exposure from drinking water and risk of premalignant skin lesions in Bangladesh: Baseline results from the health effects of arsenic longitudinal study. Americ. J. Epidemio. 2006, 163, 1138–1148. [Google Scholar] [CrossRef]
- Navas-Acien, A.; Silbergeld, E.K.; Pastor-Barriuso, R.; Guallar, E. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA 2008, 300, 814–822. [Google Scholar]
- Kozul, C.D.; Ely, K.H.; Enelow, R.I.; Hamilton, J.W. Low-dose arsenic compromises the immune response to influenza A infection in vivo. Environ. Health Perspec. 2009, 117, 1441–1447. [Google Scholar]
- Masotti, A.; Da Sacco, L.; Bottazzo, G.F.; Sturchio, E. Risk assessment of inorganic arsenic pollution on human health. Environ. Pollut. 2009, 157, 1771–1772. [Google Scholar] [CrossRef]
- Martinez, V.D.; Vucic, E.A.; Becker-Santos, D.D.; Gil, L.; Lam, W.L. Arsenic exposure and the induction of human cancers. J. Toxicol. 2011. [Google Scholar] [CrossRef]
- Rahman, A.; Vahter, M.; Ekstrom, E.C.; Rahman, M.; Golam Mustafa, A.H.; Wahed, M.A. Association of arsenic exposure during pregnancy with fetal loss and infant death: A cohort study in Bangladesh. Amer. J. Epidemiol. 2007, 165, 1389–1396. [Google Scholar]
- Karagas, M.R.; Tosteson, T.D.; Blum, J.; Klaue, B.; Weiss, J.E.; Stannard, V.; Spate, V.; Morris, J.S. Measurement of low levels of arsenic exposure: A comparison of water and toenail concentrations. Amer. J. of Epidemiol. 2000, 152, 84–90. [Google Scholar] [CrossRef]
- Lindberg, A.L.; Ekström, E.C.; Nermell, B.; Rahman, M.; Lönnerdal, B.; Persson, L.A.; Vahter, M. Gender and age differences in the metabolism of inorganic arsenic in a highly exposed population in Bangladesh. Environ. Res. 2008, 106, 110–120. [Google Scholar] [CrossRef]
- Viet, P.H.; Con, T.H.; Ha, C.T.; Ha, H.V.; Berg, M.; Giger, W.; Schertenleib, R. Investigation of Arsenic Removal Technologies for Drinking Water in Vietnam. In Arsenic Exposure and Health Effects V, Proceedings of the Fifth International Conference on Arsenic Exposure and Health Effects, San Diego, CA, USA, 14–18 July 2002; Elsevier, B.V., Ed.; pp. 459–469.
- Sarkar, S.; Blaney, L.M.; Gupta, A.; Ghosh, D.; SenGupta, A.K. Arsenic removal from groundwater and its safe containment in a rural environment: Validation of a sustainable approach. Environ. Sci. Technol. 2008, 42, 4268–4273. [Google Scholar]
- Chang, Y.-Y.; Song, K.-H.; Yang, J.-K. Removal of As(III) in a column reactor packed with iron-coated sand and manganese-coated sand. J. Hazard. Mater. 2008, 150, 565–572. [Google Scholar] [CrossRef]
- Meng, X.; Korfiatis, G.P.; Christodoulatos, C.; Bang, S. Treatment of arsenic in Bangladesh well water using a household co-precipitation and filtration system. Water Res. 2001, 35, 2805–2810. [Google Scholar] [CrossRef]
- Giles, D.E.; Mohapatra, M.; Issa, T.B.; Anand, S.; Singh, P. Iron and aluminium based adsorption strategies for removing arsenic from water. J. Environ. Manage. 2011, 92, 3011–3022. [Google Scholar] [CrossRef]
- Nieto-Delgado, C.; Rangel-Mendez, J.R. Anchorage of iron hydro(oxide) nanoparticles onto activated carbon to remove As(V) from water. Water Res. 2012, 46, 2973–2982. [Google Scholar]
- Fierro, V.; Muñiz, G.; Gonzalez-Sánchez, G.; Ballinas, M.L.; Celzard, A. Arsenic removal by iron-doped activated carbons prepared by ferric chloride forced hydrolysis. J. Hazard. Mater. 2009, 168, 430–437. [Google Scholar] [CrossRef]
- Hao, J.; Han, M.-J.; Meng, X. Preparation and evaluation of thiol-functionalized activated alumina for arsenite removal from water. J. Hazard. Mater. 2009, 167, 1215–1221. [Google Scholar] [CrossRef]
- Pillewan, P.; Mukherjee, S.; Roychowdhury, T.; Das, S.; Bansiwal, A.; Rayalu, S. Removal of As(III) and As(V) from water by copper oxide incorporated mesoporous alumina. J. Hazard. Mater. 2011, 186, 367–375. [Google Scholar]
- Grover, K.; Komarneni, S.; Katsuki, H. Uptake of arsenite by synthetic double hydroxides. Water Res. 2009, 43, 3884–3890. [Google Scholar]
- Turk, T.; Alp, I.; Deveci, H. Adsorption of As(V) from water using Mg-Fe-based hydrotalcite (FeHT). J. Hazard. Mater. 2009, 171, 665–670. [Google Scholar] [CrossRef]
- Redman, A.D.; Macalady, D.L.; Ahmann, D. Natural organic matter affects arsenic speciation and sorption onto hematite. Environ. Sci. Technol. 2002, 36, 2889–2896. [Google Scholar]
- Baskan, M.B.; Pala, A. Removal of arsenic from drinking water using modified natural zeolite. Desalination 2011, 281, 396–403. [Google Scholar] [CrossRef]
- Doušová, B.; Lhotka, M.; Grygar, T.; Machovič, V.; Herzogová, L. In situ co-adsorption of arsenic and iron/manganese ions on raw clays. Appl. Clay Sci. 2011, 54, 166–171. [Google Scholar] [CrossRef]
- Jiang, J.Q.; Xu, Y.; Quill, K.; Simon, J.; Shettle, K. Laboratory study of boron removal by mg/al double-layered hydroxides. Industrial Eng. Chem. Res. 2007, 46, 4577–4583. [Google Scholar]
- 1. Jiang, J.Q.; Ashekuzzaman, S.M. Development of novel inorganic adsorbent for water treatment. Curr. Opin. Chem. Eng. 2012, 1, 191–199. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jiang, J.-Q.; Ashekuzzaman, S.M.; Jiang, A.; Sharifuzzaman, S.M.; Chowdhury, S.R. Arsenic Contaminated Groundwater and Its Treatment Options in Bangladesh. Int. J. Environ. Res. Public Health 2013, 10, 18-46. https://doi.org/10.3390/ijerph10010018
Jiang J-Q, Ashekuzzaman SM, Jiang A, Sharifuzzaman SM, Chowdhury SR. Arsenic Contaminated Groundwater and Its Treatment Options in Bangladesh. International Journal of Environmental Research and Public Health. 2013; 10(1):18-46. https://doi.org/10.3390/ijerph10010018
Chicago/Turabian StyleJiang, Jia-Qian, S. M. Ashekuzzaman, Anlun Jiang, S. M. Sharifuzzaman, and Sayedur Rahman Chowdhury. 2013. "Arsenic Contaminated Groundwater and Its Treatment Options in Bangladesh" International Journal of Environmental Research and Public Health 10, no. 1: 18-46. https://doi.org/10.3390/ijerph10010018
APA StyleJiang, J.-Q., Ashekuzzaman, S. M., Jiang, A., Sharifuzzaman, S. M., & Chowdhury, S. R. (2013). Arsenic Contaminated Groundwater and Its Treatment Options in Bangladesh. International Journal of Environmental Research and Public Health, 10(1), 18-46. https://doi.org/10.3390/ijerph10010018






