Heterofucan from Sargassum filipendula Induces Apoptosis in HeLa Cells
Abstract
:1. Introduction
2. Results and Discussion
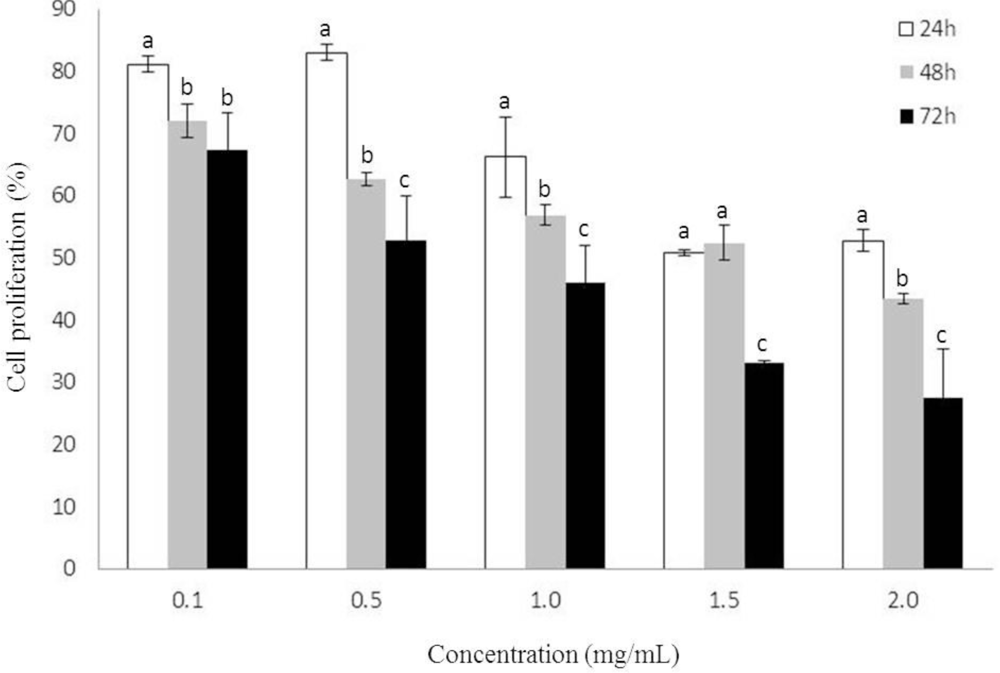
2.1. Growth Inhibition by Heterofucan SF-1.5v
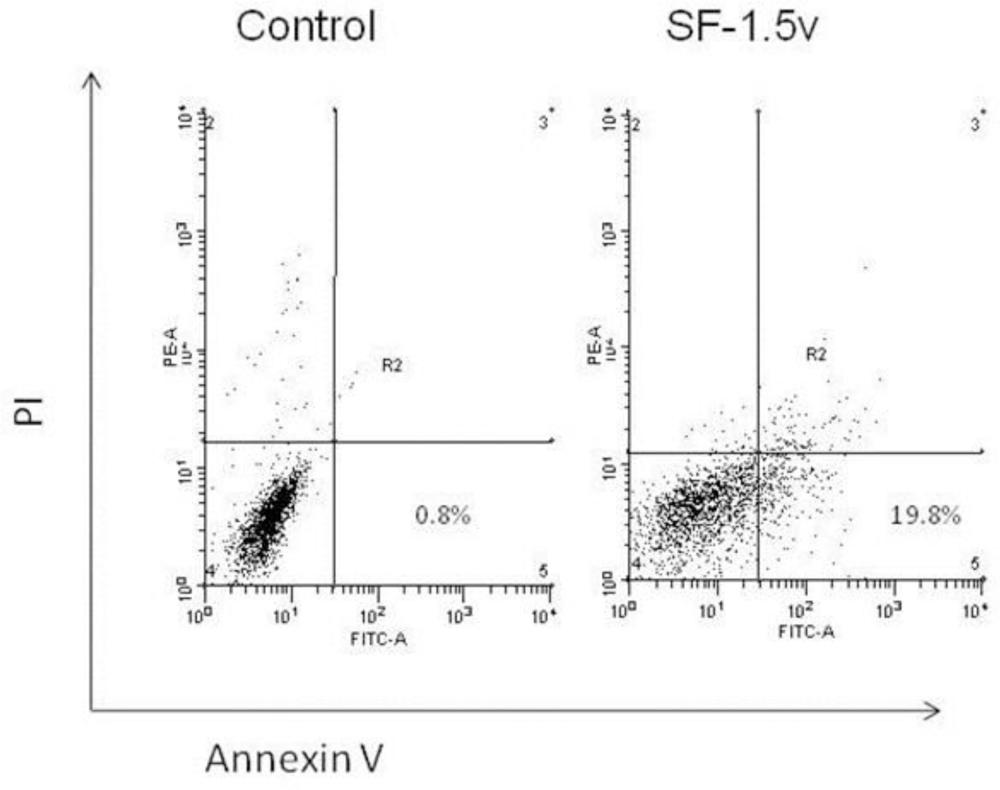
2.2. Heterofucan SF-1.5v-Induced Apoptosis in HeLa Cells
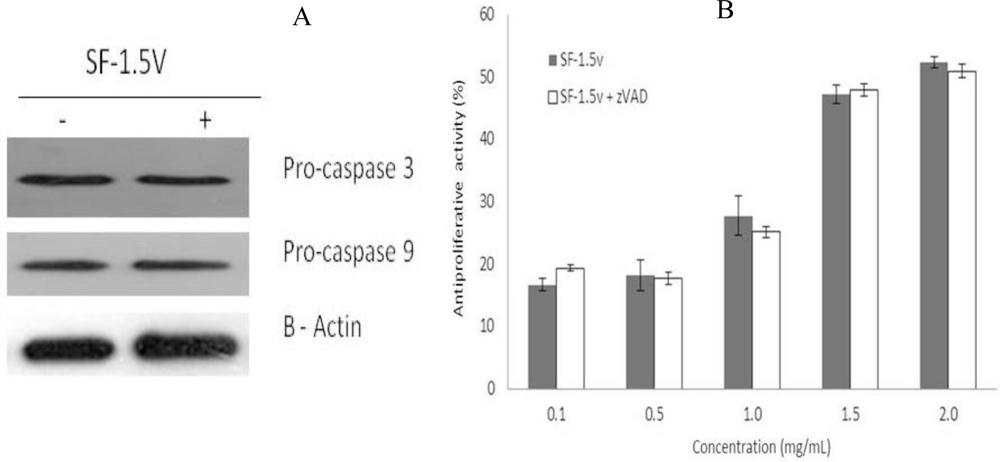
2.3. Heterofucan SF-1.5v Treatment-Induced Apoptosis Did Not Require Activation of Caspases in HeLa Cells
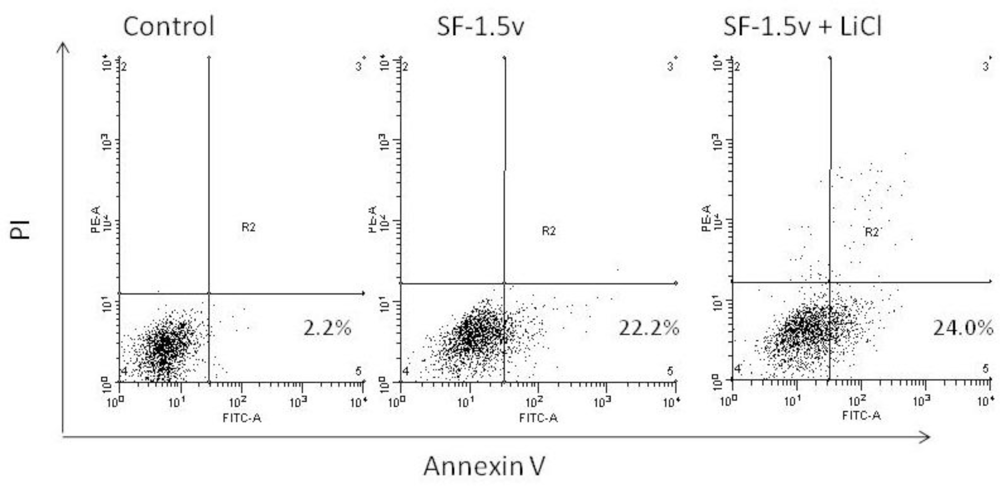
2.4. Heterofucan SF-1.5v Treatment-Induced Apoptosis in the Presence of GSK Inhibitor
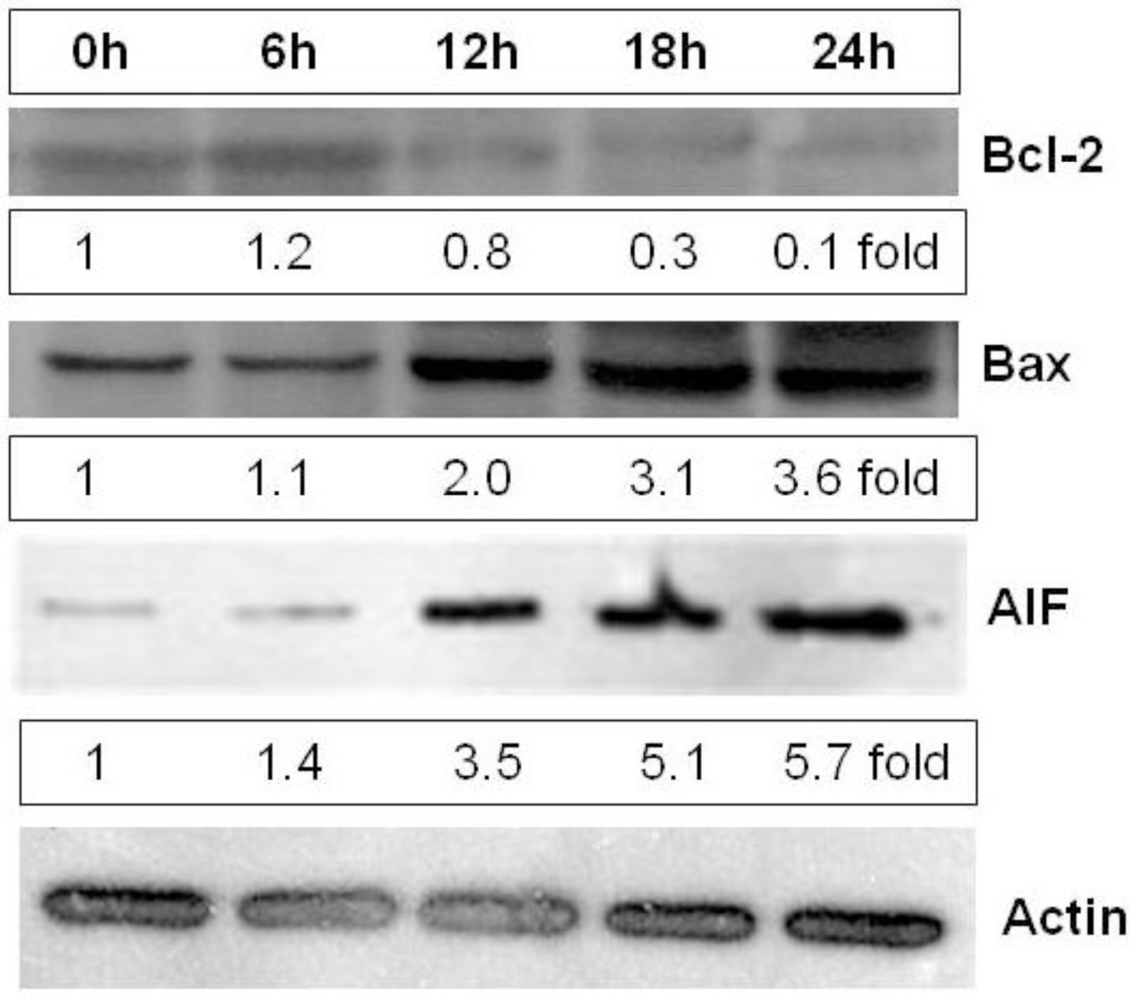
2.5. Heterofucan SF-1.5v Induces High Levels of Apoptosis-Inducing Factor (AIF) in Cytoplasm
3. Experimental Section
3.1. Materials
3.2. HeLa Cell Culture
3.3. Antiproliferative Activity
3.4. Apoptosis Assay
3.5. Western Blotting
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Scarinci, IC; Garcia, FAR; Kobetz, E; Partridge, EE; Brandt, HM; Bell, MC; Dignan, M; Ma, GX; Daye, JL; Castle, PE. Cervical cancer prevention: new tools and old barriers. Cancer 2010, 116, 2531–2542. [Google Scholar]
- Kroemer, G; Galluzzi, L; Vandenabeele, P; Abrams, J; Alnemri, ES; Baehrecke, EH; Blagosklonny, MV; El-Deiry, WS; Golstein, P; Green, DR; Hengartner, M; Knight, RA; Kumar, S; Lipton, SA; Malorni, W; Nuñez, G; Peter, ME; Tschopp, J; Yuan, J; Piacentini, M; Zhivotovsky, B; Melino, G. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 2009, 16, 3–11. [Google Scholar]
- Li, B; Lu, F; Wei, X; Zhao, R. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar]
- Pomin, VH. Review: an overview about the structure-function relationship of marine sulfated homopolysaccharides with regular chemical structures. Biopolymers 2009, 91, 601–609. [Google Scholar]
- Dietrich, CP; Farias, GGM; Abreu, LR; Leite, EL; Silva, LF; Nader, HB. A new approach for the characterization of polysaccharides from algae: presence of four main acidic polysaccharides in three species of the class Phaeophycea. Plant Sci 1995, 108, 143–153. [Google Scholar]
- Albuquerque, IRL; Queiroz, KCS; Alves, LG; Santos, EA; Leite, EL; Rocha, HAO. Heterofucans from Dictyota menstrualis have anticoagulant activity. Braz J Med Biol Res 2004, 37, 167–171. [Google Scholar]
- Choi, JI; Raghavendran, HRB; Sung, NY; Kim, JH; Chun, BS; Ahn, DH; Choi, HS; Kang, KW; Lee, JW. Effect of fucoidan on aspirin-induced stomach ulceration in rats. Chem Biol Interact 2010, 183, 249–254. [Google Scholar]
- Rocha, HAO; Franco, CRC; Trindade, ES; Carvalho, LCM; Veiga, SS; Leite, EL; Dietrich, CP; Nader, HB. A fucan from the brown seaweed Spatoglossum schröederi inhibits Chinese hamster ovary cell adhesion to several extracellular matrix proteins. Braz J Med Biol Res 2001, 34, 621–626. [Google Scholar]
- Synytsya, A; Kim, WJ; Kim, SM; Pohl, R; Synytsya, A; Kvasnička, F; Čopíková, J; Park, YL. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr Polym 2010, 81, 41–48. [Google Scholar]
- Costa, LS; Fidelis, GP; Cordeiro, SL; Oliveira, RM; Sabry, DA; Câmara, RBG; Nobre, LTDB; Costa, MSSP; Almeida-Lima, J; Farias, EHC; Leite, EL; Rocha, HAO. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed Pharmacother 2010, 64, 21–28. [Google Scholar]
- Kim, EJ; Park, SY; Lee, JY; Park, JHY. Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol 2010, 10, 1–11. [Google Scholar]
- Aisa, Y; Miyakawa, Y; Nakazato, T; Shibata, H; Saito, K; Ikeda, Y; Kizaki, M. Fucoidan induces apoptosis of human HS-sultan cells accompanied by activation of caspase-3 and down-regulation of ERK pathways. Am J Hematol 2005, 78, 7–14. [Google Scholar]
- Berteau, O; Mulloy, B. Sulfated fucans, fresh perspectives: structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29–40. [Google Scholar]
- Yamamoto, I; Takahashi, M; Suzuki, T; Seino, H; Mori, H. Antitumor effect of seaweeds. IV. Enhancement of antitumor activity by sulfation of a crude fucoidan fraction from Sargassum kjellmanianum. Jpn J Exp Med 1984, 54, 143–151. [Google Scholar]
- Stevan, FR; Oliveira, MB; Bucchi, DF; Noseda, M; Iacomini, M; Duarte, ME. Cytotoxic effects against HeLa cells of polysaccharides from seaweeds. J Submicrosc Cytol Pathol 2001, 33, 477–484. [Google Scholar]
- Hyun, JH; Kim, SC; Kang, JI; Kim, MK; Boo, HJ; Kwon, JM; Koh, YS; Hyun, JW; Park, DB; Yoo, ES; Kang, HK. Apoptosis inducing activity of fucoidan in HCT-15 colon carcinoma cells. Biol Pharm Bull 2009, 32, 1760–1764. [Google Scholar]
- Nakamura, T; Suzuki, H; Wada, Y; Kodama, T; Doi, T. Fucoidan induces nitric oxide production via p38 mitogen-activated protein kinase and NF-κB-dependent signaling pathways through macrophage scavenger receptors. Biochem Biophys Res Commun 2006, 343, 286–294. [Google Scholar]
- King, TD; Bijur, GN; Jope, RS. Caspase-3 activation induced by inhibition of mitochondrial complex I is facilitated by glycogen synthase kinase-3beta and attenuated by lithium. Brain Res 2001, 919, 106–114. [Google Scholar]
- Arboleda, G; Cárdenas, Y; Rodríguez, Y; Morales, LC; Matheus, L; Arboleda, H. Differential regulation of AKT, MAPK and GSK3β during C2-ceramide-induced neuronal death. Neurotoxicology 2010, 31, 687–693. [Google Scholar]
- Norberg, E; Orrenius, S; Zhivotovsky, B. Mitochondrial regulation of cell death: Processing of apoptosis-inducing factor (AIF). Biochem Biophys Res Commun 2010, 396, 95–100. [Google Scholar]
- Hangen, E; Blomgren, K; Bénit, P; Kroemer, G; Modjtahedi, N. Life with or without AIF. Trends Biochem Sci 2010, 35, 278–287. [Google Scholar]
- Selvakumar, E; Hsieh, T. Regulation of cell cycle transition and induction of apoptosis in HL-60 leukemia cells by lipoic acid: role in cancer prevention and therapy. J Hematol Oncol 2008, 1, 1–8. [Google Scholar]
- Almeida-Lima, J; Costa, LS; Silva, NB; Melo-Silveira, RF; Silva, FV; Felipe, MBMC; Medeiros, SRBM; Leite, EL; Rocha, HAO. Evaluating the possible genotoxic, mutagenic and tumor cell proliferation-inhibition effects of a non-anticoagulant, but antithrombotic algal heterofucan. J Appl Toxicol 2010, 30, 708–715. [Google Scholar]
- Amoli, JS; Sadighara, P; Barin, A; Yazdani, A; Satari, S. Biological screening of Amaranthus retroflexus L. (Amaranthaceae). Rev Bras Farmacogn 2009, 19, 617–620. [Google Scholar]

© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Silva Costa, L.; Silva Telles, C.B.; Medeiros Oliveira, R.; Duarte Barreto Nobre, L.T.; Dantas-Santos, N.; Barros Gomes Camara, R.; Santana Santos Pereira Costa, M.; Almeida-Lima, J.; Melo-Silveira, R.F.; Lopes Albuquerque, I.R.; et al. Heterofucan from Sargassum filipendula Induces Apoptosis in HeLa Cells. Mar. Drugs 2011, 9, 603-614. https://doi.org/10.3390/md9040603
Silva Costa L, Silva Telles CB, Medeiros Oliveira R, Duarte Barreto Nobre LT, Dantas-Santos N, Barros Gomes Camara R, Santana Santos Pereira Costa M, Almeida-Lima J, Melo-Silveira RF, Lopes Albuquerque IR, et al. Heterofucan from Sargassum filipendula Induces Apoptosis in HeLa Cells. Marine Drugs. 2011; 9(4):603-614. https://doi.org/10.3390/md9040603
Chicago/Turabian StyleSilva Costa, Leandro, Cinthia Beatrice Silva Telles, Ruth Medeiros Oliveira, Leonardo Thiago Duarte Barreto Nobre, Nednaldo Dantas-Santos, Rafael Barros Gomes Camara, Mariana Santana Santos Pereira Costa, Jailma Almeida-Lima, Raniere Fagundes Melo-Silveira, Ivan Rui Lopes Albuquerque, and et al. 2011. "Heterofucan from Sargassum filipendula Induces Apoptosis in HeLa Cells" Marine Drugs 9, no. 4: 603-614. https://doi.org/10.3390/md9040603
APA StyleSilva Costa, L., Silva Telles, C. B., Medeiros Oliveira, R., Duarte Barreto Nobre, L. T., Dantas-Santos, N., Barros Gomes Camara, R., Santana Santos Pereira Costa, M., Almeida-Lima, J., Melo-Silveira, R. F., Lopes Albuquerque, I. R., Leite, E. L., & Oliveira Rocha, H. A. (2011). Heterofucan from Sargassum filipendula Induces Apoptosis in HeLa Cells. Marine Drugs, 9(4), 603-614. https://doi.org/10.3390/md9040603





