Impact of Ocean Acidification on Energy Metabolism of Oyster, Crassostrea gigas—Changes in Metabolic Pathways and Thermal Response
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. Animal collection and maintenance
3.2. Tissue and hemolymph collection
3.3. Determination of hemolymph acid-base parameters, Peco2, Peo2 and Ceco2
3.4. Determination of metabolites
3.5. Determination of standard metabolic rate and animal condition index
3.6. Determination of cellular respiration rates and fractional cost for ion regulation via Na+/K+ ATPase
3.7. Statistical analysis
4. Conclusions
Acknowledgements
References
- Pörtner, HO; Farrell, AP. Physiology and climate change. Science 2008, 322, 690–692. [Google Scholar]
- Fabry, VJ. Marine calcifiers in a high-CO2 ocean. Science 2008, 320, 1020–1022. [Google Scholar]
- Melzner, F; Göbel, S; Langenbuch, M; Gutowska, MA; Pörtner, HO; Lucassen, M. Swimming performance in Atlantic Cod (Gadus morhua) following long-term (4–12 months) acclimation to elevated seawater Pco2. Aquat. Toxicol 2009, 92, 30–37. [Google Scholar]
- Pörtner, HO. Oxygen- and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J. Exp. Biol 2010, 213, 881–893. [Google Scholar]
- Savitz, J; Harrould-Kolieb, E. The oceans’ acid test: can our reefs be saved? Front. Ecol. Environ 2008, 6, 515. [Google Scholar]
- Royal Society, Ocean acidification due to increasing atmospheric carbon dioxide; Policy Document 12/05; The Royal Society: London, UK, 2005.
- Kleypas, JA; Feely, RA; Fabry, VJ; Langdon, C; Sabine, CL; Robbins, LL. Impacts of ocean acidification on coral reefs and other marine calcifiers: a guide for future research. Report of a workshop held on 18–20 April 2005, St Petersburg, FL, USA; 2006. [Google Scholar]
- Hall-Spencer, JM; Rodolfo-Metalpa, R; Martin, S; Ransome, E; Fine, M; Turner, SM; Rowley, SJ; Tedesco, D; Buia, MC. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 2008, 454, 96–99. [Google Scholar]
- Fabry, VJ; Seibel, BA; Feely, RA; Orr, JC. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci 2008, 65, 414–432. [Google Scholar]
- Andersson, AJ; Mackenzie, FT; Bates, NR. Life on the margin: Implications of ocean acidification on Mg-calcite, high latitude and cold water marine calcifiers. Mar. Ecol. Prog. Ser 2008, 373, 265–273. [Google Scholar]
- Reid, PC; Fischer, A; Lewis-Brown, E; Meredith, MP; Sparrow, M; Andersson, AJ; Antia, A; Bathmann, U; Beaugrand, G; Brix, H; et al. Impacts of the Oceans on Climate Change. Adv. Mar. Biol 2009, 56, 1–150. [Google Scholar]
- Riebesell, U; Zondervan, I; Rost, B; Tortell, PD; Zeebe, RE; Morel, FMM. Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature 2000, 407, 364–367. [Google Scholar]
- Caldeira, K; Wickett, ME. Oceanography: anthropogenic carbon and ocean pH. Nature 2003, 425, 365. [Google Scholar]
- Caldeira, K; Wickett, ME. Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J. Geophys. Res 2005, 110, C09S04. [Google Scholar]
- Pörtner, HO; Langenbuch, M; Reipschläger, A. Biological impact of elevated CO2 concentrations: lessons from animal physiology and earth history. J. Ozenogr 2004, 60, 705–718. [Google Scholar]
- Ishimatsu, AT; Kikkaea, T; Hayashi, M; Lee, KS; Kita, J. Effects of CO2 on marine fish: larvae and adults. J. Oceanogr 2004, 60, 731–741. [Google Scholar]
- Ishimatsu, A; Hayashi, M; Lee, KS; Kikkawa, T; Kita, J. Physiological effects on fishes in a high-CO2 world. J. Geophys. Res 2005, 110. [Google Scholar] [CrossRef]
- Pörtner, HO. Schubert, R, Schellnhuber, HJ, Buchmann, N, Epiney, A, Grieshammer, R, Kulessa, M, Messner, D, Rahmstorf, S, Schmid, J, Eds.; Auswirkungen von CO2-Eintrag und Temperaturerhöhung auf die marine Biosphäre. In Die Zukunft der Meere – zu warm, zu hoch, zu sauer; Sondergutachten des WBGU: Berlin, Germany, 2006. [Google Scholar]
- Green, MA; Waldbusser, GG; Reilly, SL; Emerson, K; O’Donnella, S. Death by dissolution: sediment saturation state as a mortality factor for juvenile bivalves. Limnol. Oceanogr 2009, 54, 1037–1047. [Google Scholar]
- Munday, PL; Dixson, DL; Donelson, JM; Jones, GP; Pratchett, MS; Devitsina, GV; Doving, KB. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc. Natl. Acad. Sci 2009, 10, 1848–1852. [Google Scholar]
- Meehl, GA; Stocker, TF; Collins, WD; Friedlingstein, P; Gaye, AT; Gregory, JM; Kitoh, A; Knutti, R; Murphy, JM; Noda, A; et al. Solomon, S, Qin, D, Manning, M, Chen, Z, Marquis, M, Averyt, KB, Tignor, M, Miller, HL, Eds.; Global climate projections. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007; pp. 747–846. [Google Scholar]
- Guppy, M; Withers, P. Metabolic depression in animals: physiological perspectives and biochemical generalizations. Biol. Rev 1999, 74, 1–40. [Google Scholar]
- Pörtner, HO. Climate-dependent evolution of Antarctic ectotherms: an integrative analysis. Deep-Sea Res. II 2006b, 53, 1071–1104. [Google Scholar]
- Sommer, A; Klein, B; Pörtner, HO. Temperature induced anaerobiosis in two populations of the polychaete worm Arenicola marina. J. Comp. Physiol. B 1997, 16, 725–735. [Google Scholar]
- van Dijk, PLM; Tesch, C; Hardewig, I; Pörtner, HO. Physiological disturbances at critically high temperatures. A comparison between stenothermal Antarctic and eurythermal temperate eelpouts (Zoarcidae). J. Exp. Biol 1999, 202, 3611–3621. [Google Scholar]
- Abele, D; Puntarulo, S. Formation of reactive species and induction of antioxidant defence systems in polar and temperate marine invertebrates and fish. Comp. Physiol. Ecol. Part A 2004, 138, 405–415. [Google Scholar]
- Lannig, G; Bock, C; Sartoris, FJ; Pörtner, HO. Oxygen limitation of thermal tolerance in cod, Gadus morhua L. studied by magnetic resonance imaging and on-line venous oxygen monitoring. Am. J. Physiol 2004, 287, R902–R910. [Google Scholar]
- Wittmann, AC; Schröer, M; Bock, C; Steeger, HU; Paul, RJ; Pörtner, HO. Indicators of oxygen- and capacity limited thermal tolerance in the lugworm Arenicola marina. Clim. Res 2008, 37, 227–240. [Google Scholar]
- Pörtner, HO; Lannig, G. Richards, JG, Farrell, AP, Brauner, CJ, Eds.; Oxygen and Capacity limited Thermal Tolerance. In Fish Physiology: Hypoxia; London Academic: London, UK, 2009; Volume 27, pp. 143–191. [Google Scholar]
- Metzger, R; Sartoris, FJ; Langenbuch, M; Pörtner, HO. Influence of elevated CO2 concentrations on thermal tolerance of the edible crab Cancer pagurus. J. Thermal. Biol 2007, 32(3), 144–151. [Google Scholar]
- Walther, K; Sartoris, FJ; Bock, C; Pörtner, HO. Impact of anthropogenic ocean acidification on thermal tolerance of the spider crab Hyas araneus. Biogeosci. Discuss 2007, 6, 2837–2861. [Google Scholar]
- Michaelidis, B; Ouzounis, C; Paleras, A; Pörtner, HO. Effects of long-term moderate hypercapnia on acid-base balance and growth rate in marine mussels Mytilus galloprovincialis. Mar. Ecol. Prog. Ser 2005, 293, 109–118. [Google Scholar]
- Pörtner, HO. Ecosystem effects of ocean acidification in times of ocean warming: a physiologist’s view. Mar. Ecol. Prog. Ser 2008, 373, 203–217. [Google Scholar]
- Shirayama, Y; Thornton, H. Effect of increased atmospheric CO2 on shallow water marine benthos. J. Geophys. Res 2005, 110, C09S08. [Google Scholar] [CrossRef]
- Pörtner, HO; Langenbuch, M; Michaelidis, B. Synergistic effects of temperature extremes, hypoxia and increases in CO2 on marine animals: from earth history to global change. J. Geophys. Res 2005, 110, C09S10. [Google Scholar] [CrossRef]
- Seibel, BA; Fabry, VJ. Marine biotic response to elevated carbon dioxide. Adv. Appl. Biodiv. Sci 2003, 4, 59–67. [Google Scholar]
- Lindinger, MI; Lawren, DJ; McDonald, DG. Acid–base balance in the sea mussel Mytilus edulis. Effects of environmental hypercapnia on intra and extracellular acid–base balance. Mar. Biol. Lett 1984, 5, 371–381. [Google Scholar]
- Nienhuis, S; Palmer, AR; Harley, CDG. Elevated CO2 affects shell dissolution rate but not calcification rate in a marine snail. Proc. R. Soc. B 2010. [Google Scholar] [CrossRef]
- DeFur, PL; McMahon, BR. Physiological compensation to short term air exposure in red rock crabs Cancer productus from littoral and sublittoral habitats: Acid–base balance. Physiol. Zool 1984, 57, 151–160. [Google Scholar]
- Gazeau, F; Quiblier, C; Jansen, JM; Gattuso, JP; Middelburg, JJ; Heip, CHR. Impact of elevated CO2 on shellfish calcification. Geophys. Res. Lett 2007, 34, L07603. [Google Scholar] [CrossRef]
- Ries, JB; Cohen, AL; McCorkle, DC. Marine calcifiers exhibit mixed responses to CO2 -induced ocean acidification. Geol 2009, 37, 1131–1134. [Google Scholar]
- Berge, JA; Bierkeng, B; Pettersen, O; Schaanning, MT; Oxnevad, S. Effects of increased water concentrations of CO2 on growth of the bivalve Mytilus edulis L. Chemosphere 2006, 62, 681–687. [Google Scholar]
- Gutowska, MA; Pörtner, HO; Melzner, F. Growth and calcification in the cephalopod Sepia officinalis under elevated seawater pCO2. MEPS 2008, 373, 303–309. [Google Scholar]
- Fennel, K; Wilkin, J; Previdi, M; Najjar, R. Denitrification effects on air-sea CO2 flux in the coastal ocean: Simulations for the northwest North Atlantic. Geophys. Res. Lett 2008, 35, L24608. [Google Scholar] [CrossRef]
- Cao, L; Caldeira, K. Atmospheric CO2 stabilization and ocean acidification. Geophys. Res. Lett 2008, 35, L19609. [Google Scholar] [CrossRef]
- Turley, C; Eby, M; Ridgwell, AJ; Schmidt, DN; Findlay, HS; Brownlee, C; Riebesell, U; Fabry, VJ; Feely, RA; Gattuso, JP. The societal challenge of ocean acidification. Mar. Poll. Bull 2010, 60, 787–792. [Google Scholar]
- Brewer, PG; Bradshow, AL; Williams, RT. Trabalka, JR, Reichle, DE, Eds.; Measurement of total carbon dioxide and alkalinity in the North Atlantic Ocean in 1981. In The Changing Carbon Cycle—A Global Analysis; Springer: New York, NY, USA, 1986; pp. 358–381. [Google Scholar]
- Lewis, E; Wallace, D. Program Development for CO2 System Calculations; Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1998. [Google Scholar]
- Mehrbach, C; Culberson, C; Hawley, J; Pytkovicz, R. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr 1973, 18, 897–907. [Google Scholar]
- Dickson, AG; Millero, FJ. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep-Sea Res 1987, 34, 1733–1743. [Google Scholar]
- Gutowska, MA; Melzner, F; Pörtner, HO; Meier, S. Cuttlebone calcification increases during exposure to elevated seawater pCO2 in the cephalopod Sepia officinalis. Mar. Biol 2010, 157, 1653–1663. [Google Scholar]
- Trimborn, S; Wolf-Gladrow, D; Richter, KU; Rost, B. The effect of pCO2 on carbon acquisition and intracellular assimilation in four marine diatoms. J. Exp. Mar. Biol. Ecol 2009, 376, 26–36. [Google Scholar]
- Pörtner, HO; Boutilier, RG; Tang, Y; Toews, DP. Determination of intracellular pH and PCO2 after metabolic inhibition by fluoride and nitrilotriacetic acid. Respir. Physiol 1990, 81, 255–274. [Google Scholar]
- Heisler, N. Heisler, N, Ed.; Buffering and transmembrane ion transfer processes. In Acid-Base Regulation in Animals; Elsevier: Amsterdam, The Netherlands, 1986; pp. 3–47. [Google Scholar]
- Wittmann, AC; Held, C; Pörtner, HO; Sartoris, FJ. Ion regulatory capacity and the biogeography of Crustacea at high southern latitudes. Polar Biol 2010, 33, 919–928. [Google Scholar]
- Lynch, MJ; Master, J; Pryor, JP; Lindon, JC; Spraul, M; Foxall, PJD; Nicholson, JK. Ultra high field NMR spectroscopic studies on human seminal fluid, seminal vesicle and prostatic secretions. J. Pharm. Biomed. Anal 1994, 12, 5–19. [Google Scholar]
- Willker, W; Engelmann, J; Brand, A; Leibfritz, D. Metabolite identification in cell extracts and culture media by proton-detected 2D-H,C-NMR spectroscopy. J. Magn. Reson. Anal 1997, 2, 21–32. [Google Scholar]
- Viant, MR; Rosenblum, ES; Tjeerdema, RS. NMR based metabolomics: A powerful approach for characterizing the effects of environmental stressors on organism health. Environ. Sci. Technol 2003, 37, 4982–4498. [Google Scholar]
- Pinero-Sagredo, E; Nunes, S; Jose, M; de los Santos, J; Celda, B; Esteve, V. NMR metabolic profile of human follicular fluid. NMR Biomed 2010, 23, 485–495. [Google Scholar]
- Bubb, WA; Wright, LC; Cagney, M; Santangelo, RT; Sorrell, TC; Kuchel, PW. Heteronuclear NMR studies of metabolites produced by Cryptococcus neoformans in culture media: identification of possible virulence factors. Magn. Reson. Med 1999, 42, 442–453. [Google Scholar]
- Sze, DY; Jardetzky, O. Determination of metabolite and nucleotide concentrations in proliferating lymphocytes by 1H NMR of acid extracts. Biochim. Biophys. Acta 1990, 1054, 181–197. [Google Scholar]
- Dabos, KJ; Parkinson, JA; Hewage, C; Nelson, LJ; Sadler, IH; Hayes, PC; Plevris, JN. 1H NMR spectroscopy as a tool to evaluate key metabolic functions of primary porcine hepatocytes after cryopreservation. NMR Biomed 2002, 15, 241–250. [Google Scholar]
- Lannig, G; Flores, FJ; Sokolova, IM. Temperature-dependent stress response in oysters, Crassostrea virginica: Pollution reduces temperature tolerance in oysters. Aquat. Toxicol 2006, 79, 278–287. [Google Scholar]
- Bougrier, S; Geairon, P; Deslous-Paoli, JM; Bacher, C; Jonquieres, G. Allometric relationships and effects of temperature on clearance and oxygen consumption rates of Crassostrea gigas (Thunberg). Aquaculture 1995, 134, 143–154. [Google Scholar]
- Lucas, AP; Beninger, G. The use of physiological condition index in marine bivalve aquaculture. Aquaculture 1985, 44, 187–200. [Google Scholar]
- Cruz-Rodriguez, LA; Baucum, AJ; Soudant, P; Chu, F-LE; Hale, RC. Effects of PCBs sorbed to algal paste and sediments on stress protein response (HSP70 family) in the eastern oyster, Crassostrea virginica. Mar. Environ. Res 2000, 50, 341–345. [Google Scholar]
- Cherkasov, AS; Biswas, PK; Ridings, DM; Ringwood, AH; Sokolova, IM. Effects of acclimation temperature and cadmium exposure on cellular energy budgets in the marine mollusk Crassostrea virginica: linking cellular and mitochondrial responses. J. Exp. Biol 2006, 209, 1274–1284. [Google Scholar]
- Reipschläger, A; Pörtner, HO. Metabolic depression during environmental stress: The role of extracellular versus intracellular pH in Sipunculus nudus. J. Exp. Biol 1996, 199, 1801–1807. [Google Scholar]
- Willson, LL; Burnett, LE. Whole animal and gill tissue oxygen uptake in the Eastern oyster, Crassostrea virginica: Effect of hypoxia, hypercapnia, air exposure, and infection with the protozoan parasite Perkinsus marinus. J. Exp. Mar. Biol. Ecol 2000, 246, 223–240. [Google Scholar]
- Krumschnabel, G; Schwarzbaum, PJ; Wieser, W. Coupling of energy supply and energy demand in isolated goldfish Hepatocytes. Physiol. Zool 1994, 67, 438–448. [Google Scholar]
- Mark, FC; Hirse, T; Pörtner, HO. Thermal sensitivity of cellular energy budgets in some Antarctic fish hepatocytes. Polar Biol 2005, 28, 805–814. [Google Scholar] [Green Version]
- Addadi, L; Joester, D; Nudelman, F; Weiner, S. Mollusk Shell Formation: A Source of New Concepts for Understanding Biomineralization Processes. Chem. Eur. J 2006, 12, 980–987. [Google Scholar]
- Palmer, AR. Relative cost of producing skeletal organic matrix versus calcification: evidence from marine gastropods. Mar. Biol 1983, 75, 287–292. [Google Scholar]
- Palmer, AR. Calcification in Marine Molluscs: How Costly is it? Proc. Natl. Acad. Sci 1992, 89, 1379–1382. [Google Scholar]
- Digby, PSB. Fretter, V, Ed.; The mechanism of calcification in the molluscan shell. In Symposium of Zoological Society of London. Studies in the Structure, Physiology and Ecology of Molluscs; Academic Press: London, UK, 1968; pp. 93–107. [Google Scholar]
- Wheeler, AP. Bonucci, E, Ed.; Mechanisms of molluscan shell formation. In Calcification in Biological Systems; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Mount, AS; Wheeler, AP; Paradkar, RP; Snider, D. Hemocyte-mediated shell mineralization in the eastern oyster. Science 2004, 304, 297–300. [Google Scholar]
- Day, EG; Branch, GM; Viljoen, C. How costly is molluscan shell erosion? A comparison of two patellid limpets with contrasting shell structures. J. Exp. Mar. Biol. Ecol 2000, 243, 185–208. [Google Scholar]
- Kurihara, H; Asai, T; Kato, S; Ishimatsu, A. Effects of elevated p CO2 on early development in the mussel Mytilus galloprovincialis. Aquat. Biol 2008, 4, 225–233. [Google Scholar]
- Wood, H; Spicer, JI; Widdicombe, S. Ocean acidification may increase calcification rates, but at a cost. Proc. R. Soc. B 2008, 275, 1767–1773. [Google Scholar]
- Thomsen, J; Melzner, F. Seawater acidification does not elicit metabolic depression in the blue mussel Mytilus edulis. Mar. Biol 2010. Submitted. [Google Scholar]
- Langenbuch, M; Pörtner, HO. Energy budget of hepatocytes from Antarctic fish (Pachycara brachycephalum and Lepidonotothen kempi) as a function of ambient CO2: pH-dependent limitations of cellular protein biosynthesis? J. Exp. Biol 2003, 206, 3895–3903. [Google Scholar]
- Langenbuch, M; Bock, C; Leibfritz, D; Pörtner, HO. Effects of environmental hypercapnia on animal physiology: A 13C NMR study of protein synthesis rates in the marine invertebrate Sipunculus nudus. Comp. Biochem. Physiol. A 2006, 144, 479–484. [Google Scholar]
- Grieshaber, MK; Hardewig, I; Kreutzer, U; Poertner, HO. Physiological and metabolic responses to hypoxia in invertebrates. Rev. Physiol. Biochem. Pharmacol 1994, 125, 143–147. [Google Scholar]
- Kurochkin, IO; Ivanina, AV; Eilers, S; Downs, CA; May, LA; Sokolova, IM. Cadmium affects metabolic responses to prolonged anoxia and reoxygenation in eastern oysters Crassostrea virginica. Am. J. Physiol 2009, R1262–R1272. [Google Scholar]
- Michaelidis, B; Haas, D; Grieshaber, MK. Extracellular and Intracellular Acid-Base Status with Regard to the Energy Metabolism in the Oyster Crassostrea gigas during Exposure to Air. Physiol. Biochem. Zool 2005, 78, 373–383. [Google Scholar]
- Deigweiher, K. Impact of high CO2 concentrations on marine life: Molecular mechanisms and physiological adaptations of pH and ion regulation in marine fish. PhD Thesis, University of Bremen, Bremen, Germany, 2009. [Google Scholar]
- Thomsen, J; Gutowska, MA; Saphörster, J; Heinemann, A; Trübenbach, K; Fietzke, J; Hiebenthal, C; Eisenhauer, A; Körtzinger, A; Wahl, M; Melzner, F. Calcifying invertebrates succeed in a naturally CO2 enriched coastal habitat but are threatened by high levels of future ocean acidification. Biogeosci. Dis 2010, 7, 5119–5156. [Google Scholar] [CrossRef]
- Zittier, ZMC; Kutsch, B; Bock, C; Pörtner, HO. Synergistic impacts of ocean acidification and temperature increase on the physiology of Mytilus edulis. Presented at the Annual Main Meeting of Society for Experimental Biology (SEB), C8: General Thermal Biology, Prague, Czech Republic, June/July 2010.
- Stark, A; Treydte, T; Heilmeyer, O; Brey, T; Pörtner, HO. Is the Greenland smoothcockle (Serripes groenlandicus) sensitive to elevated CO2 and temperature levels? Presented at the Annual Main Meeting of Society for Experimental Biology (SEB), C1: Polar adaptations & challenges, Prague, Czech Republic, June/July 2010.
- Hardewig, I; Pörtner, HO; Grieshaber, MK. Interactions of anaerobic propionate formation and acid-base status in Arenicola marina: an analysis of propionyl-CoA carboxylase. Physiol. Zool 1994, 67, 892–909. [Google Scholar]
- Pörtner, HO. Contributions of anaerobic metabolism to pH regulation in animal tissues: theory. J. Exp. Biol 1987, 131, 69–87. [Google Scholar]
- Pörtner, HO; Bock, C; Reipschläger, A. Modulation of the cost of pHi regulation during metabolic depression: a 31P-NMR study in invertebrate (Sipunculus nudus) isolated muscle. J. Exp. Biol 2000, 203, 2417–2428. [Google Scholar]
- Seidelin, M; Brauner, CJ; Jensen, FB; Madsen, SS. Vacuolar-type H+-ATPase and Na+, K+-ATPase expression in gills of Atlantic salmon (Salmo salar) during isolated and combined exposure to hyperoxia and hypercapnia in fresh water. Zoolog. Sci 2001, 18(9), 1199–1205. [Google Scholar]
- Deigweiher, K; Koschnick, N; Pörtner, HO; Lucassen, M. Acclimation of ion regulatory capacities in gills of marine fish under environmental hypercapnia. Am. J. Physiol 2008, 295, R1660–R1670. [Google Scholar]
- Pörtner, HO; Bock, C. Heldmaier, G, Klingenspor, M, Eds.; A contribution of acid-base regulation to metabolic depression in marine ectotherms. In Life in the Cold; Springer Verlag: Berlin, Germany, 2000; pp. 443–458. [Google Scholar]
- Marshall, WS. Na+, Cl−, Ca2+ and Zn2+ transport by fish gills: retrospective review and prospective synthesis. J. Exp. Zool 2002, 293(3), 264–283. [Google Scholar]
- Perry, SF; Gilmour, KM. Acid-base balance and CO2 excretion in fish: unanswered questions and emerging models. Respir. Physiol. Neurobiol 2006, 154(1–2), 199–215. [Google Scholar]
- Parker, LM; Ross, PM; O’Connor, WA. The effect of ocean acidification and temperature on the fertilization and embryonic development of the Sydney rock oyster Saccostrea glomerata (Gould 1850). Global Change Biol 2009, 15, 2123–2136. [Google Scholar]
- Sokolova, IM; Lannig, G. Interactive effects of metal pollution and temperature on metabolism in aquatic ectotherms: Implications of global climate change. Clim. Res 2008, 37, 181–201. [Google Scholar]



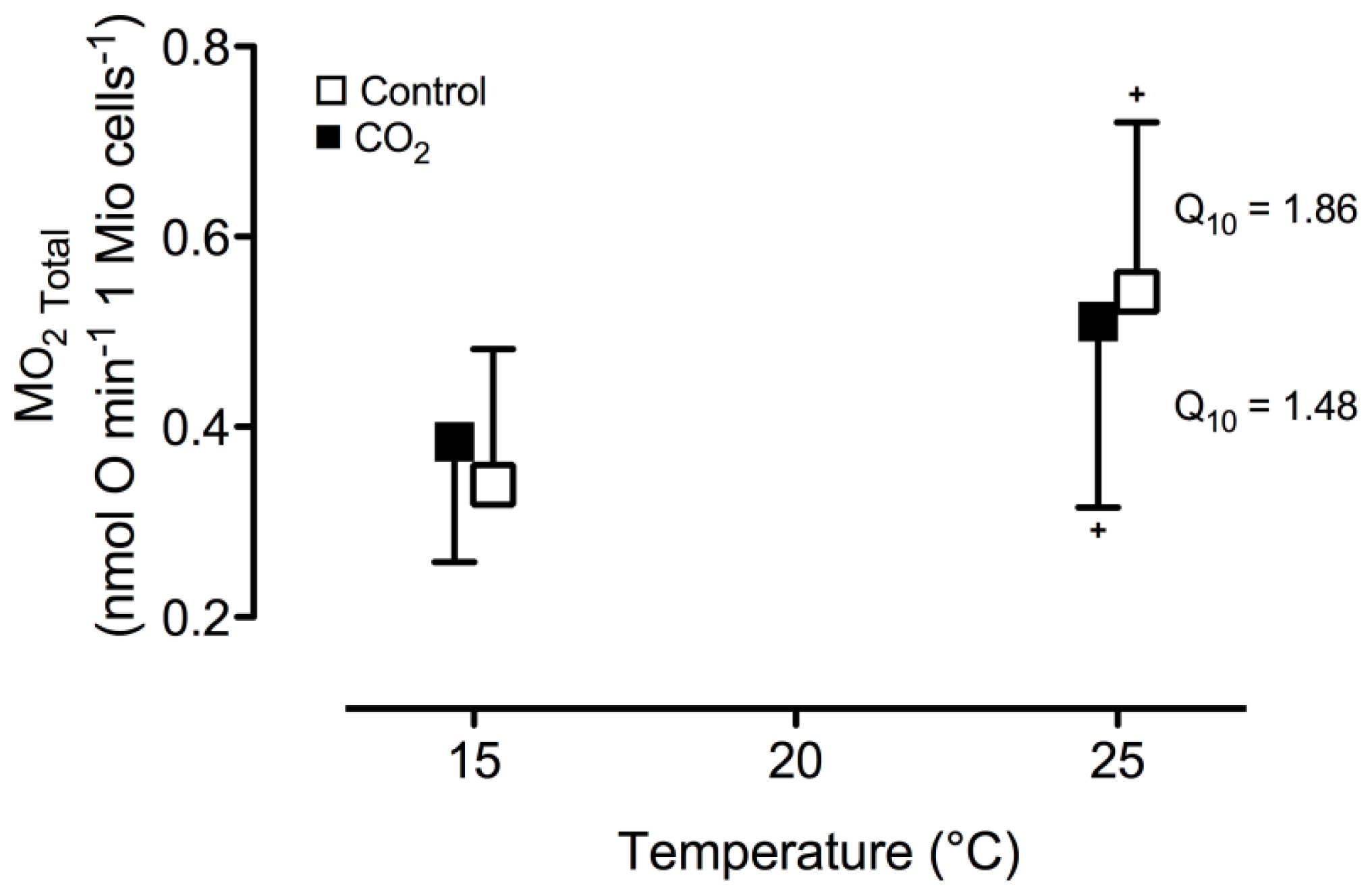
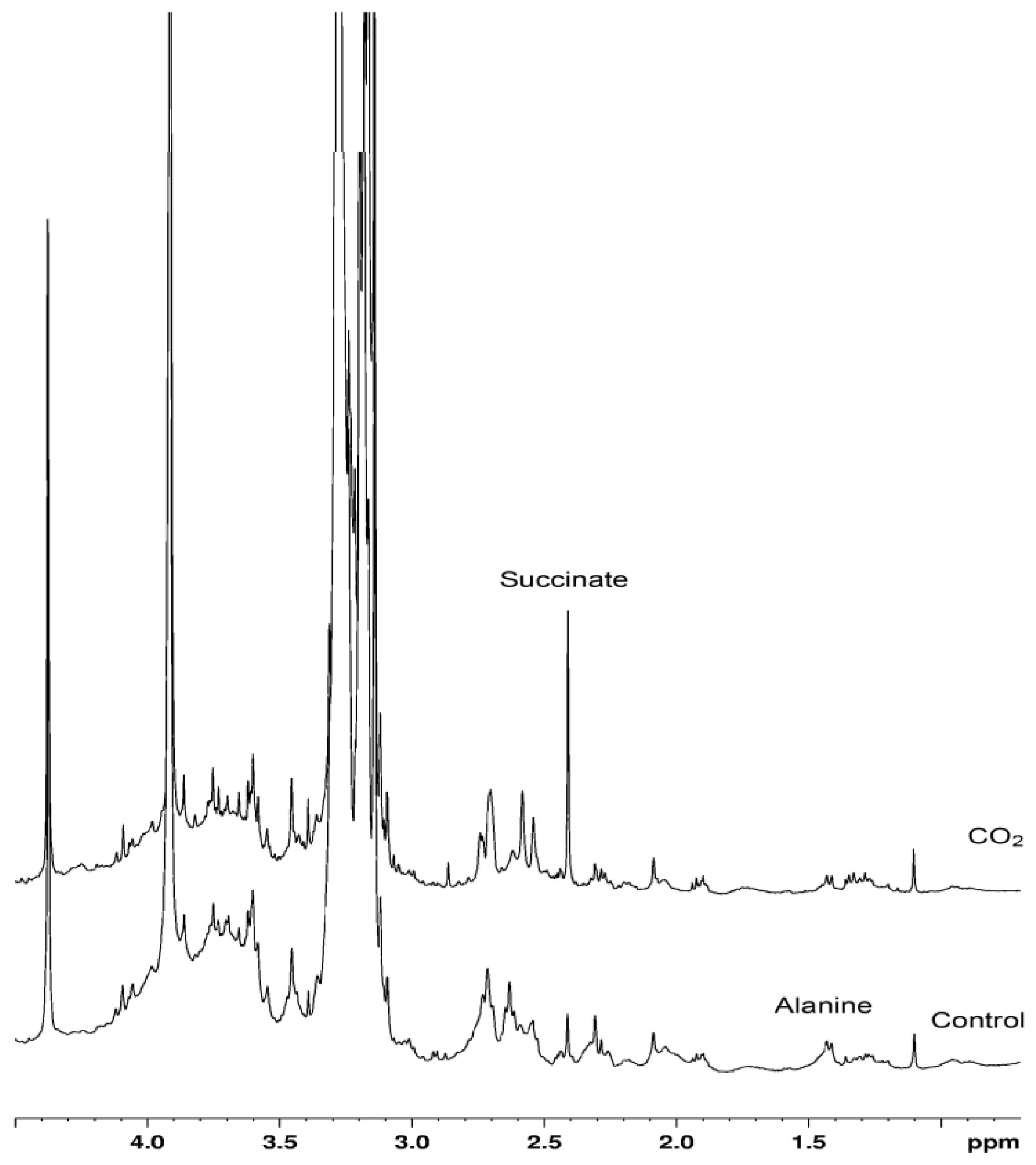
| Parameter/Group | Control | CO2-incubation |
|---|---|---|
| pHe | 7.60 ± 0.10 | 7.09 ± 0.18* |
| PeCO2 (kPa) | 0.15 ± 0.04 | 0.54 ± 0.19* |
| PeO2 (kPa) | 11.44 ± 3.67 | 9.43 ± 2.29° |
| CeCO2 (mM) | 1.67 ± 0.11 | 2.15 ± 0.30* |
| HCO−3 (mM) | 1.28 ± 0.11 | 1.78 ± 0.29* |
| Ca2+ (mM) | 7.2 ± 0.6 | 6.2 ± 0.1° |
| Na+ (mM) | 445.4 ± 16.0 | 422.3 ± 7.4* |
| K+ (mM) | 11.9 ± 0.8 | 13.0 ± 0.9* |
| Parameter/Group | Control | CO2-incubation |
|---|---|---|
| Salinity (psu) | 32.1 ± 0.5 | 31.3 ± 0.4 |
| pH NBS | 8.07 ± 0.04 | 7.68 ± 0.07 |
| PCO2 (kPa) | 0.059 ± 0.008 | 0.15 ± 0.026 |
| [HCO−3] (mmol kg−1) | 2.09 ± 0.09 | 2.24 ± 0.12 |
| Ω Ar | 2.31 ± 0.30 | 0.87 ± 0.15 |
| Ω Ca | 3.59 ± 0.44 | 1.36 ± 0.24 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lannig, G.; Eilers, S.; Pörtner, H.O.; Sokolova, I.M.; Bock, C. Impact of Ocean Acidification on Energy Metabolism of Oyster, Crassostrea gigas—Changes in Metabolic Pathways and Thermal Response. Mar. Drugs 2010, 8, 2318-2339. https://doi.org/10.3390/md8082318
Lannig G, Eilers S, Pörtner HO, Sokolova IM, Bock C. Impact of Ocean Acidification on Energy Metabolism of Oyster, Crassostrea gigas—Changes in Metabolic Pathways and Thermal Response. Marine Drugs. 2010; 8(8):2318-2339. https://doi.org/10.3390/md8082318
Chicago/Turabian StyleLannig, Gisela, Silke Eilers, Hans O. Pörtner, Inna M. Sokolova, and Christian Bock. 2010. "Impact of Ocean Acidification on Energy Metabolism of Oyster, Crassostrea gigas—Changes in Metabolic Pathways and Thermal Response" Marine Drugs 8, no. 8: 2318-2339. https://doi.org/10.3390/md8082318
APA StyleLannig, G., Eilers, S., Pörtner, H. O., Sokolova, I. M., & Bock, C. (2010). Impact of Ocean Acidification on Energy Metabolism of Oyster, Crassostrea gigas—Changes in Metabolic Pathways and Thermal Response. Marine Drugs, 8(8), 2318-2339. https://doi.org/10.3390/md8082318





