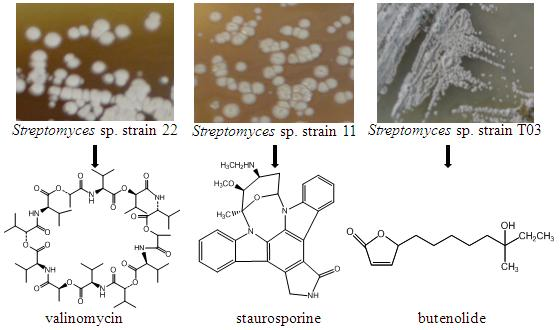
Anti-Parasitic Compounds from Streptomyces sp. Strains Isolated from Mediterranean Sponges
Abstract
:1. Introduction
2. Results and Discussion
Acknowledgments
- Samples Availability: Available from the authors.
References and Notes
- Jensen, PR; Mincer, TJ; Williams, PG; Fenical, W. Marine actinomycete diversity and natural product discovery. Anton Leeuwenhoek 2005, 87, 43–48. [Google Scholar]
- Bull, AT; Stach, JE. Marine actinobacteria: New opportunities for natural product search and discovery. Trends Microbiol 2007, 15, 491–499. [Google Scholar]
- Fenical, W; Jensen, PR. Developing a new resource for drug discovery: Marine actinomycete bacteria. Nat Chem Biol 2006, 2, 666–673. [Google Scholar]
- Mincer, TJ; Jensen, PR; Kauffman, CA; Fenical, W. Widespread and persistent populations of a major new marine actinomycete taxon in ocean sediments. Appl Environ Microbiol 2002, 68, 5005–5011. [Google Scholar]
- Maldonado, LA; Stach, JE; Pathom-aree, W; Ward, AC; Bull, AT; Goodfellow, M. Diversity of culturable actinobacteria in geographically widespread marine sediments. Anton Leeuwenhoek 2005, 87, 11–18. [Google Scholar]
- Kim, TK; Garson, MJ; Fuerst, JA. Marine actinomycetes related to the “Salinospora” group from the Great Barrier Reef sponge Pseudoceratina clavata. Environ Microbiol 2005, 7, 509–518. [Google Scholar]
- Webster, NS; Wilson, KJ; Blackall, LL; Hill, RT. Phylogenetic diversity of bacteria associated with the marine sponge Rhopaloeides odorabile. Appl Environ Microbiol 2001, 67, 434–444. [Google Scholar]
- Montalvo, NF; Mohamed, NM; Enticknap, JJ; Hill, RT. Novel actinobacteria from marine sponges. Anton Leeuwenhoek 2005, 87, 29–36. [Google Scholar]
- Zhang, H; Lee, YK; Zhang, W; Lee, HK. Culturable actinobacteria from the marine sponge Hymeniacidon perleve: Isolation and phylogenetic diversity by 16S rRNA gene-RFLP analysis. Anton Leeuwenhoek 2006, 90, 159–169. [Google Scholar]
- Jiang, S; Sun, W; Chen, M; Dai, S; Zhang, L; Liu, Y; Lee, KJ; Li, X. Diversity of culturable actinobacteria isolated from marine sponge Haliclona sp. Anton Leeuwenhoek 2007, 92, 405–416. [Google Scholar]
- Peraud, O; Biggs, JS; Hughen, RW; Light, AR; Concepcion, GP; Olivera, BM; Schmidt, EW. Microhabitats within venomous cone snails contain diverse actinobacteria. Appl Environ Microbiol 2009, 75, 6820–6826. [Google Scholar]
- Lam, KS. Discovery of novel metabolites from marine actinomycetes. Curr Opin Microbiol 2006, 9, 245–251. [Google Scholar]
- Fenical, W. Marine pharmaceuticals: past, present and future. Oceanography 2006, 19, 110–119. [Google Scholar]
- Natera, S; Machuca, C; Padron-Nieves, M; Romero, A; Diaz, E; Ponte-Sucre, A. Leishmania spp.: Proficiency of drug-resistant parasites. Int J Antimicrob Agents 2007, 29, 637–642. [Google Scholar]
- Pimentel-Elardo, SM; Scheuermayer, M; Kozytska, S; Hentschel, U. Streptomyces axinellae sp. nov., isolated from the Mediterranean sponge Axinella polypoides (Porifera). Int J Syst Evol Microbiol 2009, 59, 1433–1437. [Google Scholar]
- Pimentel-Elardo, SM; Tiro, LP; Grozdanov, L; Hentschel, U. Saccharopolyspora cebuensis sp. nov., a novel actinomycete isolated from a Philippine sponge (Porifera). Int J Syst Evol Microbiol 2008, 58, 628–632. [Google Scholar]
- Pimentel-Elardo, S; Gulder, TAM; Hentschel, U; Bringmann, G. Cebulactams A1 and A2, new macrolactams isolated from Saccharopolyspora cebuensis, the first obligate-marine strain of the genus Saccharopolyspora. Tetrahedron Lett 2008, 49, 6889–6892. [Google Scholar]
- Pimentel-Elardo, S; Wehrl, M; Friedrich, A; Jensen, PR; Hentschel, U. Isolation of planctomycetes from Aplysina sponges. Aquat Microb Ecol 2003, 33, 239–245. [Google Scholar]
- The isolation of valinomycin was carried out by semi-preparative HPLC (Phenomenex Luna SemiPrep RP18e 10 × 250 mm) using H2O (A) and CH3OH (B) as the solvents and the following gradient: flow 4.5 mL/min; 0–5 min 90% B, 11–15 min 100% B yielding 8.4 mg of the compound (Rt = 13.085 min).
- Brockmann, H; Schmidt-Kastner, G. Valinomycin I, XXVII. Mitteilung über Antibiotika aus Actinomyceten. Chem Ber 1955, 88, 57–61. [Google Scholar]
- Leishmania major promastigotes were seeded at a cell density of 1 × 107 cells/mL into 96-well plates in complete medium (RPMI with NaHCO3, 10% FCS, 2 mM glutamine, 10 mM Hepes pH 7.2, 100 U/mL penicillin, 50 μg/mL gentamicin, 50 mM 2-mercaptoethanol) without phenol red (200 mL), in the absence or presence of different concentrations of the compounds. These were then incubated for 24 h at 26 °C, 5% CO2 and 95% humidity. Following the addition of 20 mL of Alamar Blue, the plates were incubated again and the optical densities (ODs) measured 24 h and 48 h later with an enzyme-linked immunosorbent assay (ELISA) reader (Multiskan Ascent, Germany) using a test wavelength of 540 nm and a reference wavelength of 630 nm. Absorbance in the absence of compounds was set as 100% of growth. Amphotericin B was used as a reference compound and positive control. The effects of cell density, incubation time and the concentration of DMSO were examined in control experiments. The final concentration of DMSO in the medium never exceeded 1% vol/vol and had no effect on the proliferation of extracellular or intracellular parasites. For each experiment, each drug concentration was assayed in duplicate wells [31]
- Trypomastigote forms of Trypanosoma brucei brucei laboratory strain TC 221 were cultured in complete Baltz medium [80 mL Baltz medium basic solution, 0.8 mL 2 mercaptoethanol stock solution (20 mM), 0.8 mL penicillin/streptomycin (10,000 U/mL), 16 mL FCS (inactivated for 30 min at 56 °C). Baltz medium basic solution is composed of the following: 500 mL MEM with Earle’s salts and L-glutamine, 3 g Hepes, 0.5 g monohydrate glucose, 0.110 g sodium pyruvate, 0.007 g hypoxanthine, 0.002 g thymidine, 0.0107 g adenosine, 0.0141 g bathocuproine disulfonic acid disodium salt, 0.146 g glutamine, 5 mL sterile non-essential amino acid concentrate (100×, pH 7.5). A defined number of parasites (104 trypanosomes per mL) were exposed in test chambers of 96-well plates to various concentrations of the test substances (previously dissolved in DMSO) to make a final volume of 200 μL in duplicates. Positive (trypanosomes in culture medium) and negative controls (test substance without trypanosomes) were run simultaneously with each plate. The plates were then incubated at 37 °C in an atmosphere of 5% CO2 for a total time period of 72 h. After 24 h, 20 μL of Alamar Blue was added. The activity of the test substances was measured by light absorption using MR 700 Microplate Reader at a wavelength of 550 nm with a reference wavelength of 630 nm. The first reading was done at 48 h and subsequently at 72 h. The effect of the test substances was quantified in IC50 values by linear interpolation of three independent measurements [32].
- Heisey, R; Huang, J; Mishka, SK; Keller, JE; Miller, JR; Putnam, AR; D’Silva, TD. Production of valinomycin, an insecticidal antibiotic, by Streptomyces griseus var. flexipartum var. nov. J Agric Food Chem 1988, 36, 1283–1286. [Google Scholar]
- The isolation of staurosporine was carried out by semi-preparative HPLC (Phenomenex Luna SemiPrep RP18e 10 × 250 mm) using H2O + 0.1% TFA (A) and CH3OH (B) as the solvents and the following gradient: flow 4.5 mL/min; 0–5 min 70% B, 10 min 80% B, 20–25 min 100% B to yield 1.4 mg of the compound (Rt = 4.162 min).
- Fdhila, F; Vazquez, V; Sanchez, JL; Riguera, R. DD-diketopiperazines: Antibiotics active against Vibrio anguillarum isolated from marine bacteria associated with cultures of Pecten maximus. J Nat Prod 2003, 66, 1299–1301. [Google Scholar]
- Schupp, P; Proksch, P; Wray, V. Further new staurosporine derivatives from the ascidian Eudistoma toealensis and its predatory flatworm Pseudoceros sp. J Nat Prod 2002, 65, 295–298. [Google Scholar]
- Utz, I; Spitaler, M; Rybczynska, M; Ludescher, C; Hilbe, W; Regenass, U; Grunicke, H; Hofmann, J. Reversal of multidrug resistance by the staurosporine derivatives CGP 41251 and CGP 42700. Int J Cancer 1998, 77, 64–69. [Google Scholar]
- Cho, KW; Lee, HS; Rho, JR; Kim, TS; Mo, SJ; Shin, J. New lactone-containing metabolites from a marine-derived bacterium of the genus Streptomyces. J Nat Prod 2001, 64, 664–667. [Google Scholar]
- Mukku, VJ; Speitling, M; Laatsch, H; Helmke, E. New butenolides from two marine streptomycetes. J Nat Prod 2000, 63, 1570–1572. [Google Scholar]
- J774.1 macrophages were cultured in complete medium (RPMI with NaHCO3, 10% FCS, 2 mM glutamine, 10 mM Hepes pH 7.2, 100 U/mL penicillin, 50 μg/mL gentamicin, 50 μM 2-mercaptoethanol) without phenol red in the absence or presence of increasing concentrations of the compounds at a cell density of 1 × 105 cells/mL (200 μL) for 24 h at 37 °C, 5% CO2 and 95% humidity. Following the addition of 20 μL of Alamar Blue, the plates were incubated and the ODs measured at 24 h, 48 h and 72 h. The same Alamar blue assay previously described for Leishmania was followed [21]. Kidney epithelial 293T cells were also tested in the same manner as the macrophages but using complete DMEM medium (4.5 g/L solution of DMEM high glucose solution with sodium pyruvate but without L-glutamine, FBS superior at final concentration of 20%, 200 mM L-glutamine 100x) and cell density (2 × 104 cells/mL).
- Ponte-Sucre, A; Vicik, R; Schultheis, M; Schirmeister, T; Moll, H. Aziridine-2,3-dicarboxylates, peptidomimetic cysteine protease inhibitors with antileishmanial activity. Antimicrob Agents Chemother 2006, 50, 2439–2447. [Google Scholar]
- Huber, W; Koella, JC. A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop 1993, 55, 257–261. [Google Scholar]

| Compound | Leishmania major | Trypanosoma brucei brucei (48 h) | Trypanosoma brucei brucei (72 h) |
|---|---|---|---|
| valinomycin | <0.11 | 0.0032 | 0.0036 |
| staurosporine | 5.30 | 0.022 | 0.035 |
| butenolide | >100 | 31.77 | 33.08 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pimentel-Elardo, S.M.; Kozytska, S.; Bugni, T.S.; Ireland, C.M.; Moll, H.; Hentschel, U. Anti-Parasitic Compounds from Streptomyces sp. Strains Isolated from Mediterranean Sponges. Mar. Drugs 2010, 8, 373-380. https://doi.org/10.3390/md8020373
Pimentel-Elardo SM, Kozytska S, Bugni TS, Ireland CM, Moll H, Hentschel U. Anti-Parasitic Compounds from Streptomyces sp. Strains Isolated from Mediterranean Sponges. Marine Drugs. 2010; 8(2):373-380. https://doi.org/10.3390/md8020373
Chicago/Turabian StylePimentel-Elardo, Sheila Marie, Svitlana Kozytska, Tim S. Bugni, Chris M. Ireland, Heidrun Moll, and Ute Hentschel. 2010. "Anti-Parasitic Compounds from Streptomyces sp. Strains Isolated from Mediterranean Sponges" Marine Drugs 8, no. 2: 373-380. https://doi.org/10.3390/md8020373
APA StylePimentel-Elardo, S. M., Kozytska, S., Bugni, T. S., Ireland, C. M., Moll, H., & Hentschel, U. (2010). Anti-Parasitic Compounds from Streptomyces sp. Strains Isolated from Mediterranean Sponges. Marine Drugs, 8(2), 373-380. https://doi.org/10.3390/md8020373