Abstract
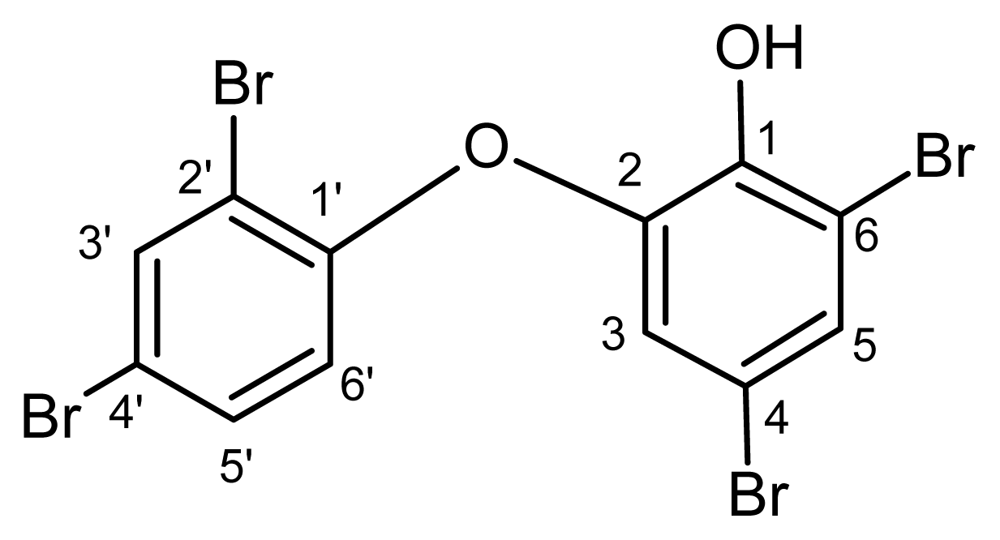
2-(2′,4′-Dibromophenoxy)-4,6-dibromophenol isolated from the marine sponge Dysidea granulosa (Bergquist) collected off the coast of Lakshadweep islands, Indian Ocean, exhibited potent and broad spectrum in-vitro antibacterial activity, especially against methicillin resistant Staphylococcus aureus (MRSA), methicillin sensitive Staphylococcus aureus (MSSA), vancomycin resistant Enterococci (VRE), vancomycin sensitive Enterococci (VSE) and Bacillus spp. Minimal inhibitory concentration (MIC) was evaluated against 57 clinical and standard strains of Gram positive and Gram negative bacteria. The observed MIC range was 0.117–2.5 μg/mL against all the Gram positive bacteria and 0.5–2 μg/mL against Gram negative bacteria. The in-vitro antibacterial activity observed was better than that of the standard antibiotic linezolid, a marketed anti-MRSA drug. The results establish 2-(2′,4′-dibromophenoxy)-4,6-dibromophenol, as a potential lead molecule for anti-MRSA and anti-VRE drug development.
1. Introduction
The striking rise in the prevalence of bacterial antibiotic resistance currently poses a serious threat to public health worldwide. Of particular concern are infections caused by methicillin-resistant Staphylococcus aureus (MRSA), penicillin-resistant Streptococcus pneumoniae, vancomycin-resistant Enterococcus [1] and Mycobacterium tuberculosis [2]. Many of these organisms have developed resistance to several classes of established antibiotics [1,2]. The most significant problem in clinical practice is the increase in incidence of MRSA infections. At present, the only effective treatment for multiple resistant MRSA infections is vancomycin. However, there are number of reports of emerging vancomycin resistance in some MRSA isolates [3]. Another group of clinically relevant multiple drug resistant bacteria that has emerged recently are Enterococci, some of which also exhibit vancomycin resistance. The appearance of vancomycin resistant Enterococci (VRE) infections has caused a dilemma upon physicians – linezolid and streptogramin combinations are the new drugs of choice for treating MRSA infections, but linezolid resistance have been reported in VRE and MRSA isolates [4]. Resistance to these oxazolidinone [5] streptogramin combinations [6,7] and various glycopeptides [5] requires expanded development of agents with alternative targets or modes of action. Staphylococcus aureus is the most clinically important of the Gram positive pathogens because of its exceptional virulence, stress tolerance, and capacity to accumulate antimicrobial resistances. MRSA is known as a major nosocomial pathogen which has also developed resistance to many other antibiotics. Moreover, MRSA and other S. aureus strains have been reported to acquire resistance to the last-resort antibiotic, vancomycin [8,9]. These facts suggest that S. aureus (MRSA) could fully acquire resistance to vancomycin in the near future.
The emergence and spread of resistant nosocomial and community-acquired pathogens is becoming a great menace to global public health. Therefore, a need is perceived to find new classes of antimicrobials. The search for new pharmacologically active agents obtained by screening natural sources has led to the discovery of many clinically useful drugs that play a major role in the treatment of human diseases. Approximately 60% of the antitumor and anti-infective agents that are commercially available or in late stages of clinical trials today are of natural product origin. Natural products are still major sources of innovative therapeutic agents for infectious diseases (both bacterial and fungal), cancer, lipid disorders and immunomodulation [10,11].
Given the diverse array of bioactive secondary metabolite chemical structures with a wide variety of biological activities isolated from marine sponges, natural product chemists have long been fascinated by these sessile marine invertebrates [11]. One of the most extensively studied species of sponge is the tropical marine sponge Dysidea sp. (family Dysideidae, Order Dendroceratida), a pinkish green sponge that occurs in a number of distinct chemotypes as a result of an association with symbiotic microorganisms. The chemistry reported from this sponge and related sponges in the genus, includes sesquiterpene spirolactol/lactones, tricyclic furans, some based on the furodysinin and furodysin skeletons and their oxidised derivatives, modified steroids, polychlorinated alkaloids, brominated diphenyl ethers and other metabolites. The polychlorinated alkaloids and brominated diphenyl ethers produced by the sponge have been shown to be associated with the filamentous cyanobacterium Oscillatoria spongeliae, and are therefore likely synthesized by the cyanobacterial symbionts [12–15].
Most halogenated phenols are found to be strongly antimicrobial. As a group, the halogenated bis(hydroxyphenyl) methanes and polybrominated diphenyl ethers exhibit a wide range of activities in bioassays, ranging from antibacterial activity (against S. aureus and T. mentagrophytes), to cytotoxicity (Ehrlich ascite tumor cells) [16,17]. Polybrominated biphenyl ethers from Dysidea herbacea are found to be active against eubacteria, as well as test strains of the unicellular marine cyanobacterium Synechococcus sp. [15]. Herein we have reported the isolation of 2-(2′,4′-dibromophenoxy)-4,6-dibromophenol from a new source D. granulosa, its potent in-vitro antibacterial activity, and as a possible lead molecule for drug development especially against strains of MRSA, methicillin sensitive S. aureus (MSSA), VRE and vancomycin sensitive Enterococci (VSE).
2. Results and Discussion
In the course of our programme of screening for new compounds from marine resources, 2-(2′,4′-dibromophenoxy)-4,6-dibromophenol was obtained from the sponge Dysidea granulosa as a colourless optically inactive crystalline solid, mp 179 °C. It showed a max. UV-Vis (MeOH) absorbance at 340 nm. IR (KBr) showed major peaks at 3,492.8 (phenolic O-H str), 3,084.0 (aromatic C-H str), 1,591.5 (aromatic C=C str), 1,463.9(C-H bend), 1,147.6, 1039.6 (C-O str), 918.1, 869.8, and 719.4 cm−1 (C-Br str). Spectral data including 1H-NMR (300 MHz, CDCl3), 13C-NMR, COSY, HMQC and HMBC of the compound is given in Table 1.

Table 1.
1H-, 13C-NMR, COSY and HMBC of 2-(2′,4′-dibromophenoxy)-4,6-dibromophenol.
A quasimolecular [M-H] − ion peak was observed at m/z 500.7023 (calculated: 500.6982); the corresponding [2M-2H]− peak was observed at m/z 1001.7274 (calculated: 1001.3964). The mass spectral fragmentation pattern with the respective spectral peaks at [M-H]− at 496.7042, 498.7054, 500.7023, 502.7010 and 504.6974 (observed) and 496.7023, 498.7003, 500.6982, 502.6962 and 504.6942 (calculated) in the ratio 17.30:68.0:100.0:65.70:16.50 was indicative of the presence of four bromine atoms.
These spectral data were in good agreement with the spectral data reported in literature [15,18]. The two dimensional NMR data is reported here for the first time. 2-(2′,4′-Dibromophenoxy)-4,6-dibromophenol was observed to be a potent inhibitor of the growth of clinically relevant Gram positive bacteria, especially MRSA and VRE. Preliminary testing by the agar diffusion method revealed that the compound is strongly antibacterial, but has no inhibitory effect against fungal strains. To evaluate its in-vitro antibacterial potency and characterize its activity spectrum, the compound was tested against 57 bacterial test strains. The efficacy is measured in terms of minimum inhibitory concentration (MIC) and expressed in μg/mL.
The spectrum of bacterial test cultures includes 20 clinically derived and 10 standard strains of MRSA, two standard strains of MSSA; 11 clinical strains of VRE, four standard strains of VRE, five standard strains of VSE; one each standard strains of E. coli, K. pneumoniae, S. typhi, S. flexneri and P. aeruginosa, including three methicillin resistant strains of S. aureus 3066, E710, ATCC 33591 and two vancomycin resistant strains of E. faecium 02 D3 IP1, R-2 and one vancomycin resistant strain of E. faecalis ATCC 51299, which were subjected to antibacterial activity tests.
The compound showed potent, broad-spectrum in-vitro Gram-positive activity, as evident from Table 2. The compound exhibited no cross-resistance to other key antibiotics, which was evident from its potent activity against key antibiotic-resistant bacteria including MRSA, erythromycin-resistant S. aureus, and VRE (Table 3). Escherichia coli and Pseudomonas sp. were the least susceptible, with MIC values above 15 μg/mL (Table 2). However it was active against other Gram negative bacteria such as Klebsiella pneumonia, Shigella flexneri and Salmonella typhi (Table 2). The bromoether was tested by the agar diffusion assay against C. albicans, A. fumigatus, C. tropicalis, C. glabrata and was found to be inactive against fungi even up to 100 μg/mL. Thus, the compound specifically shows antibacterial activity. Our work has first time revealed the wide spectrum of activity of this compound. Its activity with variety of resistant strains confirmed that 2-(2′,4′-dibromophenoxy)-4,6-dibromophenol did not show cross resistance with widely marketed antibiotics such as erythromycin, β-lactams, vancomycin, teichoplanin, gentamicin and streptomycin. Thus it reflects that the type of bacterial strains used for antibacterial efficacy testing has no resistance to this compound. Therefore it could be a potential molecule to develop further to address the resistance issue.

Table 2.
Antimicrobial activity of 2-(2′,4′-dibromophenoxy)-4,6-dibromophenol.

Table 3.
Antimicrobial activity of 2-(2′,4′-dibromophenoxy)-4,6-dibromophenol and linezolid towards resistant strains.
3. Conclusions
This is the first report of 2-(2′,4′-dibromophenoxy)-4, 6-dibromophenol from the sponge Dysidea granulosa and the first attempt to exhaustively test the in-vitro activity of this molecule. Results obtained indicate that the compound shows good lead properties for antibacterial drug development and represent a promising agent for the treatment of S. aureus as well as enterococcal infections caused by drug-resistant strains.
4. Experimental Section
4.1. General Experimental Procedures
Column chromatography was carried out on silica gel 60–120 mesh, Sisco, India. Gel filtration on Sephadex LH20, Pharmacia Biotech, Sweden. Fractions were monitored on TLC kieselgel 60 F254 aluminum backed sheets and visualized under UV (254 nm) and iodine vapors. UV-VIS spectrum was recorded in methanol using a Shimadzu UV-2401PC spectrophotometer. IR spectrum was recorded on a Shimadzu FTIR-8201 PC spectrophotometer. NMR spectra (1H, 13C, COSY, HMQC and HMBC) were recorded on a Bruker Avance 300MHz instrument. Chemical shifts, relative to TMS as internal standard, are given in δ values. J values are given in Hz. Mass spectra were obtained on a QSTAR XL MS/MS system.
4.2. Collection of Animal Material and Chromatography
The sponge was collected off the coast of Lakshadweep Islands by scuba diving at a depth of about 8–10 meters and identified by Dr. P.A. Thomas of the Vizhingam Research Center of the Central Marine Fisheries Research Institute, Kerala, India. A voucher specimen is deposited at the National Institute of Oceanography, Dona Paula, Goa, India. The frozen sample of D. granulosa was lyophilized to obtain 200 g of dried sample, which was extracted successively with ethyl acetate and methanol (200 mL × 3 times each). These extracts were concentrated under reduced pressure and temperature to obtain the crude extracts. The ethyl acetate extract exhibited antimicrobial activity against pathogenic strains and was chromatographed over Sephadex LH20 (1:1 methanol-chloroform). Fractions that were similar in composition as shown by thin-layer chromatography were combined. The partially purified sample was further purified over silica gel (60–120 mesh) (2% ethyl acetate-petroleum ether) to afford the single major polybrominated secondary metabolite 2-(2′,4′-dibromophenoxy)- 4, 6-dibromophenol.
4.3. Antimicrobial Activity
Minimum Inhibitory Concentration (MIC) values were determined in Mueller Hinton Broth by the broth macrodilution method according to NCCLS guidelines, as per document no. M7-A5 [19]. The medium with a twofold serial dilution of compound in Mueller Hinton broth was inoculated with 105 colony forming units/mL of test culture and incubated at 37 °C for 24 hrs. The MIC of the bromoether was determined against 57 test organisms including different clinical and standard strains of S. aureus, both MRSA and MSSA, clinical and inhouse strains of Enterococci, both VRE and VSE and some gram negative strains. American type culture collection (ATCC) strains were also screened. Linezolid (Glenmark Pharmaceuticals Ltd, India) was used as control agent.
Acknowledgements
The authors thank S.R. Shetye (Director, NIO) for constant encouragement. They also acknowledge the Ministry of Earth Sciences (MoES) for financial support and P.A. Thomas of Vizhingam Research Center of CMFRI, Kerala, India for identifying the sponge sample. One of the authors (D.S.M.P.) sincerely thanks CSIR for the award of a Senior Research Fellowship and another author (C.G.N.) is grateful to CSIR for an Emeritus Scientist award. The authors are very grateful to Somesh Sharma (Managing Director, Piramal Life Sciences Limited) and H. Sivaramkrishnan (President, Piramal Life Sciences Limited) for allowing the efficacy evaluation at Piramal Life Sciences Limited, Mumbai, India. This is NIO contribution number 4611.
- Samples Availability: Available from the authors.
References
- Menichetti, F. Current and emerging serious Gram-positive infections. Clin Microbiol Infect 2005, 11, 22–28. [Google Scholar]
- Loddenkemper, R; Sagebiel, D; Brendel, A. Strategies against multidrug-resistant tuberculosis. Eur Respir J 2002, 66S–77S. [Google Scholar]
- Aeschlimann, JR; Rybak, MJ. Pharmacodynamic analysis of the activity of quinupristin-dalfopristin against vancomycin-resistant Enterococcus faecium with differing MBCs via time-kill-curve and postantibiotic effect methods. Antimicrob Agents Chemother 1998, 42, 2188–2192. [Google Scholar]
- Tsiodras, S; Gold, HS; Sakoulas, G. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 2001, 358, 207–208. [Google Scholar]
- Mutnick, AH; Enne, V; Jones, RN. Linezolid resistance since 2001: SENTRY antimicrobial surveillance program. Ann Pharmacother 2003, 37, 769–774. [Google Scholar]
- Livermore, DM. Bacterial resistance: Origins, epidemiology and impact. Clin Infect Dis 2003, 36, S11–S23. [Google Scholar]
- Canu, A; Leclercq, R. Overcoming bacterial resistance by dual target inhibition: The case of streptogramins. Curr Drug Targets Infect Disord 2001, 1, 215–225. [Google Scholar]
- Shaw, SJ; Abbanat, D; Ashley, GW; Bush, K; Foleno, B; Macielag, M; Zhang, D; Myles, DC. 15-Amido erythromycins: Synthesis and in-vitro activity of a new class of macrolide antibiotics. J Antibiot 2005, 58, 167–177. [Google Scholar]
- Zheng, C-J; Park, S-H; Koshino, H; Kim, Y-H; Kim, W-G. Verticillin G, a new antibacterial compound from Bionectra byssicola. J Antibiot 2007, 60, 61–64. [Google Scholar]
- Clardy, J; Walsh, C. Lessons from natural molecules. Nature 2004, 432, 829–837. [Google Scholar]
- Cragg, GM; Newman, DJ; Snader, KM. Natural products in drug discovery and development. J Nat Prod 1997, 60, 52–60. [Google Scholar]
- Sarma, NS; Rambabu, M; Anjaneyulu, ASR; Rao, CBS; Saito, I. Structure and stereochemistry of herbacin, new furanosesquiterpene from the marine sponge, Dysidea herbacea. Indian J Chem 1986, 25B, 1001–1003. [Google Scholar]
- Sera, Y; Adachi, K; Nishida, F; Shizuri, Y. A new sesquiterpene as an antifouling substance from a Palauan marine sponge, Dysidea herbacea. J Nat Prod 1999, 62, 395–396. [Google Scholar]
- Cameron, GM; Stapleton, BL; Simonsen, SM; Brecknell, DJ; Garson, MJ. New sesquiterpene and brominated metabolites from the tropical marine sponge Dysidea sp. Tetrahedron 2000, 56, 5247–5252. [Google Scholar]
- Unson, MD; Holland, ND; Faulkner, DJ. A brominated secondary metabolite synthesized by the cyanobacterial symbiont of a marine sponge and accumulation of the crystalline metabolite in the sponge tissue. Mar Biol 1994, 119, 1–14. [Google Scholar]
- Popov, AM; Stekhova, SI; Utkina, NK; Rebachuk, NM. Antimicrobial and cytotoxic activity of sesquiterpenequinones and brominated diphenyl ethers isolated from marine sponges. Pharm Chem 1999, 33, 15–16. [Google Scholar]
- Oh, KB; Lee, JH; Lee, JW; Yoon, KM; Chung, SC; Jeon, HB; Shin, J; Lee, HS. Synthesis and antimicrobial activities of halogenated bis(hydroxyphenyl)methanes. Bioorg Med Chem Lett 2009, 19, 945–948. [Google Scholar]
- Faulkner, DJ. Marine natural products. Nat Prod Rep and earlier articles in this review series 1999, 16, 155–198. [Google Scholar]
- National Committee for Clinical Laboratory Standards, Methods for Antimicrobial Susceptibility Testing for Bacteria Which Grow Aerobically, 5th ed; Approved standard M7-A5; NCCLS: Wayne, PA, USA, 2000.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).