Differential Effects of Domoic Acid and E. coli Lipopolysaccharide on Tumor Necrosis Factor-α, Transforming Growth Factor-β1 and Matrix Metalloproteinase-9 Release by Rat Neonatal Microglia: Evaluation of the Direct Activation Hypothesis
Abstract
1. Introduction
2. Results and Discussion
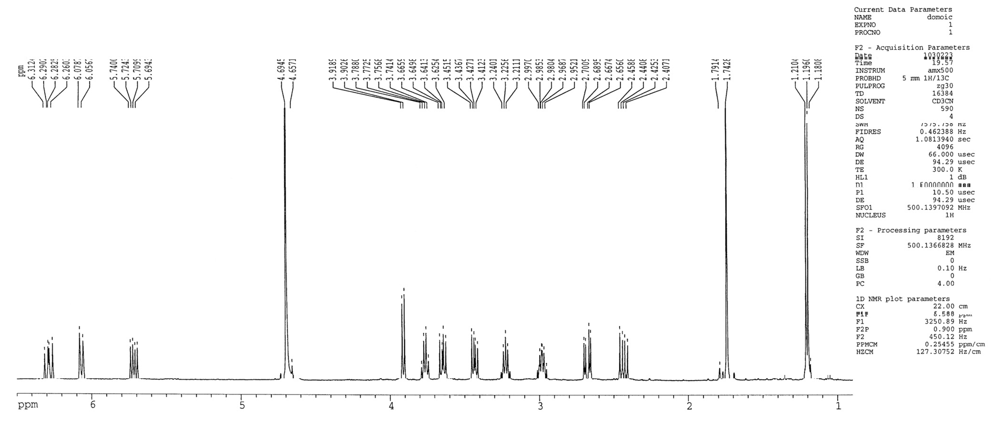
2.1 Determination of domoic acid by nuclear magnetic resonance
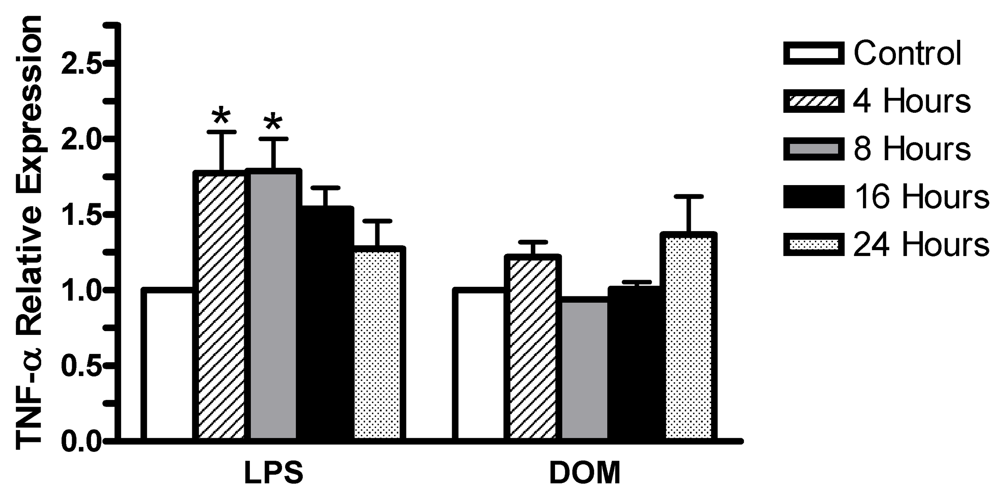
2.2 Effect of LPS and domoic acid on TNF-α mRNA expression by rat neonatal microglia
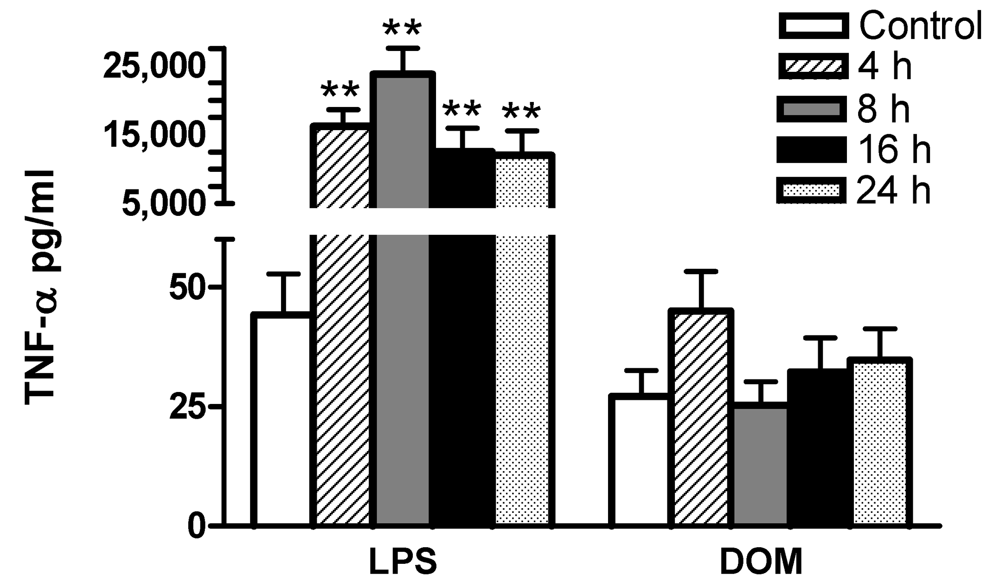
2.3 Effect of LPS and domoic acid on TNF-α release by rat neonatal microglia
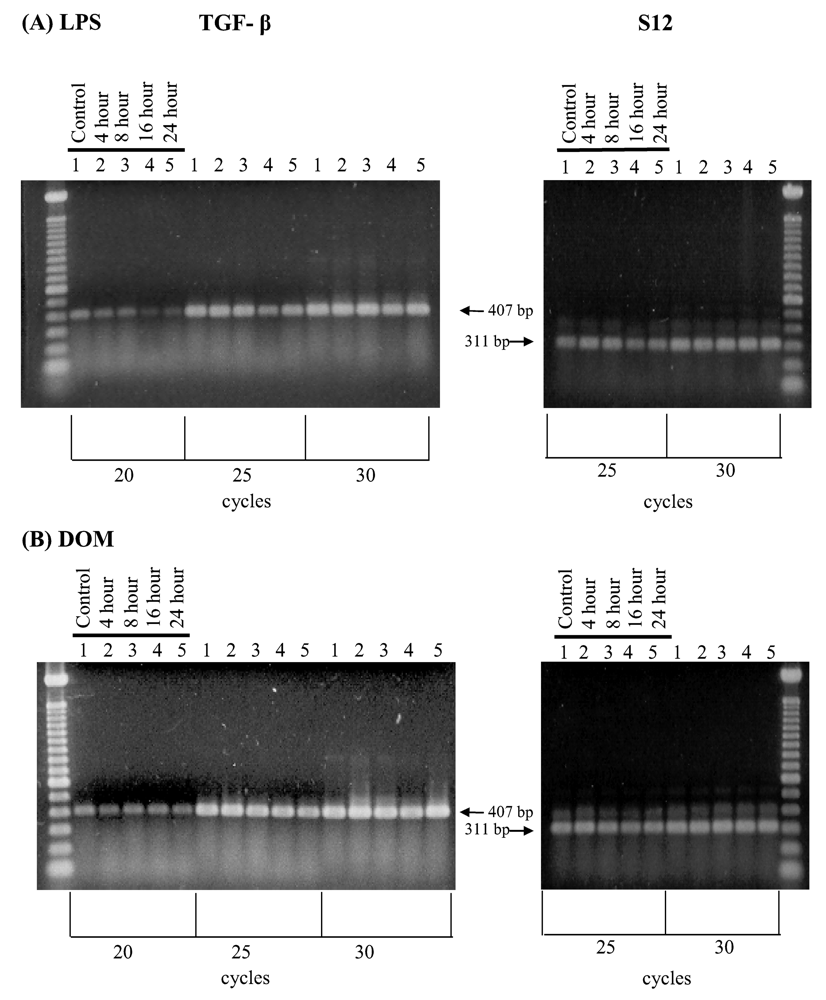
2.4 Effect of LPS and domoic acid on TGF-β1 mRNA expression by rat neonatal microglia
2.5 Effect of LPS and domoic acid on TGF-β1 release by rat neonatal microglia
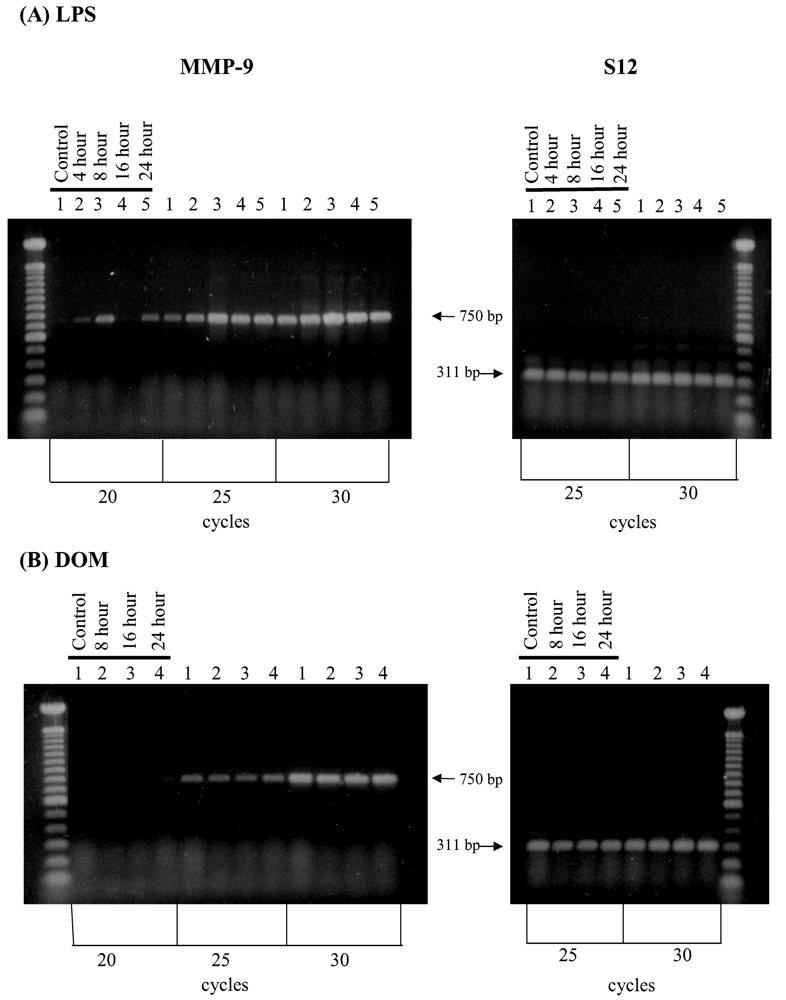
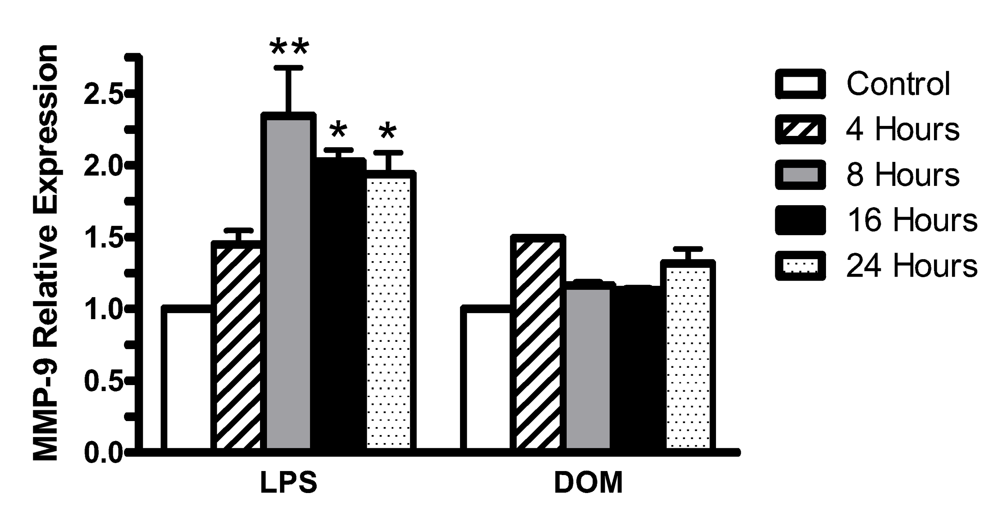
2.6. Effect of LPS and domoic acid on MMP-9 mRNA expression by rat neonatal microglia
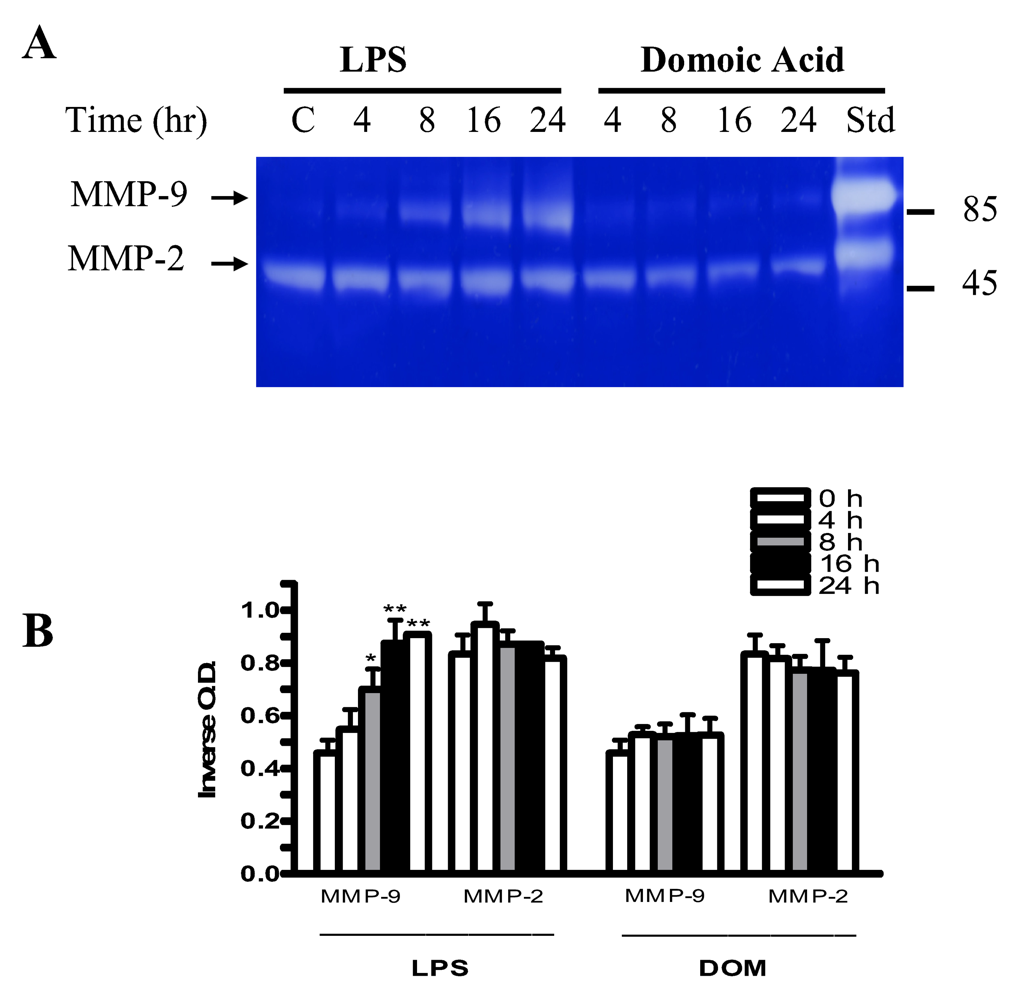
2.7 Effect of LPS and domoic acid on MMP-2 and MMP-9 release by rat neonatal microglia
3. Discussion
4. Experimental
4.1. Reagents
4.2. LPS containment
4.3. Nuclear magnetic resonance spectrometry of domoic acid
4.4. Isolation and culture of rat neonatal microglia
4.5. Experimental protocol to determine the effect of domoic acid and LPS on rat neonatal microglia TNF-α, TGF-β1 and MMP-9
4.6. Experimental protocol to determine the effect of domoic acid and LPS on rat neonatal microglia TNF- α, TGF-β1 and MMP-9 expression: RNA isolation and semiquantitative RT-PCR analysis
4.7. Assays for TNF-α and TGF-β1
4.8. SDS-PAGE gelatinase zymography for MMP-2 and MMP-9 analysis
4.9. Statistical analysis of the data
Acknowledgments
- Samples Availability: Available from the authors.
References
- Dheen, ST; Kaur, C; Ling, EA. Microglial activation and its implications in the brain diseases. Curr Med Chem 2007, 14(11), 1189–1197. [Google Scholar]
- Town, T; Nikolic, V; Tan, J. The microglial "activation" continuum: from innate to adaptive responses. J Neuroinflammation 2005, 2, 24. [Google Scholar]
- Gehrmann, J; Matsumoto, Y; Kreutzberg, GW. Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev 1995, 20(3), 269–287. [Google Scholar]
- Mayer, AMS. Therapeutic implications of microglia activation by lipopolysaccharide and reactive oxygen species generation in septic shock and central nervous system pathologies: a review. Medicina (B Aires) 1998, 58(4), 377–385. [Google Scholar]
- Mayer, AMS; Oh, S; Ramsey, KH; Jacobson, PB; Glaser, KB; Romanic, AM. Escherichia coli lipopolysaccharide potentiation and inhibition of rat neonatal microglia superoxide anion generation: correlation with prior lactic dehydrogenase, nitric oxide, tumor necrosis factor-alpha, thromboxane B2, and metalloprotease release. SHOCK 1999, 11(3), 180–186. [Google Scholar]
- Sawada, M; Kondo, N; Suzumura, A; Marunouchi, T. Production of tumor necrosis factor-alpha by microglia and astrocytes in culture. Brain Res 1989, 491(2), 394–397. [Google Scholar]
- Chao, CC; Hu, S; Sheng, WS; Tsang, M; Peterson, PK. Tumor necrosis factor-alpha mediates the release of bioactive transforming growth factor-beta in murine microglial cell cultures. Clin Immunol Immunopathol 1995, 77(3), 358–365. [Google Scholar]
- Constam, DB; Philipp, J; Malipiero, UV; ten Dijke, P; Schachner, M; Fontana, A. Differential expression of transforming growth factor-beta 1, -beta 2, and -beta 3 by glioblastoma cells, astrocytes, and microglia. J Immunol 1992, 148(5), 1404–1410. [Google Scholar]
- Gottschall, PE; Yu, X; Bing, B. Increased production of gelatinase B (matrix metalloproteinase-9) and interleukin-6 by activated rat microglia in culture. J Neurosci Res 1995, 42(3), 335–342. [Google Scholar]
- Selmaj, KW; Raine, CS. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol 1988, 23(4), 339–346. [Google Scholar]
- Banati, RB; Gehrmann, J; Schubert, P; Kreutzberg, GW. Cytotoxicity of microglia. GLIA 1993, 7(1), 111–118. [Google Scholar]
- Kreutzberg, GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci 1996, 19(8), 312–318. [Google Scholar]
- Aloisi, F. Immune function of microglia. GLIA 2001, 36(2), 165–179. [Google Scholar]
- Teitelbaum, JS; Zatorre, RJ; Carpenter, S; Gendron, D; Evans, AC; Gjedde, A; Cashman, NR. Neurologic sequelae of domoic acid intoxication due to the ingestion of contaminated mussels [see comments]. N Engl J Med 1990, 322(25), 1781–1787. [Google Scholar]
- Iverson, F; Truelove, J. Toxicology and seafood toxins: domoic acid. Nat Toxins 1994, 2(5), 334–339. [Google Scholar]
- Xi, D; Peng, YG; Ramsdell, JS. Domoic acid is a potent neurotoxin to neonatal rats. Nat Toxins 1997, 5(2), 74–79. [Google Scholar]
- Doucette, TA; Strain, SM; Allen, GV; Ryan, CL; Tasker, RA. Comparative behavioural toxicity of domoic acid and kainic acid in neonatal rats. Neurotoxicol Teratol 2000, 22(6), 863–869. [Google Scholar]
- Wang, GJ; Schmued, LC; Andrews, AM; Scallet, AC; Slikker, W, Jr; Binienda, Z. Systemic administration of domoic acid-induced spinal cord lesions in neonatal rats. J Spinal Cord Med 2000, 23(1), 31–39. [Google Scholar]
- Peng, YG; Ramsdell, JS. Brain Fos induction is a sensitive biomarker for the lowest observed neuroexcitatory effects of domoic acid. Fundam Appl Toxicol 1996, 31(2), 162–168. [Google Scholar]
- Scallet, AC; Binienda, Z; Caputo, FA; Hall, S; Paule, MG; Rountree, RL; Schmued, L; Sobotka, T; Slikker, W. , Jr. Domoic acid-treated cynomolgus monkeys (M. fascicularis): effects of dose on hippocampal neuronal and terminal degeneration. Brain Res 1993, 627(2), 307–313. [Google Scholar]
- Ross, IA; Johnson, W; Sapienza, PP; Kim, CS. Effects of the seafood toxin domoic acid on glutamate uptake by rat astrocytes. Food Chem Toxicol 2000, 38(11), 1005–1011. [Google Scholar]
- Mayer, AMS. The marine toxin domoic acid may affect the developing brain by activation of neonatal brain microglia and subsequent neurotoxic mediator generation. Med Hypotheses 2000, 54(5), 837–841. [Google Scholar]
- Grewal, RP; Yoshida, T; Finch, CE; Morgan, TE. Scavenger receptor mRNAs in rat brain microglia are induced by kainic acid lesioning and by cytokines. Neuroreport 1997, 8(5), 1077–1081. [Google Scholar]
- Rogove, AD; Tsirka, SE. Neurotoxic responses by microglia elicited by excitotoxic injury in the mouse hippocampus. Curr Biol 1997, 8, 19–25. [Google Scholar]
- Gottlieb, M; Matute, C. Expression of ionotropic glutamate receptor subunits in glial cells of the hippocampal CA1 area following transient forebrain ischemia. J Cereb Blood Flow Metab 1997, 17(3), 290–300. [Google Scholar]
- Hampson, DR; Huang, XP; Wells, JW; Walter, JA; Wright, JL. Interaction of domoic acid and several derivatives with kainic acid and AMPA binding sites in rat brain. Eur J Pharmacol 1992, 218(1), 1–8. [Google Scholar]
- Mayer, AMS; Hall, M; Fay, MJ; Lamar, P; Pearson, C; Prozialeck, WC; Lehmann, VK; Jacobson, PB; Romanic, AM; Uz, T; Manev, H. Effect of a short-term in vitro exposure to the marine toxin domoic acid on viability, tumor necrosis factor-alpha, matrix metalloproteinase-9 and superoxide anion release by rat neonatal microglia. BMC Pharmacol 2001, 1(1), 7. [Google Scholar]
- al-Sarraf, H; Preston, JE; Segal, MB. Changes in the kinetics of the acidic amino acid brain and CSF uptake during development in the rat. Brain Res Dev Brain Res 1997, 102(1), 127–134. [Google Scholar]
- Hollmann, M; Heinemann, S. Cloned glutamate receptors. Annu Rev Neurosci 1994, 17, 31–108. [Google Scholar]
- Lerma, J. Kainate receptors: an interplay between excitatory and inhibitory synapses. FEBS Lett 1998, 430(1–2), 100–104. [Google Scholar]
- Johannessen, JN. Stability of domoic acid in saline dosing solutions. J AOAC Int 2000, 83(2), 411–412. [Google Scholar]
- Sanders, P; De, KJ. Janus faces of microglia in multiple sclerosis. Brain Res Rev 2007, 54(2), 274–285. [Google Scholar]
- Campbell, IL. Cytokine-mediated inflammation and signaling in the intact central nervous system. Prog Brain Res 2001, 132, 481–498. [Google Scholar]
- Bhat, NR; Zhang, P; Lee, JC; Hogan, EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen- activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci 1998, 18(5), 1633–1641. [Google Scholar]
- Wen, LL; Chiu, CT; Huang, YN; Chang, CF; Wang, JY. Rapid glia expression and release of proinflammatory cytokines in experimental Klebsiella pneumoniae meningoencephalitis. Exp Neurol 2007, 205(1), 270–278. [Google Scholar]
- Sanna, PP; Weiss, F; Samson, ME; Bloom, FE; Pich, EM. Rapid induction of tumor necrosis factor alpha in the cerebrospinal fluid after intracerebroventricular injection of lipopolysaccharide revealed by a sensitive capture immuno-PCR assay. Proc Natl Acad Sci U S A 1995, 92(1), 272–275. [Google Scholar]
- Noda, M; Nakanishi, H; Nabekura, J; Akaike, N. AMPA-kainate subtypes of glutamate receptor in rat cerebral microglia. J Neurosci 2000, 20(1), 251–258. [Google Scholar]
- de Bock, F; Derijard, B; Dornand, J; Bockaert, J; Rondouin, G. The neuronal death induced by endotoxic shock but not that induced by excitatory amino acids requires TNF-alpha. Eur J Neurosci 1998, 10(10), 3107–3114. [Google Scholar]
- Konig, HG; Kogel, D; Rami, A; Prehn, JH. TGF-{beta}1 activates two distinct type I receptors in neurons: implications for neuronal NF-{kappa}B signaling. J Cell Biol 2005, 168(7), 1077–1086. [Google Scholar]
- Boche, D; Cunningham, C; Gauldie, J; Perry, VH. Transforming growth factor-beta 1-mediated neuroprotection against excitotoxic injury in vivo. J Cereb Blood Flow Metab 2003, 23(10), 1174–1182. [Google Scholar]
- Prehn, JH; Miller, RJ. Opposite effects of TGF-beta 1 on rapidly- and slowly-triggered excitotoxic injury. Neuropharmacology 1996, 35(3), 249–256. [Google Scholar]
- Morgan, TE; Nichols, NR; Pasinetti, GM; Finch, CE. TGF-beta 1 mRNA increases in macrophage/microglial cells of the hippocampus in response to deafferentation and kainic acid-induced neurodegeneration. Exp Neurol 1993, 120(2), 291–301. [Google Scholar]
- Franciosi, S; Choi, HB; Kim, SU; McLarnon, JG. IL-8 enhancement of amyloid-beta (Abeta 1-42)-induced expression and production of pro-inflammatory cytokines and COX-2 in cultured human microglia. J Neuroimmunol 2005, 159(1–2), 66–74. [Google Scholar]
- Mizuno, T; Sawada, M; Marunouchi, T; Suzumura, A. Production of interleukin-10 by mouse glial cells in culture. Biochem Biophys Res Commun 1994, 205(3), 1907–1915. [Google Scholar]
- Lukes, A; Mun-Bryce, S; Lukes, M; Rosenberg, GA. Extracellular matrix degradation by metalloproteinases and central nervous system diseases. Mol Neurobiol 1999, 19(3), 267–284. [Google Scholar]
- Watanabe, H; Nakanishi, I; Yamashita, K; Hayakawa, T; Okada, Y. Matrix metalloproteinase-9 (92 kDa gelatinase/type IV collagenase) from U937 monoblastoid cells: correlation with cellular invasion. J Cell Sci 1993, 104 Pt 4, 991–999. [Google Scholar]
- Rosenberg, GA; Estrada, EY; Dencoff, JE; Stetler-Stevenson, WG. Tumor necrosis factor-alpha-induced gelatinase B causes delayed opening of the blood-brain barrier: an expanded therapeutic window. Brain Res 1995, 703(1–2), 151–155. [Google Scholar]
- Novelli, A; Kispert, J; Fernandez-Sanchez, MT; Torreblanca, A; Zitko, V. Domoic acid-containing toxic mussels produce neurotoxicity in neuronal cultures through a synergism between excitatory amino acids. Brain Res 1992, 577(1), 41–48. [Google Scholar]
- Appel, NM; Rapoport, SI; O'Callaghan, JP; Bell, JM; Freed, LM. Sequelae of parenteral domoic acid administration in rats: comparison of effects on different metabolic markers in brain. Brain Res 1997, 754(1–2), 55–64. [Google Scholar]
- Mayer, AMS; Carlson, LA; Braddock, MT; McCulloch, PF. Hippocampal C-Fos expression and microglia accumulation in rats exposed to the marine toxin domoic acid. The Toxicologist 2007, 96(1), 364. [Google Scholar]
- Mayer, AMS; Hall, ML; Fay, MJ; Romanic, AM. Cytokine gene expression in rat microglia exposed to the marine toxin domoic acid. The Toxicologist 2005, 84(S-1), 293. [Google Scholar]
- Mayer, AMS; Hall, ML; Fay, MJ; Romanic, AM. cDNA array analysis of matrix metalloproteinase gene expression in rat microglia exposed to the marine toxin domoic acid. The Toxicologist 2004, 46(3), 94. [Google Scholar]
- Falk, M; Seto, PF; Walter, JA. Solubility of domoic acid in water and in non-aqueous solvents. Canadian Journal of Chemistry 1991, 69, 1740–1744. [Google Scholar]
- Ren, L. Lipopolysaccharide-induced expression of IP-10 mRNA in rat brain and in cultured rat astrocytes and microglia. Brain Res Mol Brain Res 1998, 59(2), 256–263. [Google Scholar]
- Lyn, D; Liu, X; Bennett, NA; Emmett, NL. Gene expression profile in mouse myocardium after ischemia. Physiol Genomics 2000, 2 (3), 93–100. [Google Scholar]



List of abbreviations
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid |
| DMEM | Dulbecco's modified Eagle medium |
| DOM | domoic acid |
| FBS | fetal bovine serum certified |
| FCC | ferricytochrome c type III |
| HBSS | Hank’s balanced salt solution |
| LPS | lipopolysaccharide |
| MMP-9 | matrix metalloproteinase-9 |
| P | penicillin |
| PBS | phosphate buffered saline |
| PMA | phorbol 12-myristate 13-acetate |
| RT-PCR | reverse transcriptase polymerase chain reaction |
| S | streptomycin |
| SE | standard error of the mean |
| SOD | superoxide dismutase |
| TGF-β1 | transforming growth factor β1 |
| TNF-α | tumor necrosis factor |
Share and Cite
Mayer, A.M.S.; Guzman, M.; Peksa, R.; Hall, M.; Fay, M.J.; Jacobson, P.B.; Romanic, A.M.; Gunasekera, S.P. Differential Effects of Domoic Acid and E. coli Lipopolysaccharide on Tumor Necrosis Factor-α, Transforming Growth Factor-β1 and Matrix Metalloproteinase-9 Release by Rat Neonatal Microglia: Evaluation of the Direct Activation Hypothesis. Mar. Drugs 2007, 5, 113-135. https://doi.org/10.3390/md503113
Mayer AMS, Guzman M, Peksa R, Hall M, Fay MJ, Jacobson PB, Romanic AM, Gunasekera SP. Differential Effects of Domoic Acid and E. coli Lipopolysaccharide on Tumor Necrosis Factor-α, Transforming Growth Factor-β1 and Matrix Metalloproteinase-9 Release by Rat Neonatal Microglia: Evaluation of the Direct Activation Hypothesis. Marine Drugs. 2007; 5(3):113-135. https://doi.org/10.3390/md503113
Chicago/Turabian StyleMayer, Alejandro M. S., Marcio Guzman, Renee Peksa, Mary Hall, Michael J. Fay, Peer B. Jacobson, Anne M. Romanic, and Sarath P. Gunasekera. 2007. "Differential Effects of Domoic Acid and E. coli Lipopolysaccharide on Tumor Necrosis Factor-α, Transforming Growth Factor-β1 and Matrix Metalloproteinase-9 Release by Rat Neonatal Microglia: Evaluation of the Direct Activation Hypothesis" Marine Drugs 5, no. 3: 113-135. https://doi.org/10.3390/md503113
APA StyleMayer, A. M. S., Guzman, M., Peksa, R., Hall, M., Fay, M. J., Jacobson, P. B., Romanic, A. M., & Gunasekera, S. P. (2007). Differential Effects of Domoic Acid and E. coli Lipopolysaccharide on Tumor Necrosis Factor-α, Transforming Growth Factor-β1 and Matrix Metalloproteinase-9 Release by Rat Neonatal Microglia: Evaluation of the Direct Activation Hypothesis. Marine Drugs, 5(3), 113-135. https://doi.org/10.3390/md503113




