Chlorella pyrenoidosa Polysaccharide CPP-3a Promotes M1 Polarization of Macrophages via TLR4/2-MyD88-NF-κB/p38 MAPK Signaling Pathways
Abstract
1. Introduction
2. Results
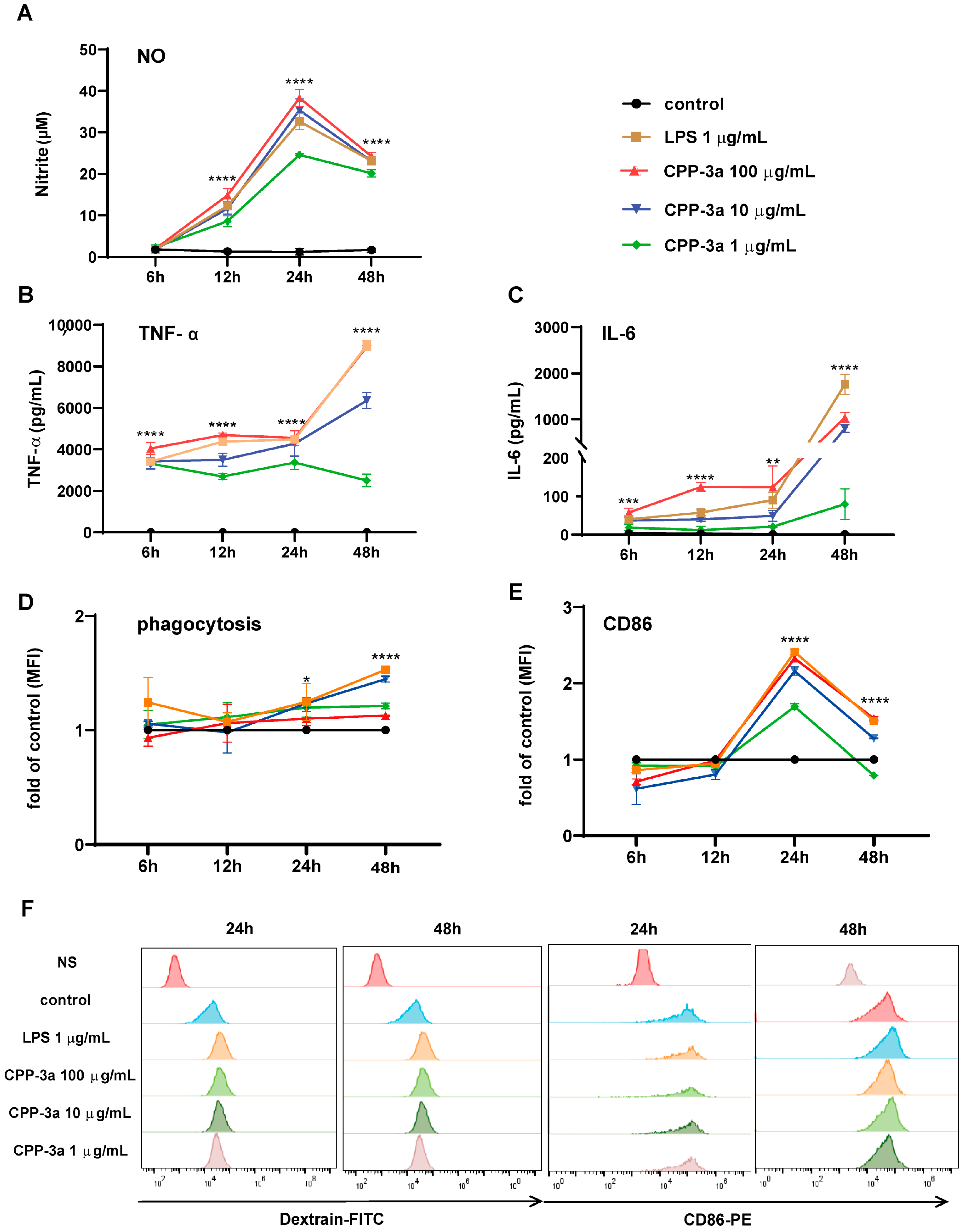
2.1. Chlorella polysaccharide CPP-3a Induces M1 Polarization of RAW264.7 Cells
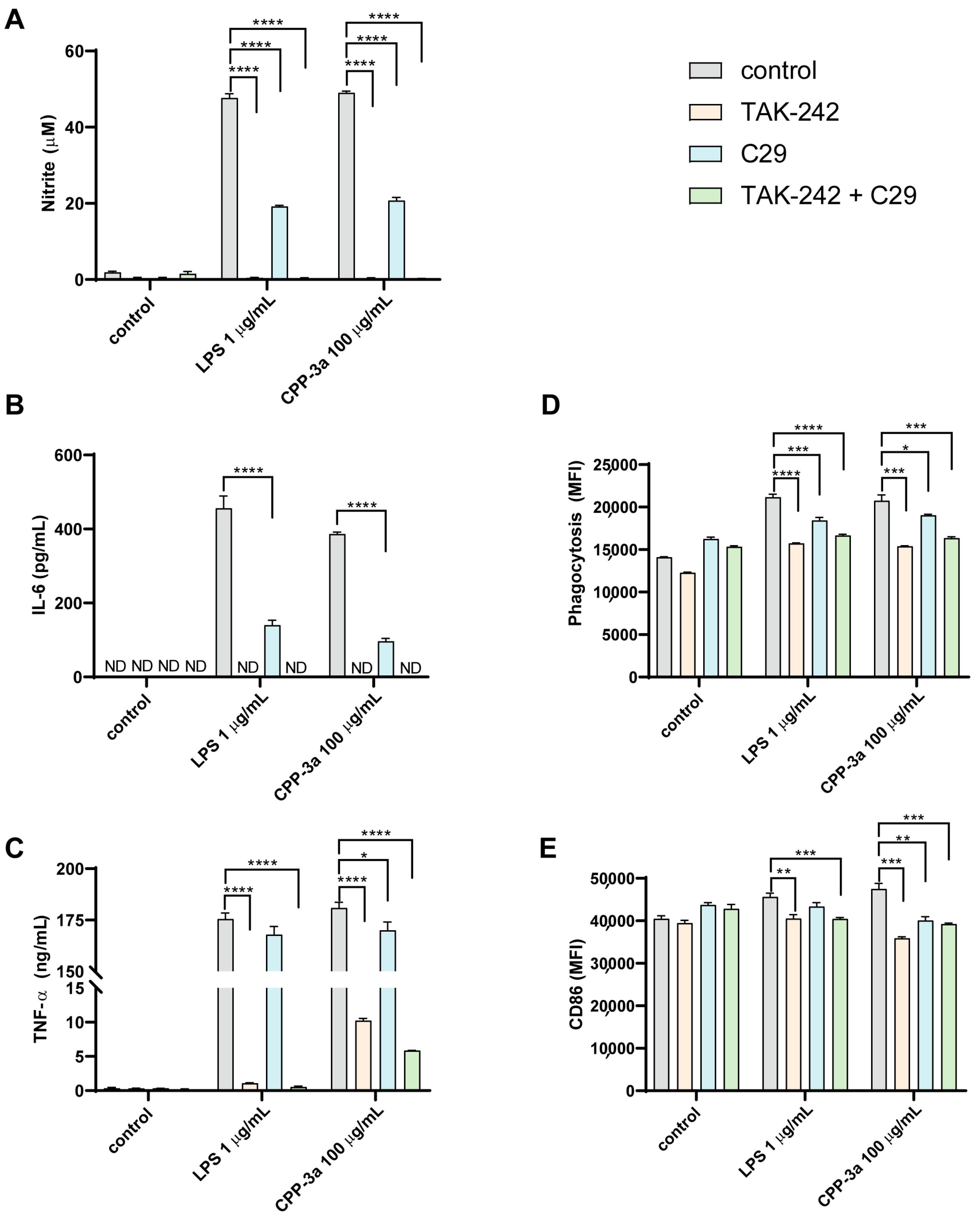
2.2. Both TLR4 Inhibitor TAK242 and TLR2 Inhibitor C29 Inhibited CPP-3a-Induced M1 Polarization in RAW264.7 Cells
2.3. TLR4-KO Inhibits CPP-3a-Induced M1 Polarization in RAW264.7 Cells
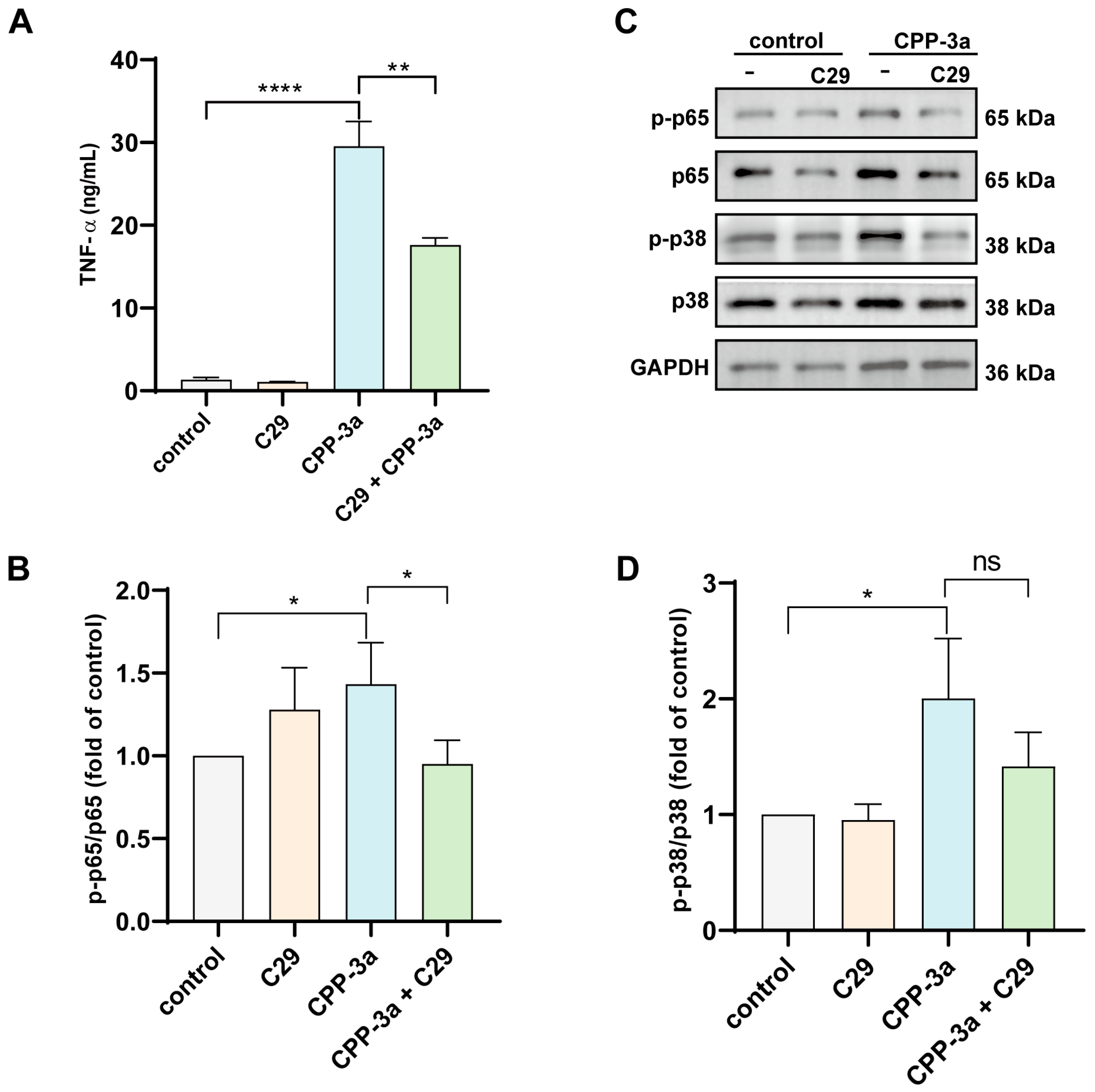
2.4. C29 Suppresses TNF-α Release in CPP-3a-Activated TLR4-KO RAW264.7 Macrophages
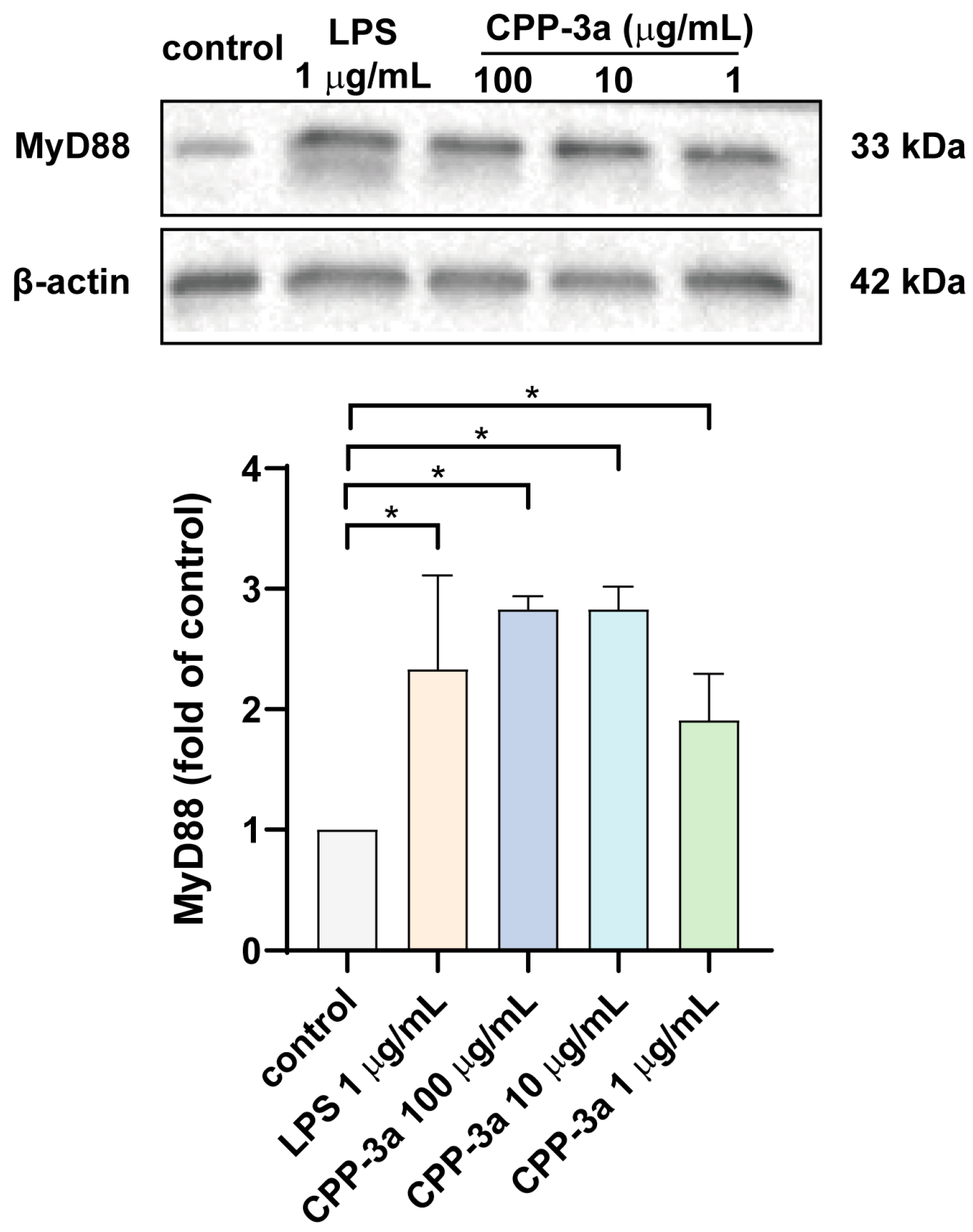
2.5. CPP-3a Activates the TLR Signaling Pathway Through MyD88
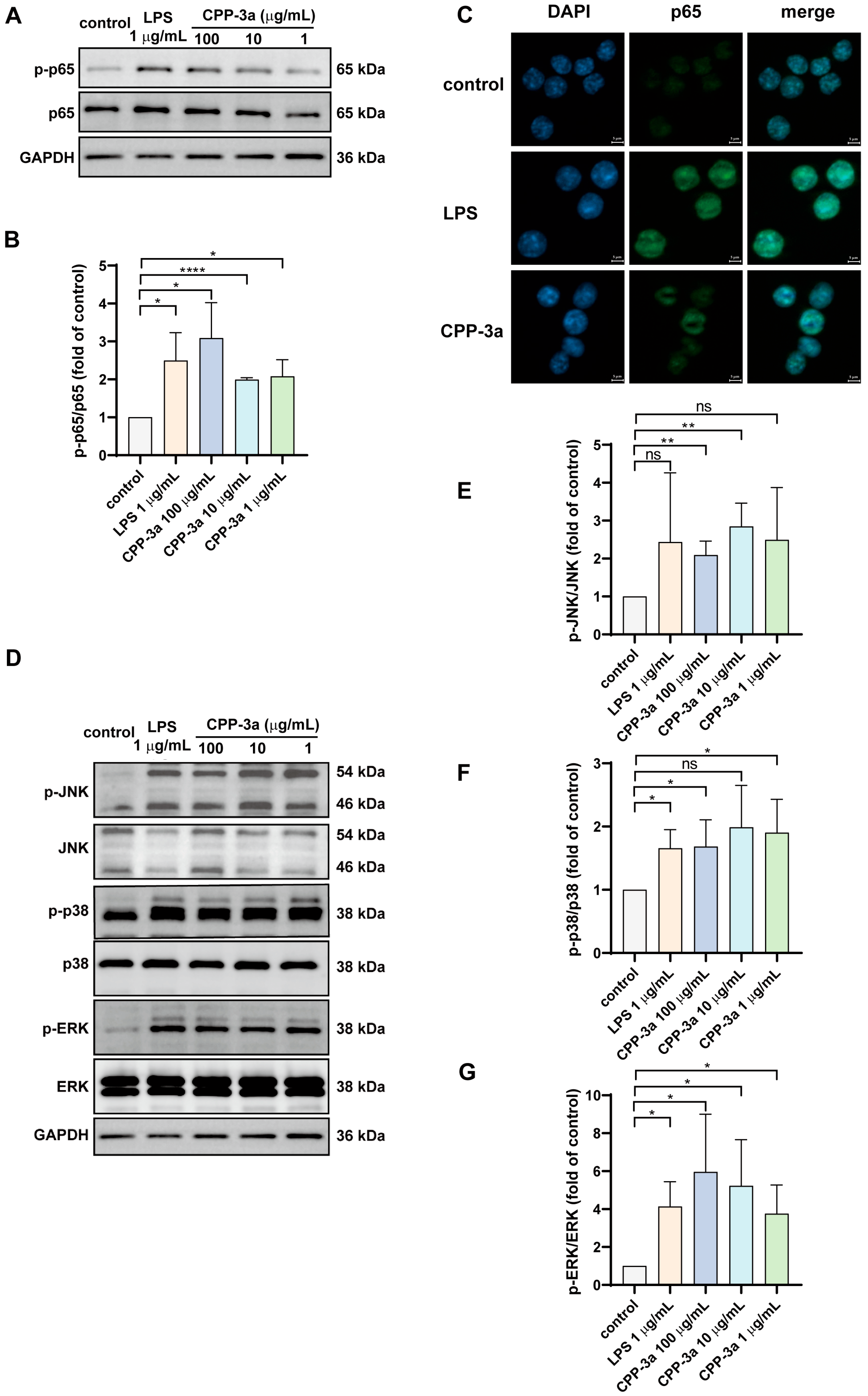
2.6. The NF-κB and p38 MAPK Signaling Are Activated in CPP-3a-Stimulated RAW264.7 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Phagocytic Function Assay
4.3. Assessment of Nitrite Levels and Cytokine Release
4.4. Flow Cytometry Analysis
4.5. Western Blotting
4.6. Establishment of Tlr4 Knockout Cell Line
4.7. Immunofluorescence Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FAO | Food and Agriculture Organization of the United Nations |
| DCs | Dendritic cells |
| TNF-α | Tumor necrosis factor-alpha |
| IL-6 | Interleukin-6 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| MAPK | Mitogen-activated protein kinases |
| TLR | Toll-like receptor |
| NO | Nitric oxide |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | Fetal bovine serum |
| LPS | Lipopolysaccharide |
| PFA | Paraformaldehyde |
| sg-RNAs | Single-guide RNAs |
References
- Kay, R.A.; Barton, L.L. Microalgae as food and supplement. Crit. Rev. Food Sci. Nutr. 1991, 30, 555–573. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, N.A. The Chlorella genome: Big surprises from a small package. Plant Cell 2010, 22, 2924. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Li, H.; Wei, Z.; Lv, K.; Gao, C.; Liu, Y.; Zhao, L. Isolation, structures and biological activities of polysaccharides from Chlorella: A review. Int. J. Biol. Macromol. 2020, 163, 2199–2209. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Yuan, Q.; Li, H.; Li, T.; Ma, H.; Gao, C.; Zhang, S.; Liu, Y.; Zhao, L. Chlorella pyrenoidosa polysaccharides as a prebiotic to modulate gut microbiota: Physicochemical properties and fermentation characteristics in vitro. Foods 2022, 11, 725. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Huang, J.; Yuan, Q.; Lv, K.; Ma, H.; Li, T.; Liu, Y.; Mi, S.; Zhao, L. A regular Chlorella mannogalactan and its sulfated derivative as a promising anticoagulant: Structural characterization and anticoagulant activity. Carbohydr. Polym. 2023, 314, 120956–120964. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Liang, R.; Lv, K.; Shi, X.; Leng, J.; Liu, Y.; Xiao, J.; Zhang, L.; Zhao, L. Structural characterization of a Chlorella heteropolysaccharide by analyzing its depolymerized product and finding an inducer of human dendritic cell maturation. Carbohydr. Polym. 2024, 333, 122000. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zhao, F.; Cheng, H.; Su, M.; Wang, Y. Macrophage polarization: An important role in inflammatory diseases. Front. Immunol. 2024, 15, 1352946. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Juhas, U.; Szargiej, P.; Myśliwska, J. Different pathways of macrophage activation and polarization. Postępy Hig. Med. Dośw. 2015, 69, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Vergadi, E.; Ieronymaki, E.; Lyroni, K.; Vaporidi, K.; Tsatsanis, C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J. Immunol. 2017, 198, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; An, Y.; Li, G.; Wang, S. Regulatory mechanism of macrophage polarization based on Hippo pathway. Front. Immunol. 2023, 14, 1279591. [Google Scholar] [CrossRef] [PubMed]
- Rumpel, N.; Riechert, G.; Schumann, J. miRNA-mediated fine regulation of TLR-induced M1 polarization. Cells 2024, 13, 701–718. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage M1-M2 polarization balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. TLR signaling pathways. Semin. Immunol. 2004, 16, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-κB Signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R. Toll-like receptor signaling and its role in cell-mediated immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef] [PubMed]
- Kralovec, J.A.; Metera, K.L.; Kumar, J.R.; Watson, L.V.; Girouard, G.S.; Guan, Y.; Carr, R.I.; Barrow, C.J.; Ewart, H.S. Immunostimulatory principles from Chlorella pyrenoidosa—Part 1: Isolation and biological assessment in vitro. Phytomedicine 2007, 14, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Jeyashoke, N.; Yeh, C.H.; Song, Y.J.; Hua, K.F.; Chao, L.K. Immunostimulatory bioactivity of algal polysaccharides from Chlorella pyrenoidosa activates macrophages via Toll-like receptor 4. J Agric Food Chem 2010, 58, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Kim, S.M. Effects of the molecular weight and protein and sulfate content of Chlorella ellipsoidea polysaccharides on their immunomodulatory activity. Int. J. Biol. Macromol. 2018, 107, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Lin, R.; Hou, X.; Wu, J.; Zhao, W.; Ma, H.; Fan, Z.; Li, S.; Zhu, Y.; Zhang, D. Immunomodulatory mechanism of a purified polysaccharide isolated from Isaria cicadae Miquel on RAW264.7 cells via activating TLR4-MAPK-NF-κB signaling pathway. Int. J. Biol. Macromol. 2020, 164, 4329–4338. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Huang, Z.; Meng, H.; Shi, X.; Xie, J. Immunomodulation effect of polysaccharides from liquid fermentation of Monascus purpureus 40269 via membrane TLR-4 to activate the MAPK and NF-κB signaling pathways. Int. J. Biol. Macromol. 2022, 201, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mo, J.; Xiang, W.; Shi, X.; Guo, L.; Li, Y.; Bao, Y.; Zheng, L. Immunoregulatory effects of Tetrastigma hemsleyanum polysaccharide via TLR4-mediated NF-κB and MAPK signaling pathways in Raw264.7 macrophages. Biomed. Pharmacother. 2023, 161, 114471–114482. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Lee, S.; Shin, K. Immunostimulating and intracellular signaling pathways mechanism on macrophage of rhamnogalacturonan-I type polysaccharide purified from radish leaves. Int. J. Biol. Macromol. 2022, 217, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Zhao, Y.; Wang, M.; Cao, J.; Chang, M.; Yun, S.; Cheng, Y.; Cheng, F.; Feng, C. Effects of Sparassis latifolia neutral polysaccharide on immune activity via TLR4-mediated MyD88-dependent and independent signaling pathways in RAW264.7 macrophages. Front. Nutr. 2022, 9, 994971. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, X.; Yang, Y.; Ayivi-Tosuh, S.; Wang, F.; Li, H.; Wang, G. A polysaccharide isolated from the fruits of Physalis alkekengi L. induces RAW264.7 macrophages activation via TLR2 and TLR4-mediated MAPK and NF-κB signaling pathways. Int. J. Biol. Macromol. 2019, 140, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Li, W.; Xu, J.; Yang, Y.; Tan, Z.; Yang, R. A novel polysaccharide isolated from fresh longan (Dimocarpus longan Lour.) activates macrophage via TLR2/4-mediated PI3/AKT and MyD88/TRAF6 pathways. Front. Pharmacol. 2021, 12, 786127. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Pan, W.; Mehmood, S.; Cheng, X.; Chen, Y. Polysaccharide isolated from Sarcodon aspratus induces RAW264.7 activity via TLR4-mediated NF-κB and MAPK signaling pathways. Int. J. Biol. Macromol. 2018, 120, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Yang, Y.; Chen, Y.; Hua, K.; Lu, C.; Sheu, F.; Lin, G.; Tsay, S.; Liang, S.; Wu, S. A Novel Exopolysaccharide from the Biofilm of Thermus aquaticus YT-1 Induces the Immune Response through Toll-like Receptor 2. J. Biol. Chem. 2011, 286, 17736–17745. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Cheng, Y.; Mu, J.; Huang, Y.; Chen, H.; Zhao, L.; Wang, K.; Hu, Z. Glucose-rich polysaccharide from dried ‘Shixia’ longan activates macrophages through Ca2+ and CR3− mediated MAPKs and PI3K-AKT pathways. Int. J. Biol. Macromol. 2021, 167, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Talapphet, N.; Palanisamy, S.; Li, C.; Ma, N.; Prabhu, N.M.; You, S. Polysaccharide extracted from Taraxacum platycarpum root exerts immunomodulatory activity via MAPK and NF-κB pathways in RAW264.7 cells. J. Ethnopharmacol. 2021, 281, 114519–114529. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Luo, Z.; Liu, D.; Ning, Z.; Yang, J.; Ren, J. Structure Characterization of a Novel Polysaccharide from Dictyophora indusiata and Its Macrophage Immunomodulatory Activities. J. Agric. Food Chem. 2015, 63, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Fu, H.; Shang, J.; Chen, J.; Xu, X. Dectin-1 mediates the immunoenhancement effect of the polysaccharide from Dictyophora indusiata. Int. J. Biol. Macromol. 2018, 109, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Lin, X.; Hu, J.; Wang, S.; Wu, J.; Xiong, W.; Wu, L. The polysaccharide from Camellia oleifera fruit shell enhances immune responses via activating MAPKs and NF-κB signaling pathways in RAW264.7 macrophages. Food Nutr. Res. 2022, 66, 8963–8972. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Yu, J.; Huang, Q.; Yu, J.; Yang, Y.; Song, H.; Liu, Y.; Xiao, X.; Cui, L.; Li, W. Immunoenhancement activity of Bletilla striata polysaccharide through MAPK and NF-κB signalling pathways in vivo and in vitro. Autoimmunity 2022, 55, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Tabarsa, M.; Jafari, A.; You, S.; Cao, R. Immunostimulatory effects of a polysaccharide from Pimpinella anisum seeds on RAW264.7 and NK-92 cells. Int. J. Biol. Macromol. 2022, 213, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, X.; Yin, Z.; Wang, M.; Wang, B.; Ma, C.; Wang, J.; Kang, W. Activation of RAW264.7 cells by PCp-I, a polysaccharide from Psoralea corylifolia L, through NF-κB/MAPK signalling pathway. Int. J. Immunopathol. Pharmacol. 2021, 35, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Perera, N.; Yang, F.; Chiu, H.; Hsieh, C.; Li, L.; Zhang, Y.; Hua, K.; Wu, S. Phagocytosis enhancement, endotoxin tolerance, and signal mechanisms of immunologically active glucuronoxylomannan from Auricularia auricula-judae. Int. J. Biol. Macromol. 2020, 165, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, F.Z.; Skwarczynski, M.; Toth, I. Developments in vaccine adjuvants. Methods Mol. Biol. 2022, 2412, 145–178. [Google Scholar] [PubMed]
- Du, B.; Meenu, M.; Liu, H.; Xu, B. A concise review on the molecular structure and function relationship of β-glucan. Int. J. Mol. Sci. 2019, 20, 4032. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.J.; Rezoagli, E.; Major, I.; Rowan, N.; Laffey, J.G. β-Glucan metabolic and immunomodulatory properties and potential for clinical application. J. Fungi 2020, 6, 356. [Google Scholar] [CrossRef] [PubMed]
- Castro, E.D.M.; Calder, P.C.; Roche, H.M. β-1,3/1,6-glucans and immunity: State of the art and future directions. Mol. Nutr. Food Res. 2021, 65, 1901071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.R.; Qi, C.H.; Guo, Y.; Zhou, W.X.; Zhang, Y.X. Toll-like receptor 4-related immunostimulatory polysaccharides: Primary structure, activity relationships, and possible interaction models. Carbohydr. Polym. 2016, 149, 186–206. [Google Scholar] [CrossRef] [PubMed]


| Target Sequence | Synthesized Oligo DNA | Primers for Verification | |
|---|---|---|---|
| Sg1 | 5’-ACACGTCCATCGGTTGATCTTGG-3’ | 5’-CACCGACACGTCCATCGGTTGATCT-3’ 3’-CTGTGCAGGTAGCCAACTAGACAAA-5’ | Left primer: GGGAATTAAGCTCCATGAACTG Right primer: GATACACCTGCCAGAGACATTG |
| Sg2 | 5’- GATCTACTCGAGTCAGAATGAGG -3’ | 5’-CACCGGATCTACTCGAGTCAGAATG-3’ 3’-CCTAGATGAGCTCAGTCTTACCAAA-5’ | Left primer: TCATCAGTGTGTCAGTGGTCAG Right primer: TGTAGTGAAGGCAGAGGTGAAA |
| Sg3 | 5’- CACGTCCATCGGTTGATCTTGGG -3’ | 5’-CACCGCACGTCCATCGGTTGATCTT-3’ 3’-CGTGCAGGTAGCCAACTAGAACAAA-5’ | Left primer: GGGAATTAAGCTCCATGAACTG Right primer: GATACACCTGCCAGAGACATTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pi, Y.; Yuan, Q.; Qin, S.; Lan, C.; Nong, Q.; Yun, C.; Tang, H.; Leng, J.; Xiao, J.; Zhao, L.; et al. Chlorella pyrenoidosa Polysaccharide CPP-3a Promotes M1 Polarization of Macrophages via TLR4/2-MyD88-NF-κB/p38 MAPK Signaling Pathways. Mar. Drugs 2025, 23, 290. https://doi.org/10.3390/md23070290
Pi Y, Yuan Q, Qin S, Lan C, Nong Q, Yun C, Tang H, Leng J, Xiao J, Zhao L, et al. Chlorella pyrenoidosa Polysaccharide CPP-3a Promotes M1 Polarization of Macrophages via TLR4/2-MyD88-NF-κB/p38 MAPK Signaling Pathways. Marine Drugs. 2025; 23(7):290. https://doi.org/10.3390/md23070290
Chicago/Turabian StylePi, Yihua, Qingxia Yuan, Shaoting Qin, Chundie Lan, Qingdong Nong, Chenxia Yun, Haibo Tang, Jing Leng, Jian Xiao, Longyan Zhao, and et al. 2025. "Chlorella pyrenoidosa Polysaccharide CPP-3a Promotes M1 Polarization of Macrophages via TLR4/2-MyD88-NF-κB/p38 MAPK Signaling Pathways" Marine Drugs 23, no. 7: 290. https://doi.org/10.3390/md23070290
APA StylePi, Y., Yuan, Q., Qin, S., Lan, C., Nong, Q., Yun, C., Tang, H., Leng, J., Xiao, J., Zhao, L., & Zhang, L. (2025). Chlorella pyrenoidosa Polysaccharide CPP-3a Promotes M1 Polarization of Macrophages via TLR4/2-MyD88-NF-κB/p38 MAPK Signaling Pathways. Marine Drugs, 23(7), 290. https://doi.org/10.3390/md23070290







