Targeted Isolation of ω-3 Polyunsaturated Fatty Acids from the Marine Dinoflagellate Prorocentrum lima Using DeepSAT and LC-MS/MS and Their High Activity in Promoting Microglial Functions
Abstract
1. Introduction
2. Results
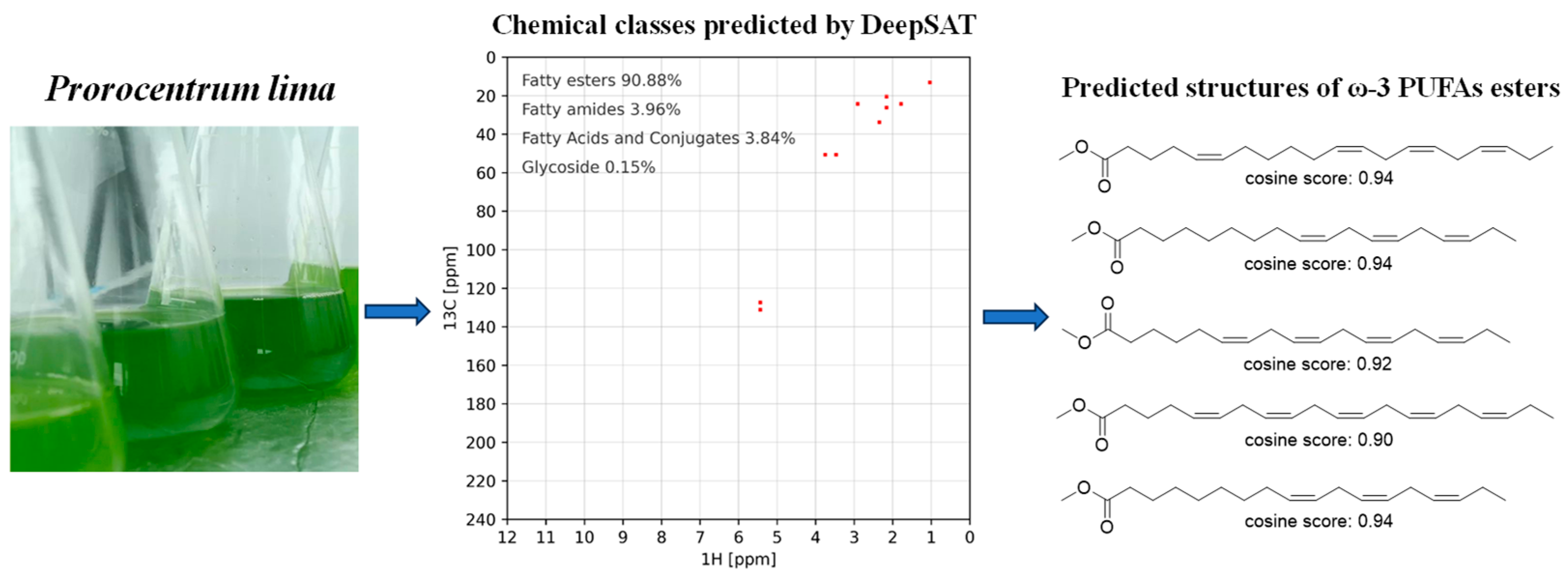
2.1. Characterization of ω-3 Polyunsaturated Fatty Esters via Integrated DeepSAT and UPLC-MS/MS
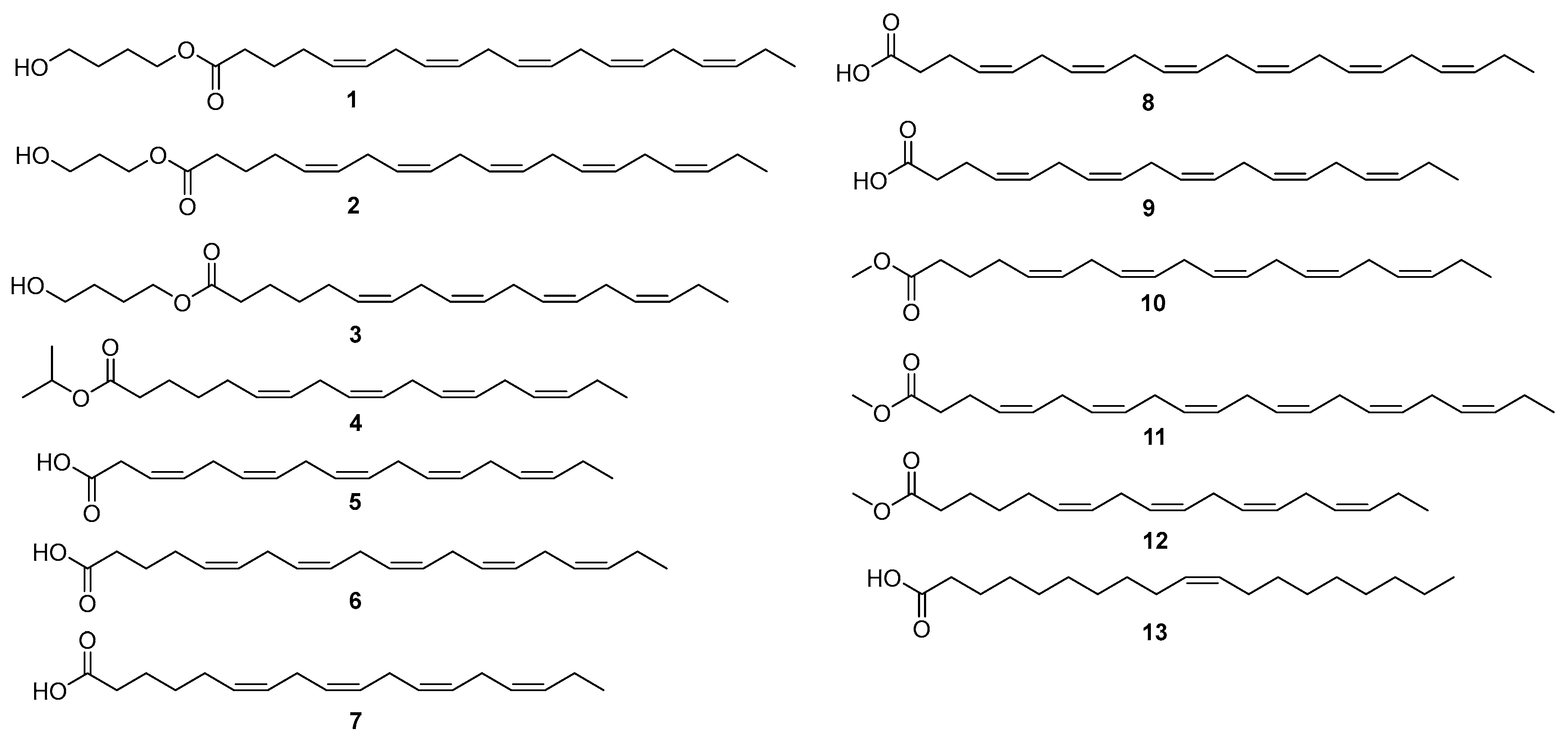
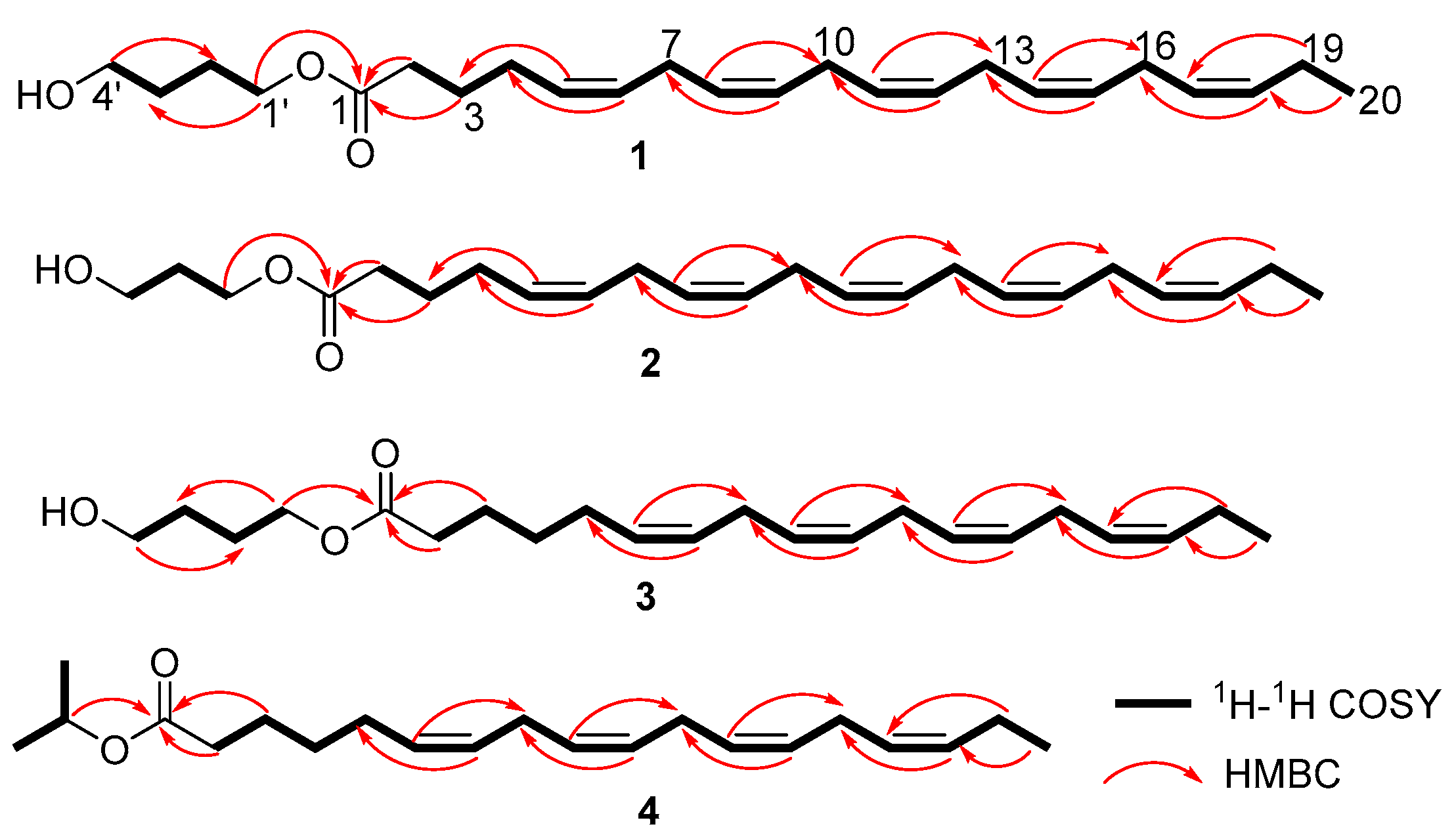
2.2. Structural Elucidation
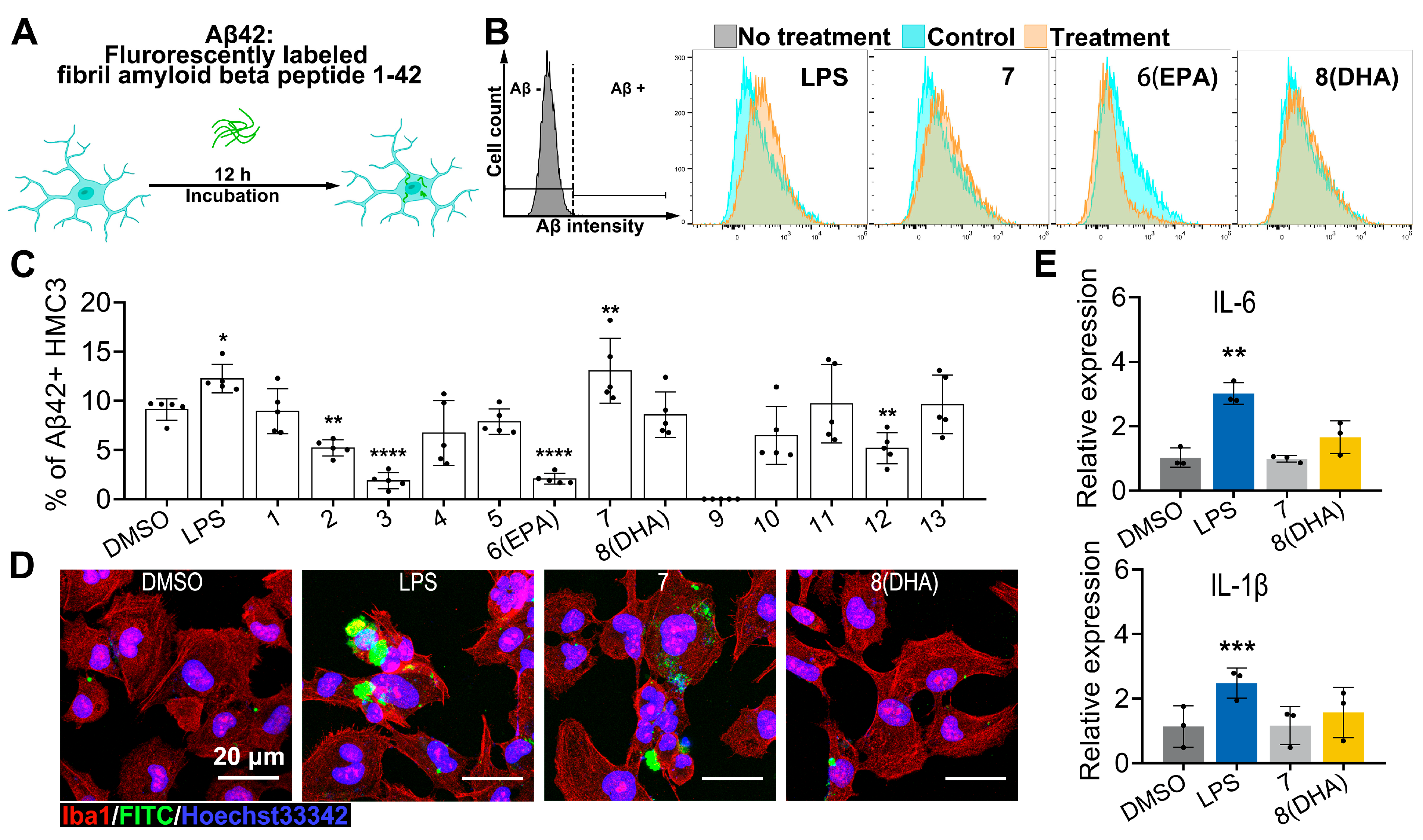
2.3. Biological Activity
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedure
4.2. Culture of Prorocentrum lima
4.3. DeepSAT Usage Procedure
4.4. UPLC-MS/MS Method
4.5. HPLC Method
4.6. SciFinder Search Procedure
4.7. Extraction and Isolation
4.8. Spectroscopic Data of New Compounds
4.9. MTT Assay
4.10. Scratch Wound Healing Assay
4.11. Aβ42 Peptide Phagocytosis Assay and Flow Cytometry
4.12. Reverse Transcription and Quantitative Real Time-PCR (qRT-PCR)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hort, V.; Abadie, E.; Arnich, N.; Dechraoui, M.Y.; Amzil, Z. Chemodiversity of brevetoxins and other potentially toxic metabolites produced by Karenia spp. and their metabolic products in marine organisms. Mar. Drugs 2021, 19, 656. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Grkovic, T.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2025, 42, 257–297. [Google Scholar] [CrossRef]
- Lai, C.R.; Dai, X.J.; Tian, D.M.; Lv, S.H.; Tang, J.S. Chemistry and bioactivity of marine algal toxins and their geographic distribution in China. Fitoterapia 2024, 178, 106193. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Muñoz, D.; Praptiwi, R.A.; Lawton, L. High value phycotoxins from the dinoflagellate Prorocentrum. Front. Mar. Sci. 2021, 8, 638739. [Google Scholar] [CrossRef]
- Lee, S.; Yang, A.R.; Yoo, Y.D.; Jeong, E.J.; Rho, J.R. Relative configurational assignment of 4-hydroxyprorocentrolide and prorocentrolide C isolated from a benthic dinoflagellate (Prorocentrum lima). J. Nat. Prod. 2019, 82, 1034–1039. [Google Scholar] [CrossRef]
- Geng, X.; Yang, B.; Li, R.; Teng, T.; Ladu, M.J.; Sun, G.Y.; Greenlief, C.M.; Lee, J.C. Effects of docosahexaenoic acid and its peroxidation product on Amyloid-β peptide-stimulated microglia. Mol. Neurobiol. 2020, 57, 1085–1098. [Google Scholar] [CrossRef]
- Bie, N.; Feng, X.; Li, C.; Meng, M.; Wang, C. The protective effect of docosahexaenoic acid on PC12 cells in oxidative stress induced by H2O2 through the TrkB-Erk1/2-CREB pathway. ACS Chem. Neurosci. 2021, 12, 3433–3444. [Google Scholar] [CrossRef]
- Yuan, S.; Li, H.; Yang, C.; Xie, W.; Wang, Y.; Zhang, J.; Cai, Z.; Mao, Z.; Xie, W.; Lü, T. DHA attenuates Aβ-induced necroptosis through the RIPK1/RIPK3 signaling pathway in THP-1 monocytes. Biomed. Pharmacother. 2020, 126, 110102. [Google Scholar] [CrossRef]
- Hjorth, E.; Zhu, M.; Toro, V.C.; Vedin, I.; Palmblad, J.; Cederholm, T.; Freund-Levi, Y.; Faxen-Irving, G.; Wahlund, L.O.; Basun, H.; et al. Omega-3 fatty acids enhance phagocytosis of Alzheimer’s disease-related amyloid-β42 by human microglia and decrease inflammatory markers. J. Alzheimers Dis. 2013, 35, 697–713. [Google Scholar] [CrossRef]
- Ding, W.J.; Tian, D.M.; Chen, M.; Xia, Z.X.; Tang, X.Y.; Zhang, S.H.; Wei, J.H.; Li, X.; Yao, X.S.; Wu, B.; et al. Molecular Networking-Guided Isolation of Cyclopentapeptides from the Hydrothermal Vent Sediment Derived Fungus Aspergillus pseudoviridinutans TW58-5 and Their Anti-inflammatory Effects. J. Nat. Prod. 2023, 86, 1919–1930. [Google Scholar] [CrossRef]
- Yu, C.X.; Xia, Z.X.; Xu, Z.P.; Tang, X.Y.; Ding, W.J.; Wei, J.H.; Tian, D.M.; Wu, B.; Tang, J.S. Curvularin derivatives from hydrothermal vent sediment fungus Penicillium sp. HL-50 guided by molecular networking and their anti-inflammatory activity. Chin. J. Nat. Med. 2025, 23, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.K.; Wang, X.; Lian, M.Y.; Guo, S.S.; Gao, Z.H.; Bai, M.; Huang, X.X.; Song, S.J. Bioassay-Guided and DeepSAT-Driven Precise Mining of Monoterpenoid Coumarin Derivatives with Antifeedant Effects from the Leaves of Ailanthus altissima. J. Agric. Food Chem. 2024, 72, 10958–10969. [Google Scholar] [CrossRef]
- Zhu, H.; He, L.; Wu, W.; Duan, H.; Chen, J.; Xiao, Q.; Lin, P.; Qin, Z.; Dai, Y.; Wu, W.; et al. A compounds annotation strategy using targeted molecular networking for offline two-dimensional liquid chromatography-mass spectrometry analysis: Yupingfeng as a case study. J. Chromatogr. A 2023, 1702, 464045. [Google Scholar] [CrossRef] [PubMed]
- Hiraga, Y.; Shikano, T.; Widianti, T.; Ohkata, K. Three new glycolipids with cytolytic activity from cultured marine dinoflagellate Heterocapsa circularisquama. Nat. Prod. Res. 2008, 22, 649–657. [Google Scholar] [CrossRef]
- Vatele, J.M.; Fenet, B.; Eynard, T. Complete 13C assignments and structural elucidation of n-3 polyunsaturated fatty acids by the use of a new 2D NMR technique: SAPHIR-HSQC. Chem. Phys. Lipids 1998, 94, 239–250. [Google Scholar] [CrossRef]
- Fu, M.; Koulman, A.; Rijssel, M.; Boer, M.K.; Liebezeit, G. Chemical characterisation of three haemolytic compounds from the microalgal species Fibrocapsa japonica (Raphidophyceae). Toxicon 2004, 43, 355–363. [Google Scholar] [CrossRef]
- Alamsjah, M.A.; Hirao, S.; Ishibashi, F.; Fujita, Y. Isolation and structure determination of algicidal compounds from Ulva fasciata. Biosci. Biotechnol. Biochem. 2005, 69, 2186–2192. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Liu, D.; Yang, J. Study on the chemical components of Sargassum thunbergii. Chin. J. Pharm. Sci. 2012, 47, 948–952. [Google Scholar]
- Rezanka, T.; Temina, M.; Hanus, L.; Dembitsky, V.M. The tornabeatins, four tetrahydro-2-furanone derivatives from the lichenized ascomycete Tornabea scutellifera (With.) J.R. Laundon. Phytochemistry 2004, 65, 2605–2612. [Google Scholar] [CrossRef]
- Prinz, M.; Jung, S.; Priller, J. Microglia Biology: One Century of Evolving Concepts. Cell 2019, 179, 292–311. [Google Scholar] [CrossRef]
- Prinz, M.; Mildner, A. Microglia in the CNS: Immigrants from another world. Glia 2011, 59, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Chausse, B.; Kakimoto, P.A.; Kann, O. Microglia and lipids: How metabolism controls brain innate immunity. Semin. Cell Dev. Biol. 2021, 112, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.L.; Ahmad, N.N.; Ooi, Y.Y.; Ramasamy, R.; Vidyadaran, S. Response Profiles of BV2 Microglia to IFN-γ and LPS Co-Stimulation and Priming. Biomedicines 2023, 11, 2648. [Google Scholar] [CrossRef]
- Hwang, B.S.; Yoon, E.Y.; Jeong, E.J.; Park, J.; Kim, E.H.; Rho, J.R. Determination of the absolute configuration of polyhydroxy compound Ostreol B isolated from the dinoflagellate Ostreopsis cf. ovata. J. Org. Chem. 2018, 83, 194–202. [Google Scholar] [CrossRef]
- Sugahara, K.; Kitamura, Y.; Murata, M.; Satake, M.; Tachibana, K. Prorocentrol, a polyoxy linear carbon chain compound isolated from the toxic dinoflagellate Prorocentrum hoffmannianum. J. Org. Chem. 2011, 76, 3131–3138. [Google Scholar] [CrossRef]
- Takaaki, K.; Takahiro, I.; Haruaki, I. Amphidinin G, a putative biosynthetic precursor of amphidinin A from marine dinoflagellate Amphidinium sp. Tetrahedron Lett. 2015, 56, 990–993. [Google Scholar]
- Leutou, A.S.; McCall, J.R.; York, R.; Govindapur, R.R.; Bourdelais, A.J. Anti-inflammatory activity of glycolipids and a polyunsaturated fatty acid methyl ester isolated from the marine dinoflagellate Karenia mikimotoi. Mar. Drugs 2020, 18, 138. [Google Scholar] [CrossRef]
- Tian, L.; Chi, G.; Lin, S. Marine microorganisms: Natural factories for polyunsaturated fatty acid production. Blue Biotechnol. 2024, 1, 15. [Google Scholar] [CrossRef]
- Du, F.; Xu, Q.; Li, X.; Hang, Y.; Zhang, D.; Zhang, F.; Ma, W.; Sun, X.; Huang, H. Regulating triacylglycerol cycling for high-efficiency production of polyunsaturated fatty acids and derivatives. Nat. Commun. 2025, 16, 4262. [Google Scholar] [CrossRef]
- Huang, L.; Zhou, Y.; Gou, Z.X.; Zhang, F.; Lu, L.Q. Docosahexaenoic acid reduces hypoglycemia-induced neuronal necroptosis via the peroxisome proliferator-activated receptor γ/nuclear factor-κB pathway. Brain Res. 2022, 1774, 147708. [Google Scholar] [CrossRef]
- Abdi, J.; Garssen, J.; Faber, J.; Redegeld, F.A. Omega-3 fatty acids, EPA and DHA induce apoptosis and enhance drug sensitivity in multiple myeloma cells but not in normal peripheral mononuclear cells. J. Nutr. Biochem. 2014, 25, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Salsinha, A.S.; Socodato, R.; Rodrigues, A.; Vale-Silva, R.; Relvas, J.B.; Pintado, M.; Rodríguez-Alcalá, L.M. Potential of omega-3 and conjugated fatty acids to control microglia inflammatory imbalance elicited by obesogenic nutrients. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 2023, 1868, 159331. [Google Scholar] [CrossRef] [PubMed]
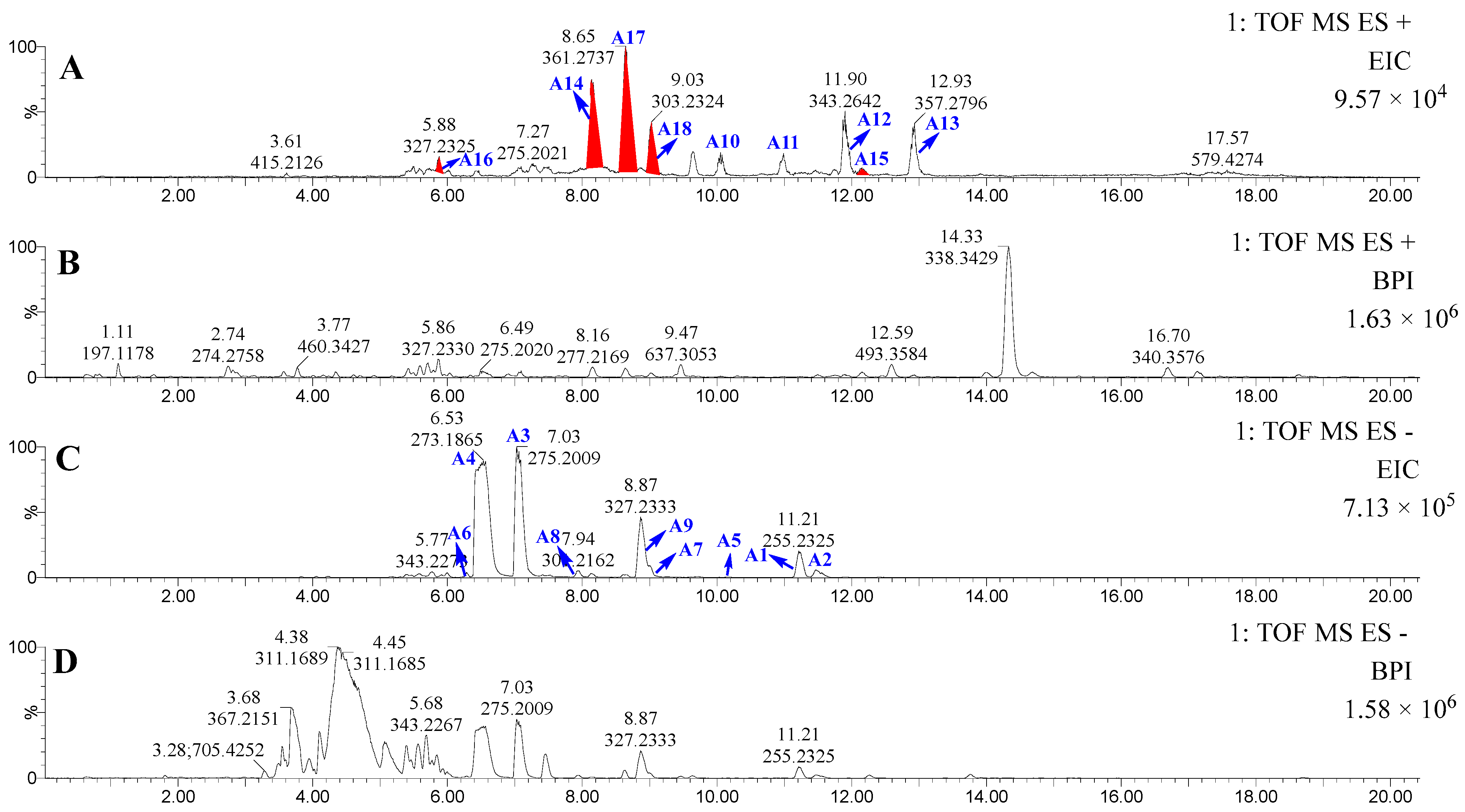

| No. | Lipid Type (C:N) | tR (min) | Formula | Ion Type | Calculated Ion m/z | Observed Ion m/z | Mass Accuracy Error (ppm) | Possible MS/MS Fragments |
|---|---|---|---|---|---|---|---|---|
| A1 | FA16:0 | 11.21 | C16H32O2 | [M − H]− | 255.2324 | 255.2325 | 0.4 | 239.2011 |
| A2 | FA18:1 | 11.47 | C18H34O2 | [M − H]− | 281.2481 | 281.2482 | 0.4 | 265.2168 |
| A3 | FA18:4 | 7.03 | C18H28O2 | [M − H]− | 275.2011 | 275.2010 | −0.4 | 231.2113 |
| A4 | FA18:5 | 6.28 | C18H26O2 | [M − H]− | 273.1855 | 273.1865 | 3.7 | 229.1958 |
| A5 | FA19:1 | 10.16 | C19H36O2 | [M − H]− | 295.2637 | 295.2645 | 2.7 | 279.2321 |
| A6 | FA19:5 | 6.98 | C19H28O2 | [M − H]− | 287.2011 | 287.2017 | 2.1 | 243.2113 |
| A7 | FA20:3 | 9.00 | C20H34O2 | [M − H]− | 305.2481 | 305.2492 | 3.6 | 261.2582 |
| A8 | FA20:5 | 7.94 | C20H30O2 | [M − H]− | 301.2168 | 301.2166 | −0.7 | 257.2310 |
| A9 | FA22:6 | 8.87 | C22H32O2 | [M − H]− | 327.2324 | 327.2333 | 2.8 | 283.2430 |
| A10 | FA18:4 methyl ester | 10.05 | C19H30O2 | [M + H]+ | 291.2324 | 291.2326 | 0.7 | 277.2188, 259.2075 |
| A11 | FA20:5 methyl ester | 10.99 | C21H32O2 | [M + H]+ | 317.2481 | 317.2491 | 3.2 | 303.2322, 285.2230 |
| A12 | FA22:6 methyl ester | 11.90 | C23H34O2 | [M + H]+ | 343.2637 | 343.2640 | 0.9 | 329.2458, 311.2375 |
| A13 | FA22:6 ethyl ester | 12.91 | C24H36O2 | [M + H]+ | 357.2794 | 357.2792 | −0.6 | 329.2467, 311.2376 |
| A14 * | FA18:4 ester | 8.14 | C22H36O3 | [M + H]+ | 349.2743 | 349.2745 | 0.8 | 277.2171, 259.2068 |
| A15 * | FA18:4 ester | 12.15 | C21H34O2 | [M + H]+ | 319.2637 | 319.2630 | −2.2 | 277.2159, 259.2058 |
| A16 * | FA20:5 ester | 5.88 | C22H30O2 | [M + H]+ | 327.2324 | 327.2325 | 0.3 | 303.2325, 285.2246 |
| A17 * | FA20:5 ester | 8.65 | C23H36O3 | [M + H]+ | 361.2743 | 361.2737 | −0.6 | 303.2322, 285.2216 |
| A18 * | FA20:5 ester | 9.03 | C24H38O3 | [M + H]+ | 375.2899 | 375.2899 | −0.0 | 303.2322, 285.2216 |
| No. | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 173.8 | 174.2 | ||
| 2 | 33.9 | 2.31 (2H, t, 15.2) | 33.8 | 2.32 (2H, t, 15.2) |
| 3 | 25.0 | 1.69 (2H, m) | 25.0 | 1.69 (2H, m) |
| 4 | 26.7 | 2.12 (2H, m) | 26.7 | 2.12 (2H, m) |
| 5, 6, 8, 9, 11, 12, 14, 15, 17, 18 | 127.1–132.2 | 5.28–5.43 (10H, m) | 127.2–132.2 | 5.28–5.43 (10H, m) |
| 7, 10, 13, 16 | 25.7–25.8 | 2.79–2.85 (8H, m) | 25.7–25.8 | 2.79–2.86 (8H, m) |
| 19 | 20.7 | 2.05 (2H, m) | 20.7 | 2.05 (2H, m) |
| 20 | 14.4 | 0.97 (3H, t, 15.2) | 14.4 | 0.97 (3H, t, 15.2) |
| 1′ | 64.2 | 4.10 (2H, t, 12.8) | 61.4 | 4.24 (2H, t, 12.0) |
| 2′ | 25.3 | 1.75 (2H, m) | 31.9 | 1.87 (2H, m) |
| 3′ | 29.3 | 1.60 (2H, m) | 59.4 | 3.69 (2H, t, 12.0) |
| 4′ | 62.5 | 3.67 (2H, t, 12.4) | ||
| No. | 3 | 4 | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 173.9 | 173.4 | ||
| 2 | 34.4 | 2.31 (2H, t, 14.8) | 34.7 | 2.31 (2H, t, 15.2) |
| 3 | 24.8 | 1.66 (2H, m) | 24.8 | 1.66 (2H, m) |
| 4 | 29.3 | 1.39 (2H, m) | 29.2 | 1.42 (2H, m) |
| 5 | 27.0 | 2.09 (2H, m) | 27.0 | 2.12 (2H, m) |
| 6, 7, 9, 10, 12, 13, 15, 16 | 127.2–132.2 | 5.28–5.43 (8H, m) | 127.2–132.2 | 5.31–5.46 (8H, m) |
| 8, 11, 14 | 25.7–25.8 | 2.83 (6H, m) | 25.7–25.8 | 2.86 (6H, m) |
| 17 | 20.7 | 2.05 (2H, m) | 20.7 | 2.08 (2H, m) |
| 18 | 14.4 | 0.98 (3H, t, 15.2) | 14.4 | 1.00 (3H, t, 15.2) |
| 1′ | 64.2 | 4.11 (2H, t, 12.8) | 67.5 | 5.03 (1H, m) |
| 2′ | 25.3 | 1.75 (2H, m) | 22.0 | 1.24 (3H, s) |
| 3′ | 29.3 | 1.60 (2H, m) | 22.0 | 1.26 (3H, s) |
| 4′ | 62.6 | 3.68 (2H, t, 12.4) | ||
| Gene | Sequences (5′-3′) |
|---|---|
| Il-1β | F: ATGATGGCTTATTACAGTGGCAA R: GTCGGAGATTCGTAGCTGGA |
| Il-6 | F: TCCTTCTCCACAAACATGTAACAA R: TCACCAGGCAAGTCTCCTCA |
| GAPDH | F: ACACCCACTCCTCCACCTTTG R: TCCACCACCCTGTTGCTGTAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, C.-R.; Jiang, M.-X.; Tian, D.-M.; Lu, W.; Wu, B.; Tang, J.-S.; Zou, Y.; Lv, S.-H.; Yao, X.-S. Targeted Isolation of ω-3 Polyunsaturated Fatty Acids from the Marine Dinoflagellate Prorocentrum lima Using DeepSAT and LC-MS/MS and Their High Activity in Promoting Microglial Functions. Mar. Drugs 2025, 23, 286. https://doi.org/10.3390/md23070286
Lai C-R, Jiang M-X, Tian D-M, Lu W, Wu B, Tang J-S, Zou Y, Lv S-H, Yao X-S. Targeted Isolation of ω-3 Polyunsaturated Fatty Acids from the Marine Dinoflagellate Prorocentrum lima Using DeepSAT and LC-MS/MS and Their High Activity in Promoting Microglial Functions. Marine Drugs. 2025; 23(7):286. https://doi.org/10.3390/md23070286
Chicago/Turabian StyleLai, Chang-Rong, Meng-Xing Jiang, Dan-Mei Tian, Wei Lu, Bin Wu, Jin-Shan Tang, Yi Zou, Song-Hui Lv, and Xin-Sheng Yao. 2025. "Targeted Isolation of ω-3 Polyunsaturated Fatty Acids from the Marine Dinoflagellate Prorocentrum lima Using DeepSAT and LC-MS/MS and Their High Activity in Promoting Microglial Functions" Marine Drugs 23, no. 7: 286. https://doi.org/10.3390/md23070286
APA StyleLai, C.-R., Jiang, M.-X., Tian, D.-M., Lu, W., Wu, B., Tang, J.-S., Zou, Y., Lv, S.-H., & Yao, X.-S. (2025). Targeted Isolation of ω-3 Polyunsaturated Fatty Acids from the Marine Dinoflagellate Prorocentrum lima Using DeepSAT and LC-MS/MS and Their High Activity in Promoting Microglial Functions. Marine Drugs, 23(7), 286. https://doi.org/10.3390/md23070286








