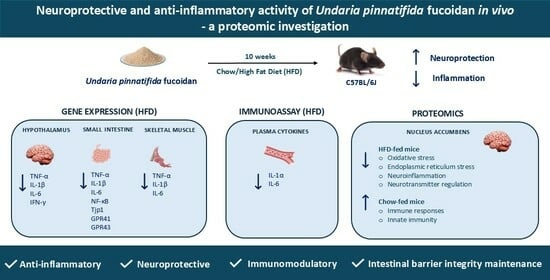
Neuroprotective and Anti-Inflammatory Activity of Undaria pinnatifida Fucoidan In Vivo—A Proteomic Investigation
Abstract
1. Introduction
2. Results
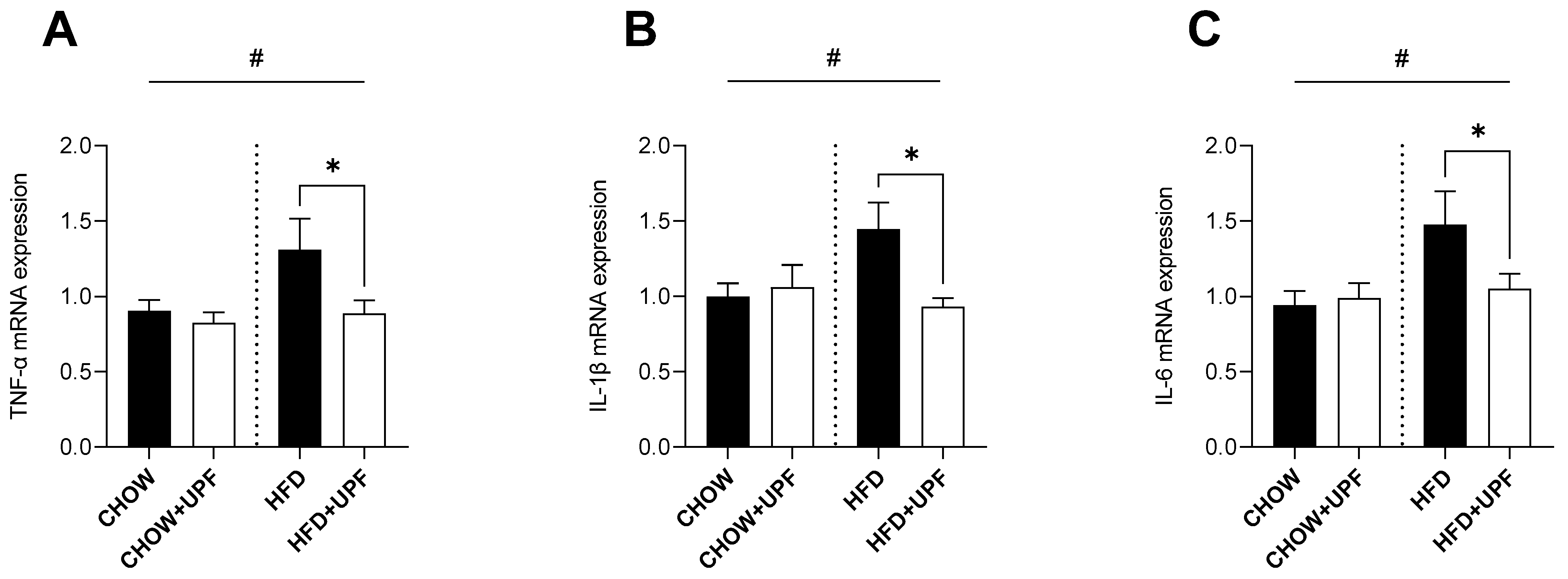
2.1. Effects of HFD and UPF on Skeletal Muscle Gene Expression
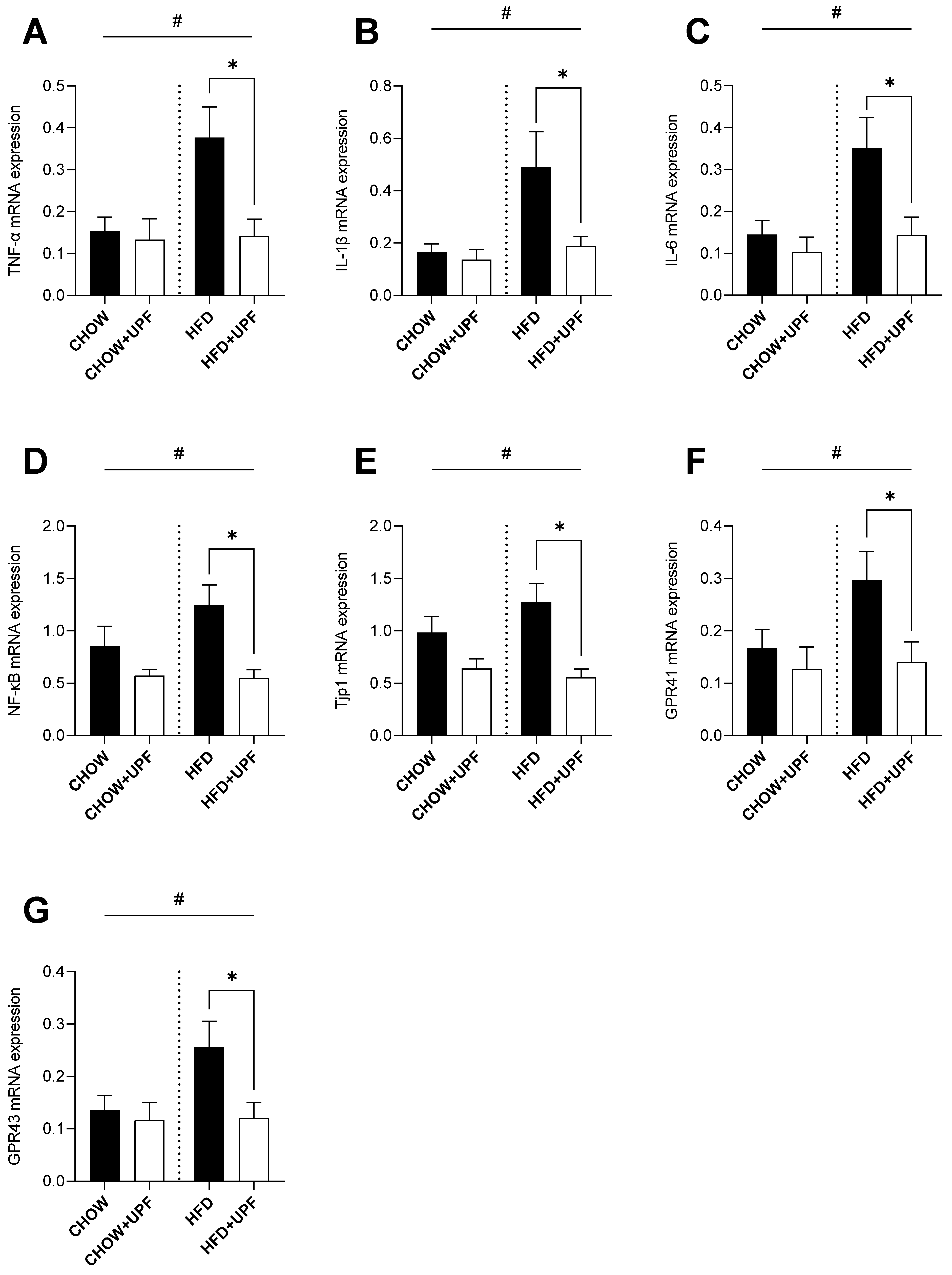
2.2. Effects of HFD and UPF on Small Intestine Gene Expression
2.3. Effects of HFD and UPF on Hypothalamic Gene Expression
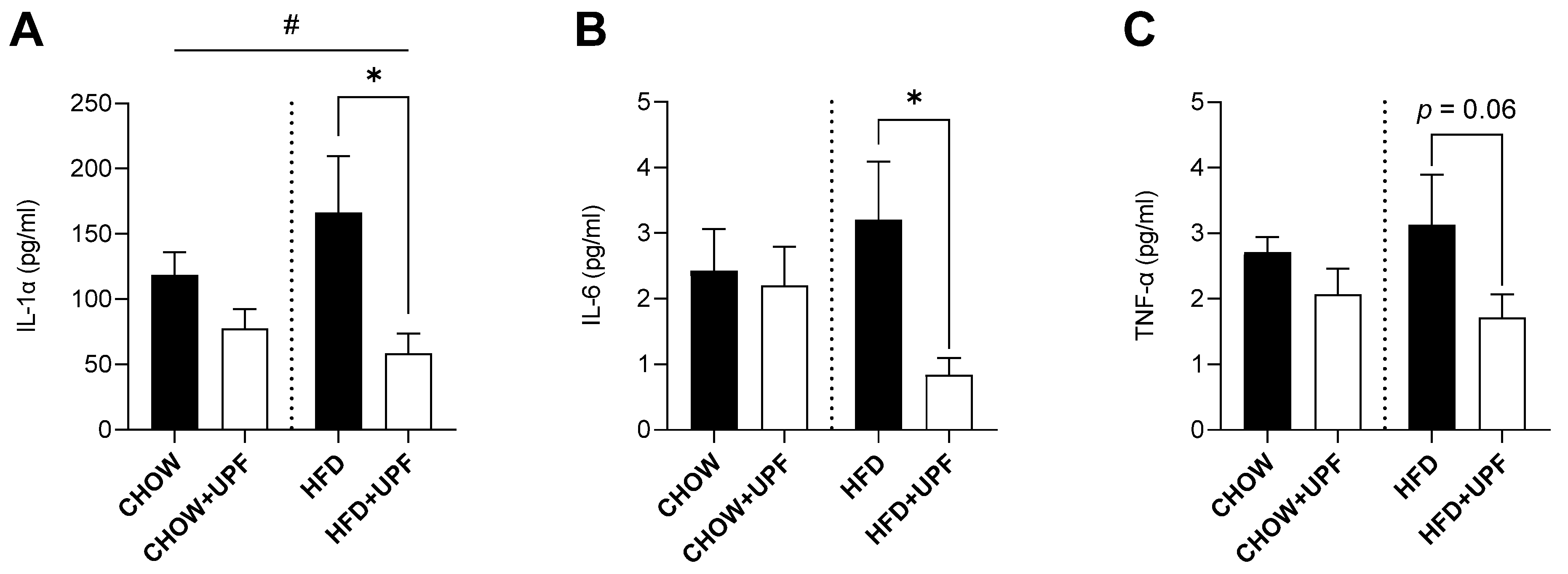
2.4. Effects of HFD and UPF on Pro-Inflammatory Plasma Cytokine Levels
2.5. Effects of UPF on NAc Protein Abundance
2.5.1. Expression Profiles of Differentially Expressed Proteins (DEPs) in the NAc
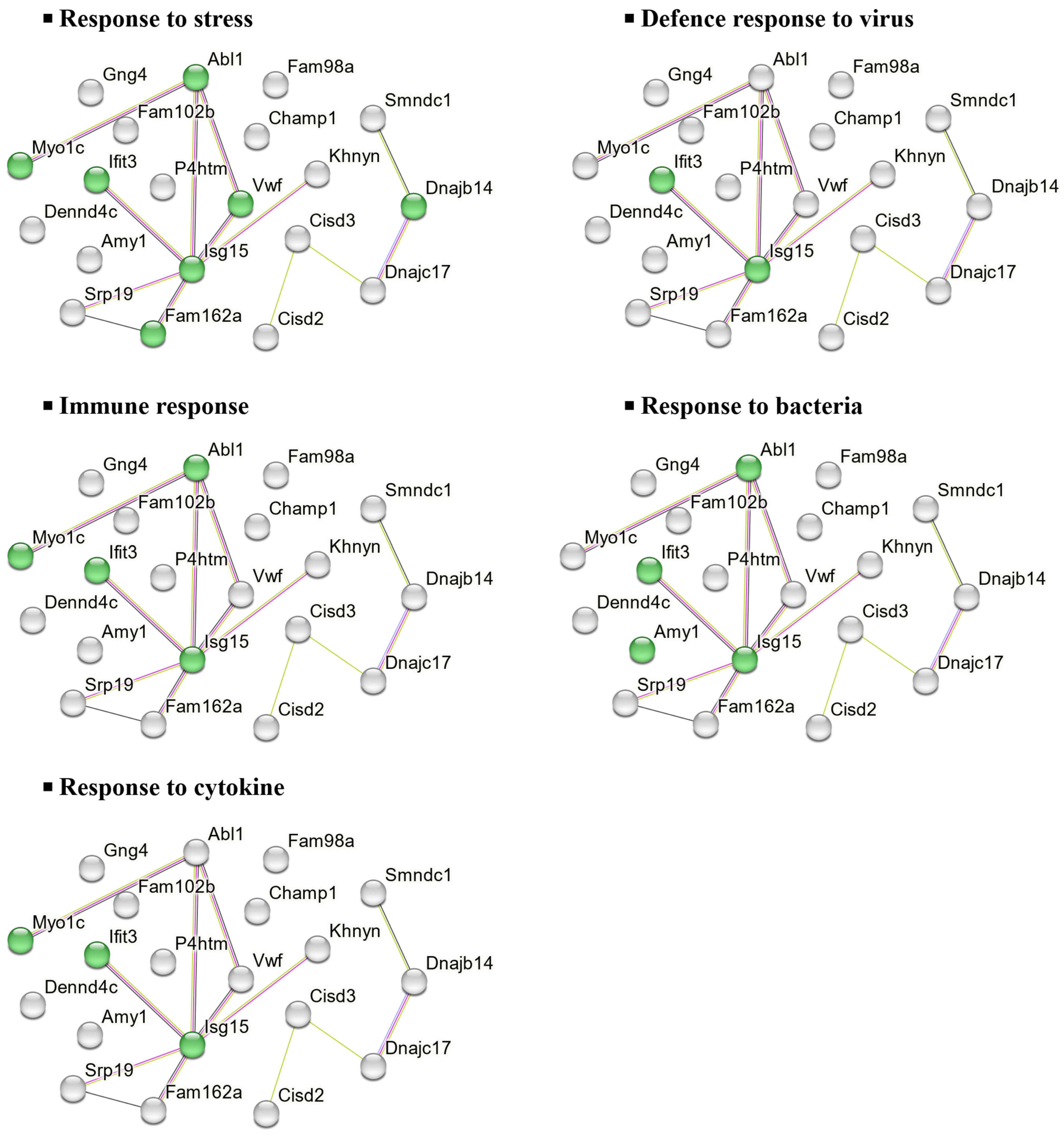
2.5.2. Molecular Functions and Biological Implications of DEPs in the NAc
3. Discussion
3.1. Inflammatory Markers and Gene Expression Studies
3.2. Proteomics: Effects of UPF in Mice Consuming Standard Chow
3.3. Proteomics: Effects of UPF in Mice Consuming HFD
3.4. Limitations
3.5. Conclusions
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals and Diet
4.3. Fucoidan Administration and Experimental Groups
4.4. Sample Collection
4.5. Real-Time Quantitative PCR (RT-qPCR) Assay
4.6. Proteomic Analysis
4.7. Proteomics Data Processing and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, J.; Alves, C.; Soledade, F.; Martins, A.; Pinteus, S.; Gaspar, H.; Alfonso, A.; Pedrosa, R. Marine-Derived Components: Can They Be a Potential Therapeutic Approach to Parkinson’s Disease? Mar. Drugs 2023, 21, 451. [Google Scholar] [CrossRef]
- Elbandy, M. Anti-Inflammatory Effects of Marine Bioactive Compounds and Their Potential as Functional Food Ingredients in the Prevention and Treatment of Neuroinflammatory Disorders. Molecules 2023, 28, 2. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Li, Y.; White, W.L.; Lu, J. Extracts from New Zealand Undaria pinnatifida Containing Fucoxanthin as Potential Functional Biomaterials against Cancer in Vitro. J. Funct. Biomater. 2014, 5, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, P.; Wang, H.; Li, Q.; Teng, H.; Liu, Z.; Yang, W.; Hou, L.; Zou, X. Fucoidan Derived from Undaria pinnatifida Induces Apoptosis in Human Hepatocellular Carcinoma SMMC-7721 Cells via the ROS-Mediated Mitochondrial Pathway. Mar. Drugs 2013, 11, 1961–1976. [Google Scholar] [CrossRef]
- Mak, W.; Hamid, N.; Liu, T.; Lu, J.; White, W.L. Fucoidan from New Zealand Undaria pinnatifida: Monthly variations and determination of antioxidant activities. Carbohydr. Polym. 2013, 95, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Phull, A.-R.; Majid, M.; Haq, I.U.; Khan, M.R.; Kim, S.J. In vitro and in vivo evaluation of anti-arthritic, antioxidant efficacy of fucoidan from Undaria pinnatifida (Harvey) Suringar. Int. J. Biol. Macromol. 2017, 97, 468–480. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Y.; Wang, J.; Ma, S.; Yu, Y.; White, W.L.; Yang, S.; Yang, F.; Lu, J. Fucoidan Extracted from Undaria pinnatifida: Source for Nutraceuticals/Functional Foods. Mar. Drugs 2018, 16, 321. [Google Scholar] [CrossRef]
- Lee, J.B.; Hayashi, K.; Hashimoto, M.; Nakano, T.; Hayashi, T. Novel antiviral fucoidan from sporophyll of Undaria pinnatifida (Mekabu). Chem. Pharm. Bull. 2004, 52, 1091–1094. [Google Scholar] [CrossRef]
- Cooper, R.; Dragar, C.; Elliot, K.; Fitton, J.H.; Godwin, J.; Thompson, K. GFS, A Preparation of Tasmanian Undaria pinnatifida Is Associated with Healing and Inhibition of Reactivation of Herpes. BMC Complement. Altern. Med. 2002, 2, 11. [Google Scholar] [CrossRef]
- Herath, K.H.I.N.M.; Kim, H.J.; Kim, A.; Sook, C.E.; Lee, B.-Y.; Jee, Y. The Role of Fucoidans Isolated from the Sporophylls of Undaria pinnatifida against Particulate-Matter-Induced Allergic Airway Inflammation: Evidence of the Attenuation of Oxidative Stress and Inflammatory Responses. Molecules 2020, 25, 2869. [Google Scholar] [CrossRef]
- Shanmugasundaram, D.; Dwan, C.; Wimmer, B.C.; Srivastava, S. Fucoidan Ameliorates Testosterone-Induced Benign Prostatic Hyperplasia (BPH) in Rats. Res. Rep. Urol. 2024, 16, 283–297. [Google Scholar] [CrossRef]
- Ahmad, T.; Eapen, M.S.; Ishaq, M.; Park, A.Y.; Karpiniec, S.S.; Stringer, D.N.; Sohal, S.S.; Fitton, J.H.; Guven, N.; Caruso, V.; et al. Anti-Inflammatory Activity of Fucoidan Extracts In Vitro. Mar. Drugs 2021, 19, 702. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zeng, L.; Zheng, C.; Song, B.; Li, F.; Kong, X.; Xu, K. Inflammatory Links Between High Fat Diets and Diseases. Front. Immunol. 2018, 9, 2649. [Google Scholar] [CrossRef]
- Bae, H.R.; Shin, S.-K.; Yoo, J.-H.; Kim, S.; Young, H.A.; Kwon, E.-Y. Chronic inflammation in high-fat diet-fed mice: Unveiling the early pathogenic connection between liver and adipose tissue. J. Autoimmun. 2023, 139, 103091. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, T.; Wu, J.; Zhao, L.; Li, Q.; Varghese, Z.; Moorhead, J.F.; Powis, S.H.; Chen, Y.; Ruan, X.Z. Chronic inflammation exacerbates glucose metabolism disorders in C57BL/6J mice fed with high-fat diet. J. Endocrinol. 2013, 219, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Foundations of Immunometabolism and Implications for Metabolic Health and Disease. Immunity 2017, 47, 406–420. [Google Scholar] [CrossRef]
- Heydemann, A. An Overview of Murine High Fat Diet as a Model for Type 2 Diabetes Mellitus. J. Diabetes Res. 2016, 2016, 2902351. [Google Scholar] [CrossRef]
- Yang, C.; Dwan, C.; Wimmer, B.C.; Wilson, R.; Johnson, L.; Caruso, V. Fucoidan from Undaria pinnatifida Enhances Exercise Performance and Increases the Abundance of Beneficial Gut Bacteria in Mice. Mar. Drugs 2024, 22, 485. [Google Scholar] [CrossRef]
- Kim, J.; Moon, I.S.; Goo, T.W.; Moon, S.S.; Seo, M. Algae Undaria pinnatifida Protects Hypothalamic Neurons against Endoplasmic Reticulum Stress through Akt/mTOR Signaling. Molecules 2015, 20, 20998–21009. [Google Scholar] [CrossRef]
- Azzini, E.; Peña-Corona, S.I.; Hernández-Parra, H.; Chandran, D.; Saleena, L.A.K.; Sawikr, Y.; Peluso, I.; Dhumal, S.; Kumar, M.; Leyva-Gómez, G.; et al. Neuroprotective and anti-inflammatory effects of curcumin in Alzheimer’s disease: Targeting neuroinflammation strategies. Phytother. Res. 2024, 38, 3169–3189. [Google Scholar] [CrossRef]
- Wei, H.; Gao, Z.; Zheng, L.; Zhang, C.; Liu, Z.; Yang, Y.; Teng, H.; Hou, L.; Yin, Y.; Zou, X. Protective Effects of Fucoidan on Aβ25-35 and d-Gal-Induced Neurotoxicity in PC12 Cells and d-Gal-Induced Cognitive Dysfunction in Mice. Mar. Drugs 2017, 15, 77. [Google Scholar] [CrossRef] [PubMed]
- Alghazwi, M.; Smid, S.; Karpiniec, S.; Zhang, W. Comparative study on neuroprotective activities of fucoidans from Fucus vesiculosus and Undaria pinnatifida. Int. J. Biol. Macromol. 2019, 122, 255–264. [Google Scholar] [CrossRef]
- Gueven, N.; Spring, K.J.; Holmes, S.; Ahuja, K.; Eri, R.; Park, A.Y.; Fitton, J.H. Micro RNA Expression after Ingestion of Fucoidan; A Clinical Study. Mar. Drugs 2020, 18, 143. [Google Scholar] [CrossRef]
- McFadden, B.A.; Vincenty, C.S.; Chandler, A.J.; Cintineo, H.P.; Lints, B.S.; Mastrofini, G.F.; Arent, S.M. Effects of fucoidan supplementation on inflammatory and immune response after high-intensity exercise. J. Int. Soc. Sports Nutr. 2023, 20, 2224751. [Google Scholar] [CrossRef] [PubMed]
- Tay, A.; Jiang, Y.; Signal, N.; O’Brien, D.; Chen, J.; Murphy, R.; Lu, J. Combining mussel with fucoidan as a supplement for joint pain and prediabetes: Study protocol for a randomized, double-blinded, placebo-controlled trial. Front. Nutr. 2022, 9, 1000510. [Google Scholar] [CrossRef]
- Barbier, L.; Ferhat, M.; Salamé, E.; Robin, A.; Herbelin, A.; Gombert, J.-M.; Silvain, C.; Barbarin, A. Interleukin-1 Family Cytokines: Keystones in Liver Inflammatory Diseases. Front. Immunol. 2019, 10, 2014. [Google Scholar] [CrossRef]
- Bleau, C.; Karelis, A.D.; St-Pierre, D.H.; Lamontagne, L. Crosstalk between intestinal microbiota, adipose tissue and skeletal muscle as an early event in systemic low-grade inflammation and the development of obesity and diabetes. Diabetes/Metab. Res. Rev. 2015, 31, 545–561. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.; Kesper, M.S.; Marschner, J.A.; Konrad, L.; Ryu, M.; Kumar VR, S.; Kulkarni, O.P.; Mulay, S.R.; Romoli, S.; Demleitner, J.; et al. Intestinal Dysbiosis, Barrier Dysfunction, and Bacterial Translocation Account for CKD–Related Systemic Inflammation. J. Am. Soc. Nephrol. 2017, 28, 76–83. [Google Scholar] [CrossRef]
- Cole, C.L.; Kleckner, I.R.; Jatoi, A.; Schwarz, E.M.; Dunne, R.F. The Role of Systemic Inflammation in Cancer-Associated Muscle Wasting and Rationale for Exercise as a Therapeutic Intervention. JCSM Clin. Rep. 2018, 3, 1–19. [Google Scholar] [CrossRef]
- Burfeind, K.G.; Michaelis, K.A.; Marks, D.L. The central role of hypothalamic inflammation in the acute illness response and cachexia. Semin. Cell Dev. Biol. 2016, 54, 42–52. [Google Scholar] [CrossRef]
- Perry, V.H. The influence of systemic inflammation on inflammation in the brain: Implications for chronic neurodegenerative disease. Brain Behav. Immun. 2004, 18, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, C.S.L.; Thang, L.A.N.; Maier, A.B. Markers of inflammation and their association with muscle strength and mass: A systematic review and meta-analysis. Ageing Res. Rev. 2020, 64, 101185. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Hsu, W.L.; Hwang, P.A.; Chen, Y.L.; Chou, T.C. Combined administration of fucoidan ameliorates tumor and chemotherapy-induced skeletal muscle atrophy in bladder cancer-bearing mice. Oncotarget 2016, 7, 51608–51618. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, T.; Chen, X.; You, H.; Zhang, Q.; Xue, J.; Zheng, Y.; Luo, D. Low molecular weight fucoidan ameliorates hindlimb ischemic injury in type 2 diabetic rats. J. Ethnopharmacol. 2018, 210, 434–442. [Google Scholar] [CrossRef]
- Iraha, A.; Chinen, H.; Hokama, A.; Yonashiro, T.; Kinjo, T.; Kishimoto, K.; Nakamoto, M.; Hirata, T.; Kinjo, N.; Higa, F.; et al. Fucoidan enhances intestinal barrier function by upregulating the expression of claudin-1. World J. Gastroenterol. 2013, 19, 5500–5507. [Google Scholar] [CrossRef]
- Lean, Q.Y.; Eri, R.D.; Fitton, J.H.; Patel, R.P.; Gueven, N. Fucoidan Extracts Ameliorate Acute Colitis. PLoS ONE 2015, 10, e0128453. [Google Scholar] [CrossRef]
- Choi, J.-I.; Raghavendran, H.R.B.; Sung, N.-Y.; Kim, J.-H.; Chun, B.S.; Ahn, D.H.; Choi, H.-S.; Kang, K.-W.; Lee, J.-W. Effect of fucoidan on aspirin-induced stomach ulceration in rats. Chem. Biol. Interact. 2010, 183, 249–254. [Google Scholar] [CrossRef]
- Tang, S.; Wang, L.; Ren, X.; Song, S.; Ai, C. Fucoidan Alleviates Colitis and Metabolic Disorder by Protecting the Intestinal Barrier, Suppressing the MAPK/NF-κB Pathways, and Regulating the Gut Microbiota. J. Food Biochem. 2024, 2024, 7955190. [Google Scholar] [CrossRef]
- Selim, H.M.; Negm, W.A.; Hawwal, M.F.; Hussein, I.A.; Elekhnawy, E.; Ulber, R.; Zayed, A. Fucoidan mitigates gastric ulcer injury through managing inflammation, oxidative stress, and NLRP3-mediated pyroptosis. Int. Immunopharmacol. 2023, 120, 110335. [Google Scholar] [CrossRef]
- Zong, X.; Cao, X.; Wang, H.; Xiao, X.; Wang, Y.; Lu, Z. Cathelicidin-WA Facilitated Intestinal Fatty Acid Absorption Through Enhancing PPAR-γ Dependent Barrier Function. Front. Immunol. 2019, 10, 1674. [Google Scholar] [CrossRef]
- Landy, J.; Ronde, E.; English, N.; Clark, S.K.; Hart, A.L.; Knight, S.C.; Ciclitira, P.J.; Al-Hassi, H.O. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J. Gastroenterol. 2016, 22, 3117–3126. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Liang, H.; Xue, M.; Liu, Y.; Gong, A.; Jiang, Y.; Qin, Y.; Yang, J.; Meng, D. Protective effect and mechanism of fucoidan on intestinal mucosal barrier function in NOD mice. Food Agric. Immunol. 2020, 31, 939–953. [Google Scholar] [CrossRef]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-Chain Fatty Acids Activate GPR41 and GPR43 on Intestinal Epithelial Cells to Promote Inflammatory Responses in Mice. Gastroenterology 2013, 145, 396–406.e310. [Google Scholar] [CrossRef] [PubMed]
- Ang, Z.; Ding, J.L. GPR41 and GPR43 in Obesity and Inflammation - Protective or Causative? Front. Immunol. 2016, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Di, Y.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Chang, G.; Zhang, H.; Wang, Y.; Ma, N.; Chandra, R.A.; Ye, G.; Zhuang, S.; Zhu, W.; Shen, X. Microbial community shifts elicit inflammation in the caecal mucosa via the GPR41/43 signalling pathway during subacute ruminal acidosis. BMC Vet. Res. 2019, 15, 298. [Google Scholar] [CrossRef]
- Wang, J.J.; Zhang, Q.M.; Ni, W.W.; Zhang, X.; Li, Y.; Li, A.L.; Du, P.; Li, C.; Yu, S.S. Modulatory effect of Lactobacillus acidophilus KLDS 1.0738 on intestinal short-chain fatty acids metabolism and GPR41/43 expression in β-lactoglobulin-sensitized mice. Microbiol. Immunol. 2019, 63, 303–315. [Google Scholar] [CrossRef]
- Lee, C.H.; Suk, K.; Yu, R.; Kim, M.S. Cellular Contributors to Hypothalamic Inflammation in Obesity. Mol. Cells 2020, 43, 431–437. [Google Scholar] [CrossRef]
- Cavaliere, G.; Viggiano, E.; Trinchese, G.; De Filippo, C.; Messina, A.; Monda, V.; Valenzano, A.; Cincione, R.I.; Zammit, C.; Cimmino, F.; et al. Long Feeding High-Fat Diet Induces Hypothalamic Oxidative Stress and Inflammation, and Prolonged Hypothalamic AMPK Activation in Rat Animal Model. Front. Physiol. 2018, 9, 818. [Google Scholar] [CrossRef]
- Ishaq, M.; Tran, D.; Yang, C.; Ng, M.J.; Kackanattil, A.; Tata, K.; Deans, B.J.; Bleasel, M.; Vicenzi, S.; Randall, C.; et al. The Anti-Obesity Compound Asperuloside Reduces Inflammation in the Liver and Hypothalamus of High-Fat-Fed Mice. Endocrines 2022, 3, 641–653. [Google Scholar] [CrossRef]
- Jais, A.; Brüning, J.C. Hypothalamic inflammation in obesity and metabolic disease. J. Clin. Investig. 2017, 127, 24–32. [Google Scholar] [CrossRef]
- Le Thuc, O.; Stobbe, K.; Cansell, C.; Nahon, J.-L.; Blondeau, N.; Rovère, C. Hypothalamic Inflammation and Energy Balance Disruptions: Spotlight on Chemokines. Front. Endocrinol. 2017, 8, 197. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, K.; Ding, X.; Wang, Y.; Bai, H.; Yang, Q.; Ben, J.; Zhang, H.; Li, X.; Chen, Q.; et al. Fucoidan antagonizes diet-induced obesity and inflammation in mice. J. Biomed. Res. 2020, 35, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Huang, J.; Li, Y.; Lv, H.; Yin, T.; Fan, S.; Zhang, C.; Li, H. Fucoidan Protects Against High-Fat Diet-Induced Obesity and Modulates Gut Microbiota in Institute of Cancer Research Mice. J. Med. Food. 2021, 24, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Ambroggi, F.; Ghazizadeh, A.; Nicola, S.M.; Fields, H.L. Roles of Nucleus Accumbens Core and Shell in Incentive-Cue Responding and Behavioral Inhibition. J. Neurosci. 2011, 31, 6820–6830. [Google Scholar] [CrossRef]
- Perng, Y.-C.; Lenschow, D.J. ISG15 in antiviral immunity and beyond. Nat. Rev. Microbiol. 2018, 16, 423–439. [Google Scholar] [CrossRef]
- Przanowski, P.; Loska, S.; Cysewski, D.; Dabrowski, M.; Kaminska, B. ISG’ylation increases stability of numerous proteins including Stat1, which prevents premature termination of immune response in LPS-stimulated microglia. Neurochem. Int. 2018, 112, 227–233. [Google Scholar] [CrossRef]
- Hwang, M.; Bergmann, C.C. Alpha/Beta Interferon (IFN-α/β) Signaling in Astrocytes Mediates Protection against Viral Encephalomyelitis and Regulates IFN-γ-Dependent Responses. J. Virol. 2018, 92, e01901-17. [Google Scholar] [CrossRef]
- Swaim, C.D.; Scott, A.F.; Canadeo, L.A.; Huibregtse, J.M. Extracellular ISG15 Signals Cytokine Secretion through the LFA-1 Integrin Receptor. Mol. Cell. 2017, 68, 581–590.e585. [Google Scholar] [CrossRef]
- Mears, H.V.; Sweeney, T.R. Better together: The role of IFIT protein–protein interactions in the antiviral response. J. Gen. Virol. 2018, 99, 1463–1477. [Google Scholar] [CrossRef]
- Gutiérrez, D.A.; Chandía-Cristi, A.; Yáñez, M.J.; Zanlungo, S.; Álvarez, A.R. c-Abl kinase at the crossroads of healthy synaptic remodeling and synaptic dysfunction in neurodegenerative diseases. Neural Regen. Res. 2023, 18, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Hebron, M.L.; Lonskaya, I.; Olopade, P.; Selby, S.T.; Pagan, F.; Moussa, C.E. Tyrosine Kinase Inhibition Regulates Early Systemic Immune Changes and Modulates the Neuroimmune Response in α-Synucleinopathy. J. Clin. Cell Immunol. 2014, 5, 259. [Google Scholar] [CrossRef]
- Brahmachari, S.; Ge, P.; Lee, S.H.; Kim, D.; Karuppagounder, S.S.; Kumar, M.; Mao, X.; Shin, J.H.; Lee, Y.; Pletnikova, O.; et al. Activation of tyrosine kinase c-Abl contributes to α-synuclein–induced neurodegeneration. J. Clin. Investig. 2016, 126, 2970–2988. [Google Scholar] [CrossRef]
- Petri, B.; Broermann, A.; Li, H.; Khandoga, A.G.; Zarbock, A.; Krombach, F.; Goerge, T.; Schneider, S.W.; Jones, C.; Nieswandt, B.; et al. von Willebrand factor promotes leukocyte extravasation. Blood 2010, 116, 4712–4719. [Google Scholar] [CrossRef] [PubMed]
- Gragnano, F.; Sperlongano, S.; Golia, E.; Natale, F.; Bianchi, R.; Crisci, M.; Fimiani, F.; Pariggiano, I.; Diana, V.; Carbone, A.; et al. The Role of von Willebrand Factor in Vascular Inflammation: From Pathogenesis to Targeted Therapy. Mediat. Inflamm. 2017, 2017, 5620314. [Google Scholar] [CrossRef] [PubMed]
- El-Mansi, S.; Mitchell, T.P.; Mobayen, G.; McKinnon, T.A.J.; Miklavc, P.; Frick, M.; Nightingale, T.D. Myosin-1C augments endothelial secretion of von Willebrand factor by linking contractile actomyosin machinery to the plasma membrane. Blood. Adv. 2024, 8, 4714–4726. [Google Scholar] [CrossRef]
- Suidan, G.L.; Brill, A.; De Meyer, S.F.; Voorhees, J.R.; Cifuni, S.M.; Cabral, J.E.; Wagner, D.D. Endothelial Von Willebrand factor promotes blood-brain barrier flexibility and provides protection from hypoxia and seizures in mice. Arter. Thromb. Vasc. Biol. 2013, 33, 2112–2120. [Google Scholar] [CrossRef]
- Bongers, T.N.; de Maat, M.P.M.; van Goor, M.-L.P.J.; Bhagwanbali, V.; van Vliet, H.H.D.M.; Gómez García, E.B.; Dippel, D.W.J.; Leebeek, F.W.G. High von Willebrand Factor Levels Increase the Risk of First Ischemic Stroke. Stroke 2006, 37, 2672–2677. [Google Scholar] [CrossRef]
- Zhu, X.; Cao, Y.; Wei, L.; Cai, P.; Xu, H.; Luo, H.; Bai, X.; Lu, L.; Liu, J.-R.; Fan, W.; et al. von Willebrand factor contributes to poor outcome in a mouse model of intracerebral haemorrhage. Sci. Rep. 2016, 6, 35901. [Google Scholar] [CrossRef]
- Maravillas-Montero, J.L.; Gillespie, P.G.; Patiño-López, G.; Shaw, S.; Santos-Argumedo, L. Myosin 1c Participates in B Cell Cytoskeleton Rearrangements, Is Recruited to the Immunologic Synapse, and Contributes to Antigen Presentation. J. Immunol. 2011, 187, 3053–3063. [Google Scholar] [CrossRef]
- Liu, J.; Han, D.; Xuan, J.; Xie, J.; Wang, W.; Zhou, Q.; Chen, K. COP9 signalosome complex is a prognostic biomarker and corresponds with immune infiltration in hepatocellular carcinoma. Aging 2024, 16, 5264–5287. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Sugiyama, A.; Uzawa, K.; Fujisawa, N.; Tashiro, Y.; Tashiro, F. Implication of Trip15/CSN2 in early stage of neuronal differentiation of P19 embryonal carcinoma cells. Dev. Brain Res. 2003, 140, 45–56. [Google Scholar] [CrossRef]
- Zhang, W.; Ni, P.; Mou, C.; Zhang, Y.; Guo, H.; Zhao, T.; Loh, Y.-H.; Chen, L. Cops2 promotes pluripotency maintenance by Stabilizing Nanog Protein and Repressing Transcription. Sci. Rep. 2016, 6, 26804. [Google Scholar] [CrossRef] [PubMed]
- Lykke-Andersen, K.; Schaefer, L.; Menon, S.; Deng, X.W.; Miller, J.B.; Wei, N. Disruption of the COP9 signalosome Csn2 subunit in mice causes deficient cell proliferation, accumulation of p53 and cyclin E, and early embryonic death. Mol. Cell. Biol. 2003, 23, 6790–6797. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, M.A.; Gomez-Cavazos, J.S.; Mei, A.; Lackner, D.H.; Hetzer, M.W. A change in nuclear pore complex composition regulates cell differentiation. Dev. Cell. 2012, 22, 446–458. [Google Scholar] [CrossRef]
- Ding, B.; Sepehrimanesh, M. Nucleocytoplasmic Transport: Regulatory Mechanisms and the Implications in Neurodegeneration. Int. J. Mol. Sci. 2021, 22, 4165. [Google Scholar] [CrossRef]
- Moehle, M.S.; Webber, P.J.; Tse, T.; Sukar, N.; Standaert, D.G.; DeSilva, T.M.; Cowell, R.M.; West, A.B. LRRK2 Inhibition Attenuates Microglial Inflammatory Responses. J. Neurosci. 2012, 32, 1602–1611. [Google Scholar] [CrossRef]
- Mendivil-Perez, M.; Velez-Pardo, C.; Jimenez-Del-Rio, M. Neuroprotective Effect of the LRRK2 Kinase Inhibitor PF-06447475 in Human Nerve-Like Differentiated Cells Exposed to Oxidative Stress Stimuli: Implications for Parkinson’s Disease. Neurochem. Res. 2016, 41, 2675–2692. [Google Scholar] [CrossRef]
- Kim, J.; Pajarillo, E.; Rizor, A.; Son, D.S.; Lee, J.; Aschner, M.; Lee, E. LRRK2 kinase plays a critical role in manganese-induced inflammation and apoptosis in microglia. PLoS ONE 2019, 14, e0210248. [Google Scholar] [CrossRef]
- Wallings, R.L.; Tansey, M.G. LRRK2 regulation of immune-pathways and inflammatory disease. Biochem. Soc. Trans. 2019, 47, 1581–1595. [Google Scholar] [CrossRef]
- Takeda, K.; Inoue, H.; Tanizawa, Y.; Matsuzaki, Y.; Oba, J.; Watanabe, Y.; Shinoda, K.; Oka, Y. WFS1 (Wolfram syndrome 1) gene product: Predominant subcellular localization to endoplasmic reticulum in cultured cells and neuronal expression in rat brain. Hum. Mol. Genet. 2001, 10, 477–484. [Google Scholar] [CrossRef]
- Fonseca, S.G.; Fukuma, M.; Lipson, K.L.; Nguyen, L.X.; Allen, J.R.; Oka, Y.; Urano, F. WFS1 Is a Novel Component of the Unfolded Protein Response and Maintains Homeostasis of the Endoplasmic Reticulum in Pancreatic β-Cells*. J. Biol. Chem. 2005, 280, 39609–39615. [Google Scholar] [CrossRef]
- Rigoli, L.; Bramanti, P.; Di Bella, C.; De Luca, F. Genetic and clinical aspects of Wolfram syndrome 1, a severe neurodegenerative disease. Pediatr. Res. 2018, 83, 921–929. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Endoplasmic Reticulum Stress and the Inflammatory Basis of Metabolic Disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef]
- Li, R.C.; Morris, M.W.; Lee, S.K.; Pouranfar, F.; Wang, Y.; Gozal, D. Neuroglobin protects PC12 cells against oxidative stress. Brain Res. 2008, 1190, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Fiocchetti, M.; Fernandez, V.S.; Montalesi, E.; Marino, M. Neuroglobin: A Novel Player in the Oxidative Stress Response of Cancer Cells. Oxid. Med. Cell. Longev. 2019, 2019, 6315034. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Takahashi, N.; Uchida, H.; Wakasugi, K. Human Neuroglobin Functions as an Oxidative Stress-responsive Sensor for Neuroprotection. J. Biol. Chem. 2012, 287, 30128–30138. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yu, Z.; Cho, K.-S.; Chen, H.; Malik, M.T.A.; Chen, X.; Lo, E.H.; Wang, X.; Chen, D.F. Neuroglobin Is an Endogenous Neuroprotectant for Retinal Ganglion Cells against Glaucomatous Damage. Am. J. Pathol. 2011, 179, 2788–2797. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Z.; Guo, S.; Lee, S.R.; Xing, C.; Zhang, C.; Gao, Y.; Nicholls, D.G.; Lo, E.H.; Wang, X. Effects of neuroglobin overexpression on mitochondrial function and oxidative stress following hypoxia/reoxygenation in cultured neurons. J. Neurosci. Res. 2009, 87, 164–170. [Google Scholar] [CrossRef]
- Alrafiah, A. Thymoquinone Protects Neurons in the Cerebellum of Rats through Mitigating Oxidative Stress and Inflammation Following High-Fat Diet Supplementation. Biomolecules 2021, 11, 165. [Google Scholar] [CrossRef]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohydr. Polym. 2019, 212, 178–185. [Google Scholar] [CrossRef]
- van de Pol, V.; Vos, M.; DeRuiter, M.C.; Goumans, M.J.; de Vries, C.J.M.; Kurakula, K. LIM-only protein FHL2 attenuates inflammation in vascular smooth muscle cells through inhibition of the NFκB pathway. Vasc. Pharmacol. 2020, 125-126, 106634. [Google Scholar] [CrossRef] [PubMed]
- Wixler, V.; Cromme, C.; Retser, E.; Meyer, L.-H.; Smyth, N.; Mühlenberg, K.; Korb-Pap, A.; Koers-Wunrau, C.; Sotsios, Y.; Bassel-Duby, R.; et al. FHL2 regulates the resolution of tissue damage in chronic inflammatory arthritis. Ann. Rheum. Dis. 2015, 74, 2216–2223. [Google Scholar] [CrossRef] [PubMed]
- Alnajar, A.; Nordhoff, C.; Schied, T.; Chiquet-Ehrismann, R.; Loser, K.; Vogl, T.; Ludwig, S.; Wixler, V. The LIM-only protein FHL2 attenuates lung inflammation during bleomycin-induced fibrosis. PLoS ONE 2013, 8, e81356. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, M.; Møller, S.; Hansen, T.; Moens, U.; Van Ghelue, M. The multifunctional roles of the four-and-a-half-LIM only protein FHL2. Cell. Mol. Life. Sci. 2006, 63, 268–284. [Google Scholar] [CrossRef]
- Verset, L.; Feys, L.; Trépant, A.L.; De Wever, O.; Demetter, P. FHL2: A scaffold protein of carcinogenesis, tumour-stroma interactions and treatment response. Histol. Histopathol. 2016, 31, 469–478. [Google Scholar] [CrossRef]
- Kamada, R.; Kudoh, F.; Ito, S.; Tani, I.; Janairo, J.I.B.; Omichinski, J.G.; Sakaguchi, K. Metal-dependent Ser/Thr protein phosphatase PPM family: Evolution, structures, diseases and inhibitors. Pharmacol. Ther. 2020, 215, 107622. [Google Scholar] [CrossRef]
- Health, N.; Council, M.R. Australian Code of Practice for the Care and Use of Animals for Scientific Purposes; Australian Government Publishing Service: Canberra City, Australia, 1997. [Google Scholar]
- Zhang, L. Method for voluntary oral administration of drugs in mice. STAR Protoc. 2021, 2, 100330. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Caruso, V.; Chen, H.; Morris, M.J. Early hypothalamic FTO overexpression in response to maternal obesity–potential contribution to postweaning hyperphagia. PLoS ONE 2011, 6, e25261. [Google Scholar] [CrossRef]
- Bahari, H.; Caruso, V.; Morris, M.J. Late-Onset Exercise in Female Rat Offspring Ameliorates the Detrimental Metabolic Impact of Maternal Obesity. Endocrinology 2013, 154, 3610–3621. [Google Scholar] [CrossRef] [PubMed]
- Caruso, V.; Bahari, H.; Morris, M. The Beneficial Effects of Early Short-Term Exercise in the Offspring of Obese Mothers are Accompanied by Alterations in the Hypothalamic Gene Expression of Appetite Regulators and FTO (Fat Mass and Obesity Associated) Gene. J. Neuroendocrinol. 2013, 25, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.S.; Moggridge, S.; Müller, T.; Sorensen, P.H.; Morin, G.B.; Krijgsveld, J. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 2019, 14, 68–85. [Google Scholar] [CrossRef]
- Chear, S.; Perry, S.; Wilson, R.; Bindoff, A.; Talbot, J.; Ware, T.L.; Grubman, A.; Vickers, J.C.; Pébay, A.; Ruddle, J.B.; et al. Lysosomal alterations and decreased electrophysiological activity in CLN3 disease patient-derived cortical neurons. Dis. Model. Mech. 2022, 15, dmm049651. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bandla, C.; Kundu, D.J.; Kamatchinathan, S.; Bai, J.; Hewapathirana, S.; John, N.S.; Prakash, A.; Walzer, M.; Wang, S.; et al. The PRIDE database at 20 years: 2025 update. Nucleic Acids Res. 2025, 53, D543–D553. [Google Scholar] [CrossRef]




| Protein | UniProt Accession | Gene Name | FDR | FC |
|---|---|---|---|---|
| COP9 signalosome complex subunit 2 | A2AQE4 | Cops2 | 0.030 | 1.9 |
| Nuclear pore membrane glycoprotein 210 | Q9QY81 | Nup210 | 0.031 | 1.8 |
| Centromere protein V | Q9CXS4 | Cenpv | 0.047 | 1.7 |
| Interferon-induced protein with tetratricopeptide repeats 3 | Q64345 | Ifit3 | 0.004 | 0.7 |
| DnaJ homolog subfamily C member 17 | Q91WT4 | Dnajc17 | 0.002 | 0.7 |
| Signal recognition particle 19 kDa protein | Q9D104 | Srp19 | 0.013 | 0.7 |
| DENN domain-containing protein 4C | A6H8H2 | Dennd4c | 0.032 | 0.7 |
| Survival of motor neuron-related-splicing factor 30 | Q8BGT7 | Smndc1 | 0.009 | 0.7 |
| CDGSH iron–sulfur domain-containing protein 2 | Q9CQB5 | Cisd2 | 0.004 | 0.7 |
| Protein FAM102B | Q8BQS4 | Fam102b | 0.003 | 0.6 |
| CDGSH iron–sulfur domain-containing protein 3, mitochondrial | B1AR13 | Cisd3 | 0.044 | 0.6 |
| Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-4 | P50153 | Gng4 | 0.021 | 0.6 |
| Protein KHNYN | Q80U38 | Khnyn | 0.005 | 0.6 |
| Alpha-amylase 1 | P00687 | Amy1 | 0.046 | 0.6 |
| Ubiquitin-like protein ISG15 | Q64339 | Isg15 | 0.036 | 0.5 |
| DnaJ homolog subfamily B member 14 | Q149L6 | Dnajb14 | 0.050 | 0.5 |
| Chromosome alignment-maintaining phosphoprotein 1 | Q8K327 | Champ1 | 0.025 | 0.4 |
| Protein FAM162A | Q9D6U8 | Fam162a | 0.011 | 0.4 |
| Tyrosine-protein kinase ABL1 | P00520 | Abl1 | 0.006 | 0.4 |
| Transmembrane prolyl 4-hydroxylase | Q8BG58 | P4htm | 0.034 | 0.3 |
| Unconventional myosin-Ic | Q9WTI7 | Myo1c | 0.037 | 0.3 |
| von Willebrand factor | Q8CIZ8 | Vwf | 0.009 | 0.2 |
| Protein FAM98A | Q3TJZ6 | Fam98a | 0.015 | 0.2 |
| Protein | UniProt Accession | Gene Name | FDR | FC |
|---|---|---|---|---|
| Protein phosphatase 1J | Q149T7 | Ppm1j | 0.004 | 2.1 |
| Four and a half LIM domains protein 2 | O70433 | Fhl2 | 0.043 | 1.9 |
| YjeF N-terminal domain-containing protein 3 | F6W8I0 | Yjefn3 | 0.044 | 1.9 |
| Muscular LMNA-interacting protein | V9GWW6 | Mlip | 0.047 | 1.7 |
| Rho GTPase-activating protein 6, isoform CRA_b | G3UZI7 | Arhgap6 | 0.012 | 0.7 |
| Synaptic vesicle glycoprotein 2C | Q69ZS6 | Sv2c | 0.022 | 0.7 |
| Sodium-dependent dopamine transporter | Q61327 | Slc6a3 | 0.040 | 0.7 |
| Dehydrogenase/reductase SDR family member 13 | Q5SS80 | Dhrs13 | 0.026 | 0.7 |
| Gamma-synuclein | Q9Z0F7 | Sncg | 0.001 | 0.7 |
| Hephaestin | Q9Z0Z4 | Heph | 0.003 | 0.6 |
| Wolframin | Q3UN10 | Wfs1 | 0.008 | 0.6 |
| ATP-sensitive inward rectifier potassium channel 10 | Q9JM63 | Kcnj10 | 0.014 | 0.6 |
| Lipid phosphate phosphatase-related protein type 1 | Q8BFZ2 | Lppr1 | 0.001 | 0.6 |
| Epidermal growth factor receptor kinase substrate 8-like protein 1 | Q8R5F8 | Eps8l1 | 0.020 | 0.6 |
| Lysosome-associated membrane glycoprotein 5 | Q9D387 | Lamp5 | 0.009 | 0.6 |
| Synaptic vesicular amine transporter | Q8BRU6 | Slc18a2 | 0.049 | 0.6 |
| Inter-alpha-trypsin inhibitor heavy chain H3 | Q61704 | Itih3 | 0.002 | 0.6 |
| Nucleosome assembly protein 1-like 5 | Q9JJF0 | Nap1l5 | 0.001 | 0.6 |
| ProSAAS | Q9QXV0 | Pcsk1n | 0.004 | 0.6 |
| Synaptic vesicle membrane protein VAT-1 homolog-like | Q80TB8 | Vat1l | 0.007 | 0.6 |
| Leucine-rich repeat serine/threonine-protein kinase 2 | Q5S006 | Lrrk2 | 0.036 | 0.6 |
| Proto-oncogene tyrosine-protein kinase receptor Ret | P35546 | Ret | 0.019 | 0.6 |
| Peptidyl-prolyl cis-trans isomerase-like 4 | Q9CXG3 | Ppil4 | 0.042 | 0.6 |
| ERC protein 2 | Q6PH08 | Erc2 | 0.029 | 0.6 |
| Proenkephalin-A | P22005 | Penk | 0.029 | 0.6 |
| Cellular retinoic acid-binding protein 1 | P62965 | Crabp1 | 0.020 | 0.5 |
| Protein FAM102B | Q8BQS4 | Fam102b | 0.038 | 0.5 |
| Protein AW551984 | Q8BGF0 | AW551984 | 0.013 | 0.5 |
| 3-hydroxybutyrate dehydrogenase type 2 | Q8JZV9 | Bdh2 | 0.007 | 0.5 |
| Leukocyte elastase inhibitor B | Q8VHP7 | Serpinb1b | 0.007 | 0.5 |
| BAI1-associated protein 3 | S4R1E7 | Baiap3 | 0.021 | 0.5 |
| Neuroglobin | Q9ER97 | Ngb | 0.001 | 0.4 |
| Protein Krt78 | E9Q0F0 | Krt78 | 0.021 | 0.4 |
| Neurotensin/neuromedin N | Q9D3P9 | Nts | 0.006 | 0.4 |
| Immunoglobulin superfamily member 1 | Q7TQA1 | Igsf1 | 0.010 | 0.3 |
| Rab GTPase-binding effector protein 2 | Q91WG2 | Rabep2 | 0.028 | 0.1 |
| Fucoidan Extract | Neutral Carbohydrates (%) | Sulfate (%) | Fucoidan (%) | Polyphenols (%) |
|---|---|---|---|---|
| UPF2022532 | 46.1 | 28.3 | 89.3 | <2 |
| Fucoidan Extract | Fucose (%) | Xylose (%) | Galactose (%) | Arabinose (%) | Rhamnose (%) |
|---|---|---|---|---|---|
| UPF2022532 | 22.5 | 0.3 | 19 | 0.6 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Dwan, C.; Wimmer, B.C.; Ronci, M.; Wilson, R.; Johnson, L.; Caruso, V. Neuroprotective and Anti-Inflammatory Activity of Undaria pinnatifida Fucoidan In Vivo—A Proteomic Investigation. Mar. Drugs 2025, 23, 189. https://doi.org/10.3390/md23050189
Yang C, Dwan C, Wimmer BC, Ronci M, Wilson R, Johnson L, Caruso V. Neuroprotective and Anti-Inflammatory Activity of Undaria pinnatifida Fucoidan In Vivo—A Proteomic Investigation. Marine Drugs. 2025; 23(5):189. https://doi.org/10.3390/md23050189
Chicago/Turabian StyleYang, Cheng, Corinna Dwan, Barbara C. Wimmer, Maurizio Ronci, Richard Wilson, Luke Johnson, and Vanni Caruso. 2025. "Neuroprotective and Anti-Inflammatory Activity of Undaria pinnatifida Fucoidan In Vivo—A Proteomic Investigation" Marine Drugs 23, no. 5: 189. https://doi.org/10.3390/md23050189
APA StyleYang, C., Dwan, C., Wimmer, B. C., Ronci, M., Wilson, R., Johnson, L., & Caruso, V. (2025). Neuroprotective and Anti-Inflammatory Activity of Undaria pinnatifida Fucoidan In Vivo—A Proteomic Investigation. Marine Drugs, 23(5), 189. https://doi.org/10.3390/md23050189