Bioassay-Guided Procedure Coupled with HR-ESIMS Dereplication for Isolation of Antiproliferative Bromo-Tyramine Derivative from Aplysina cauliformis
Abstract
:1. Introduction
2. Results
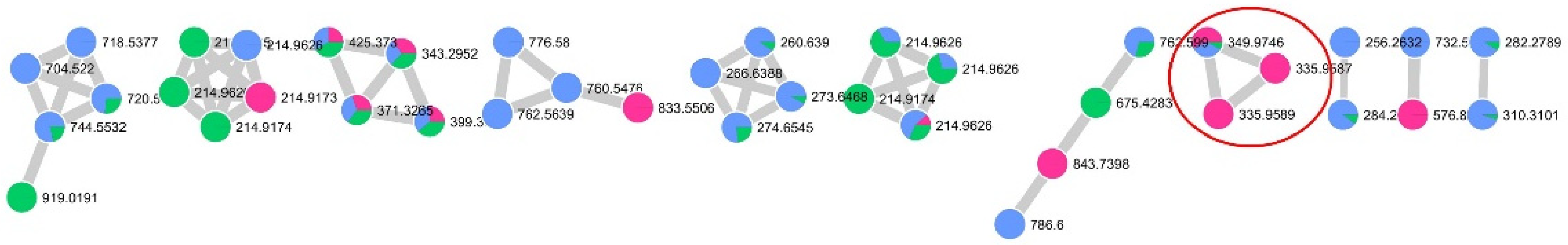
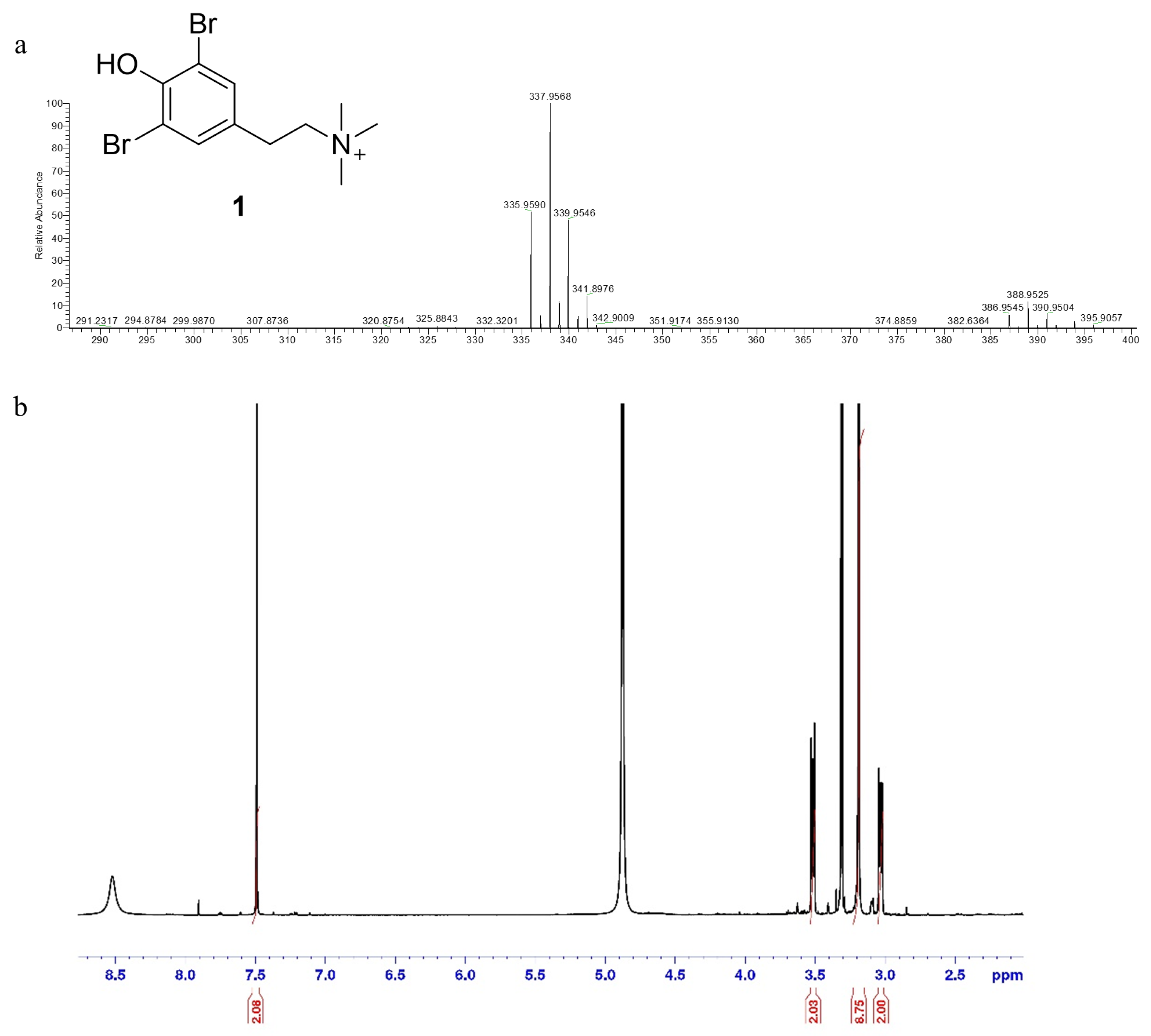
2.1. Bioassay-Guided Procedure Coupled with HR-ESIMS Dereplication for the Isolation of a Bioactive Compound from Aplysina cauliformis
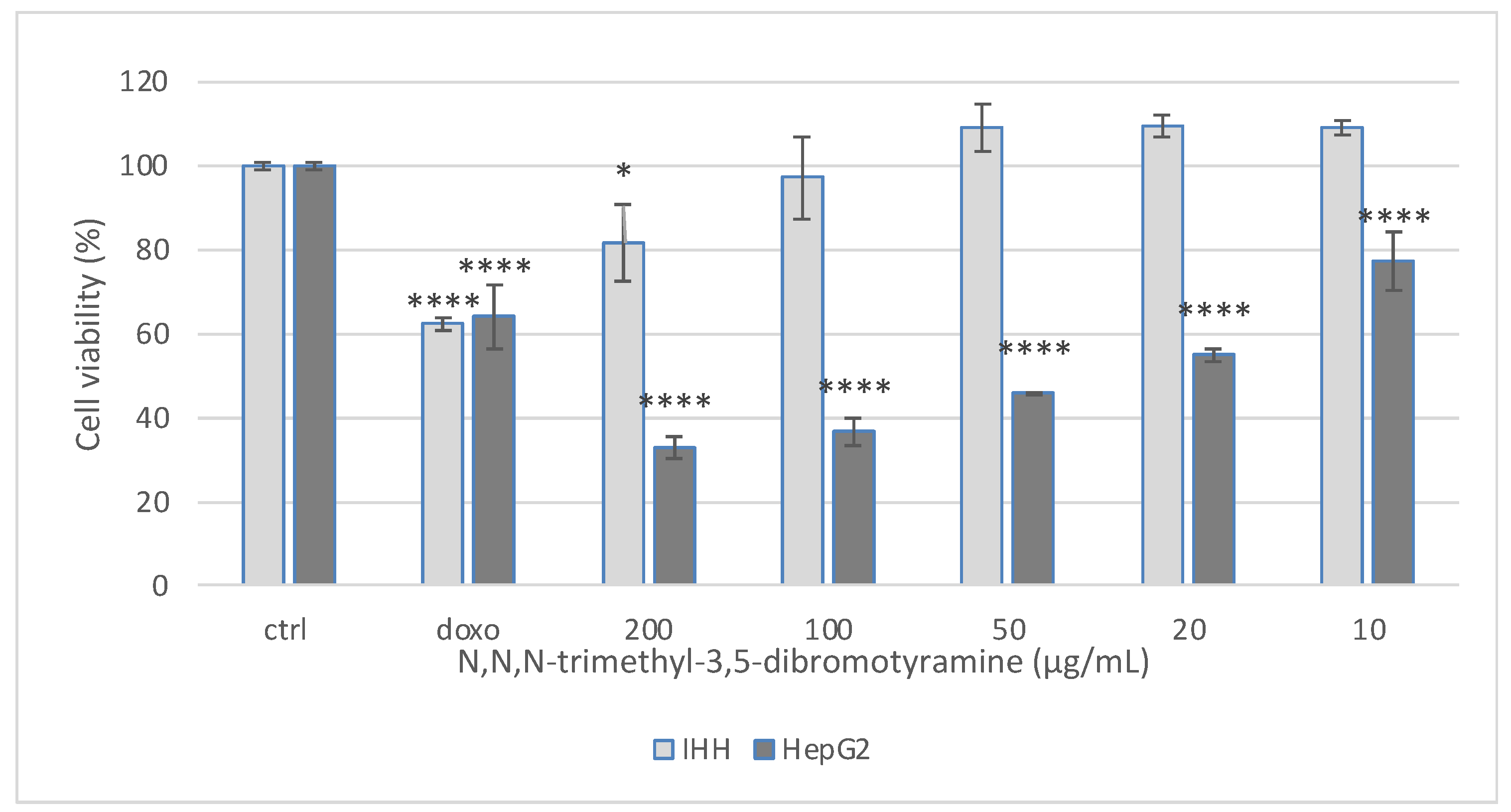
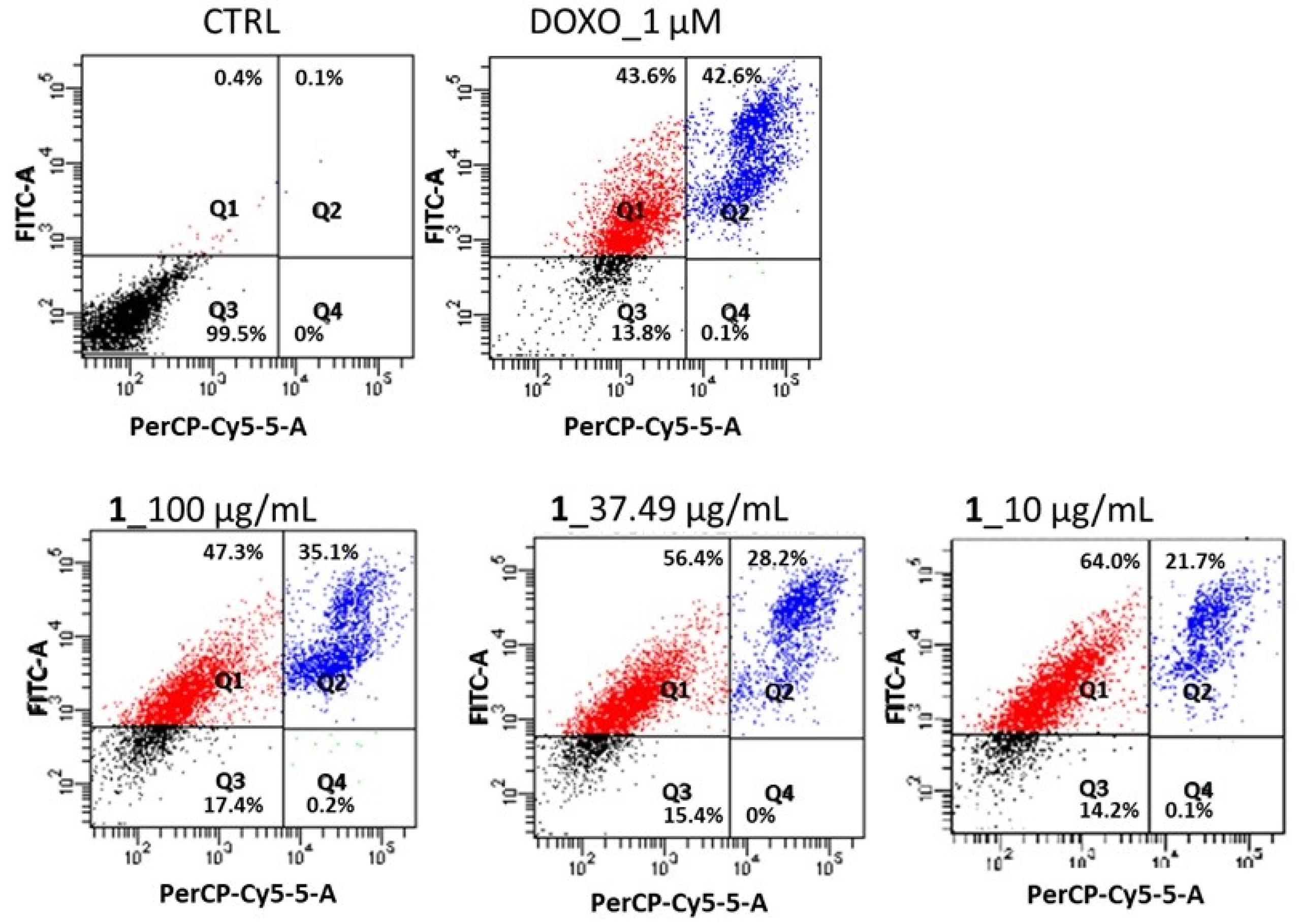
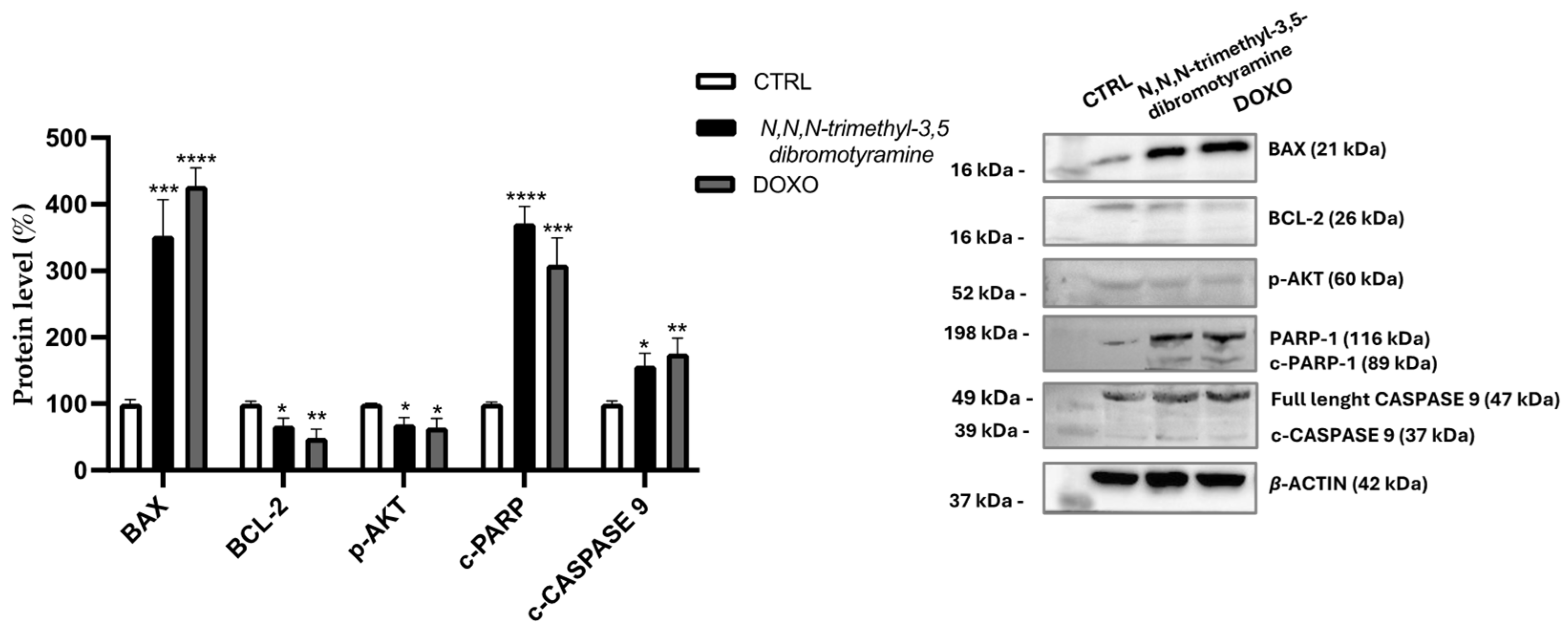
2.2. Effect of N,N,N-Trimethyl-3,5-Dibromotyramine on Apoptosis and Apoptotic Markers in HepG2 Cells
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Marine Sponge Material
4.3. Extraction and Isolation
4.4. LC-HR-ESIMS and LC-HR-MS/MS Analysis
4.5. LC-HRMS/MS Data Processing and Molecular Networking
4.6. Cell Culture and Cytotoxicity Assay
4.7. Apoptosis Analysis by Flow Cytometer
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esposito, G.; Teta, R.; Della Sala, G.; Pawlik, J.R.; Mangoni, A.; Costantino, V. Isolation of Smenopyrone, a Bis-γ-Pyrone Polypropionate from the Caribbean Sponge Smenospongia Aurea. Mar. Drugs 2018, 16, 285. [Google Scholar] [CrossRef]
- Mehbub, M.; Lei, J.; Franco, C.; Zhang, W. Marine Sponge Derived Natural Products between 2001 and 2010: Trends and Opportunities for Discovery of Bioactives. Mar. Drugs 2014, 12, 4539–4577. [Google Scholar] [CrossRef]
- Abdelaleem, E.R.; Samy, M.N.; Desoukey, S.Y.; Liu, M.; Quinn, R.J.; Abdelmohsen, U.R. Marine Natural Products from Sponges (Porifera) of the Order Dictyoceratida (2013 to 2019); a Promising Source for Drug Discovery. RSC Adv. 2020, 10, 34959–34976. [Google Scholar] [CrossRef]
- Calcabrini, C.; Catanzaro, E.; Bishayee, A.; Turrini, E.; Fimognari, C. Marine Sponge Natural Products with Anticancer Potential: An Updated Review. Mar. Drugs 2017, 15, 310. [Google Scholar] [CrossRef]
- Caso, A.; da Silva, F.B.; Esposito, G.; Teta, R.; Della Sala, G.; Nunes Cavalcanti, L.P.A.; Valverde, A.L.; Martins, R.C.C.; Costantino, V. Exploring Chemical Diversity of Phorbas Sponges as a Source of Novel Lead Compounds in Drug Discovery. Mar. Drugs 2021, 19, 667. [Google Scholar] [CrossRef]
- Nagabhishek, S.N.; Madankumar, A. A Novel Apoptosis-Inducing Metabolite Isolated from Marine Sponge Symbiont Monascus sp. NMK7 Attenuates Cell Proliferation, Migration and ROS Stress-Mediated Apoptosis in Breast Cancer Cells. RSC Adv. 2019, 9, 5878–5890. [Google Scholar] [CrossRef]
- Puyana, M.; Pawlik, J.; Blum, J.; Fenical, W. Metabolite Variability in Caribbean Sponges of the Genus Aplysina. Rev. Bras. Farmacognosia 2015, 25, 592–599. [Google Scholar] [CrossRef]
- Loh, T.-L.; Pawlik, J.R. Chemical Defenses and Resource Trade-Offs Structure Sponge Communities on Caribbean Coral Reefs. Proc. Natl. Acad. Sci. USA 2014, 111, 4151–4156. [Google Scholar] [CrossRef]
- Elissawy, A.M.; Soleiman Dehkordi, E.; Mehdinezhad, N.; Ashour, M.L.; Mohammadi Pour, P. Cytotoxic Alkaloids Derived from Marine Sponges: A Comprehensive Review. Biomolecules 2021, 11, 258. [Google Scholar] [CrossRef]
- Neumann, C.S.; Fujimori, D.G.; Walsh, C.T. Halogenation Strategies In Natural Product Biosynthesis. Chem. Biol. 2008, 15, 99–109. [Google Scholar] [CrossRef]
- Bechmann, N.; Ehrlich, H.; Eisenhofer, G.; Ehrlich, A.; Meschke, S.; Ziegler, C.G.; Bornstein, S.R. Anti-Tumorigenic and Anti-Metastatic Activity of the Sponge-Derived Marine Drugs Aeroplysinin-1 and Isofistularin-3 against Pheochromocytoma In Vitro. Mar. Drugs 2018, 16, 172. [Google Scholar] [CrossRef]
- Drechsel, A.; Helm, J.; Ehrlich, H.; Pantovic, S.; Bornstein, S.R.; Bechmann, N. Anti-Tumor Activity vs. Normal Cell Toxicity: Therapeutic Potential of the Bromotyrosines Aerothionin and Homoaerothionin In Vitro. Mar. Drugs 2020, 18, 236. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular Framework for Processing, Visualizing, and Analyzing Mass Spectrometry-Based Molecular Profile Data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Reihani, R.Z.; Doustvandi, M.A.; Amini, M.; Zargari, F.; Baradaran, B.; Yari, A.; Hashemi, M.; Tohidast, M.; Mokhtarzadeh, A. Synergistic Anticancer Effects of Curcumin and Crocin on Human Colorectal Cancer Cells. Mol. Biol. Rep. 2022, 49, 8741–8752. [Google Scholar] [CrossRef]
- El-fayoumy, E.A.; Shanab, S.M.M.; Gaballa, H.S.; Tantawy, M.A.; Shalaby, E.A. Evaluation of Antioxidant and Anticancer Activity of Crude Extract and Different Fractions of Chlorella Vulgaris Axenic Culture Grown under Various Concentrations of Copper Ions. BMC Complement. Med. Ther. 2021, 21, 51. [Google Scholar] [CrossRef]
- Ciminiello, P.; Dell’Aversano, C.; Fattorusso, E.; Magno, S.; Pansini, M. Chemistry of Verongida Sponges. 10. Secondary Metabolite Composition of the Caribbean Sponge Verongula Gigantea. J. Nat. Prod. 2000, 63, 263–266. [Google Scholar] [CrossRef]
- Fu, X.; Schmitz, F.J. 7-Hydroxyceratinamine, a New Cyanoformamide-Containing Metabolite from a Sponge, Aplysinella sp. J. Nat. Prod. 1999, 62, 1072–1073. [Google Scholar] [CrossRef]
- Peng, J.; Li, J.; Hamann, M.T. The Marine Bromotyrosine Derivatives. In The Alkaloids: Chemistry and Biology; Elsevier: Amsterdam, The Netherlands, 2005; Volume 61, pp. 59–262. ISBN 978-0-12-469561-0. [Google Scholar]
- Orfanoudaki, M.; Hartmann, A.; Alilou, M.; Mehic, N.; Kwiatkowski, M.; Jöhrer, K.; Nguyen Ngoc, H.; Hensel, A.; Greil, R.; Ganzera, M. Cytotoxic Compounds of Two Demosponges (Aplysina aerophoba and Spongia sp.) from the Aegean Sea. Biomolecules 2021, 11, 723. [Google Scholar] [CrossRef]
- Teta, R.; Della Sala, G.; Esposito, G.; Via, C.W.; Mazzoccoli, C.; Piccoli, C.; Bertin, M.J.; Costantino, V.; Mangoni, A. A Joint Molecular Networking Study of a: Smenospongia Sponge and a Cyanobacterial Bloom Revealed New Antiproliferative Chlorinated Polyketides. Org. Chem. Front. 2019, 6, 1762–1774. [Google Scholar] [CrossRef]
- Caso, A.; Esposito, G.; Sala, G.D.; Pawlik, J.R.; Teta, R.; Mangoni, A.; Costantino, V. Fast Detection of Two Smenamide Family Members Using Molecular Networking. Mar. Drugs 2019, 17, 618. [Google Scholar] [CrossRef]
- Binnewerg, B.; Schubert, M.; Voronkina, A.; Muzychka, L.; Wysokowski, M.; Petrenko, I.; Djurović, M.; Kovalchuk, V.; Tsurkan, M.; Martinovic, R.; et al. Marine Biomaterials: Biomimetic and Pharmacological Potential of Cultivated Aplysina Aerophoba Marine Demosponge. Mater. Sci. Eng. C 2020, 109, 110566. [Google Scholar] [CrossRef]
- Göthel, Q.; Sirirak, T.; Köck, M. Bromotyrosine-Derived Alkaloids from the Caribbean Sponge Aplysina Lacunosa. Beilstein J. Org. Chem. 2015, 11, 2334–2342. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- De, A.K.; Muthiyan, R.; Mondal, S.; Mahanta, N.; Bhattacharya, D.; Ponraj, P.; Muniswamy, K.; Kundu, A.; Kundu, M.S.; Sunder, J.; et al. A Natural Quinazoline Derivative from Marine Sponge Hyrtios Erectus Induces Apoptosis of Breast Cancer Cells via ROS Production and Intrinsic or Extrinsic Apoptosis Pathways. Mar. Drugs 2019, 17, 658. [Google Scholar] [CrossRef]
- Campbell, K.J.; Tait, S.W.G. Targeting BCL-2 Regulated Apoptosis in Cancer. Open Biol. 2018, 8, 180002. [Google Scholar] [CrossRef]
- Hafezi, S.; Rahmani, M. Targeting BCL-2 in Cancer: Advances, Challenges, and Perspectives. Cancers 2021, 13, 1292. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Garcia-Saez, A.J. Mechanisms of BCL-2 Family Proteins in Mitochondrial Apoptosis. Nat. Rev. Mol. Cell Biol. 2023, 24, 732–748. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The Role of BCL-2 Family Proteins in Regulating Apoptosis and Cancer Therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
- Li, Q.; He, Z.; Liu, J.; Wu, J.; Tan, G.; Jiang, J.; Su, Z.; Cao, M. Paris Polyphylla 26 Triggers G2/M Phase Arrest and Induces Apoptosis in HepG2 Cells via Inhibition of the Akt Signaling Pathway. J. Int. Med. Res. 2019, 47, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E. Curcumin Combination Chemotherapy: The Implication and Efficacy in Cancer. Molecules 2019, 24, 2527. [Google Scholar] [CrossRef] [PubMed]
- Nothias, L.-F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-Based Molecular Networking in the GNPS Analysis Environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Sinisgalli, C.; Faraone, I.; Vassallo, A.; Caddeo, C.; Bisaccia, F.; Armentano, M.F.; Milella, L.; Ostuni, A. Phytochemical Profile of Capsicum Annuum L. Cv Senise, Incorporation into Liposomes, and Evaluation of Cellular Antioxidant Activity. Antioxidants 2020, 9, 428. [Google Scholar] [CrossRef]
- Russo, D.; Miglionico, R.; Carmosino, M.; Bisaccia, F.; Andrade, P.; Valentão, P.; Milella, L.; Armentano, M. A Comparative Study on Phytochemical Profiles and Biological Activities of Sclerocarya Birrea (A.Rich.) Hochst Leaf and Bark Extracts. Int. J. Mol. Sci. 2018, 19, 186. [Google Scholar] [CrossRef]
| Extract | IC50 (µg/mL) |
|---|---|
| MeOH | 237.99 ± 2.04 a |
| Partitioning fractions | |
| A. cauliformis ex MeOH R/H2O | n.d. |
| A. cauliformis ex MeOH R/BuOH—CHCl3 | 214.29 ± 2.06 b |
| RP-C18 Silica gel CC fractions | |
| fr. A1 | n.d. |
| fr. A2 | n.d. |
| fr. A3 | 292.42 ± 2.23 c |
| fr. A4 | 134.28 ± 1.82 d |
| fr. A5 | 234.42 ± 1.34 a |
| fr. A5 * | 204.17 ± 1.89 e |
| fr. B | 218.78 ± 2.12 b |
| fr. C | 202.77 ± 2.10 e |
| HPLC fractions from fr. A4 | |
| fr. A4_HPLC 3 (pure compound) | 37.49 ± 1.94 f |
| fr. A4_HPLC 4 | 559.24 ± 3.36 g |
| fr. A4_HPLC 9 | n.d. |
| fr. A4_HPLC 13 | 44.35 ± 2.06 h |
| fr. A4_HPLC 19 | 236.66 ± 2.14 a |
| fr. A4_HPLC 20 | 245.89 ± 2.09 i |
| fr. A4_HPLC 22 | n.d. |
| fr. A4_HPLC 28 | 277.24 ± 2.45 j |
| fr. A4_HPLC 28 * | n.d. |
| fr. A4_HPLC 30 | n.d. |
| HPLC fractions from fr. A4_HPLC 13 | |
| fr. A4_HPLC 13/fr.9_HPLC 20/05/2021 | n.d. |
| fr. A4_HPLC 13/fr.11_HPLC 20/05/2021 | n.d. |
| fr. A4_HPLC 13/fr.13_HPLC 20/05/2021 | 25.89 ± 1.94 k |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, G.; Ponticelli, M.; Milella, L.; Lela, L.; Teta, R.; Pawlik, J.R.; Russo, D.; Costantino, V. Bioassay-Guided Procedure Coupled with HR-ESIMS Dereplication for Isolation of Antiproliferative Bromo-Tyramine Derivative from Aplysina cauliformis. Mar. Drugs 2025, 23, 187. https://doi.org/10.3390/md23050187
Esposito G, Ponticelli M, Milella L, Lela L, Teta R, Pawlik JR, Russo D, Costantino V. Bioassay-Guided Procedure Coupled with HR-ESIMS Dereplication for Isolation of Antiproliferative Bromo-Tyramine Derivative from Aplysina cauliformis. Marine Drugs. 2025; 23(5):187. https://doi.org/10.3390/md23050187
Chicago/Turabian StyleEsposito, Germana, Maria Ponticelli, Luigi Milella, Ludovica Lela, Roberta Teta, Joseph R. Pawlik, Daniela Russo, and Valeria Costantino. 2025. "Bioassay-Guided Procedure Coupled with HR-ESIMS Dereplication for Isolation of Antiproliferative Bromo-Tyramine Derivative from Aplysina cauliformis" Marine Drugs 23, no. 5: 187. https://doi.org/10.3390/md23050187
APA StyleEsposito, G., Ponticelli, M., Milella, L., Lela, L., Teta, R., Pawlik, J. R., Russo, D., & Costantino, V. (2025). Bioassay-Guided Procedure Coupled with HR-ESIMS Dereplication for Isolation of Antiproliferative Bromo-Tyramine Derivative from Aplysina cauliformis. Marine Drugs, 23(5), 187. https://doi.org/10.3390/md23050187