1. Introduction
Azaspiracids (AZAs) are a class of lipophilic polyether and diarrheal marine toxins. They are currently known to be generated in the native organisms
Azadinium [
1,
2] and
Amphidoma [
3,
4] of the flagellate order, and certain species of
Protoperidium, such as
P. crassipes [
5] (all of which belong to the phylum dinoflagellate), and can bioaccumulate in shellfish, such as oysters, scallops, clams, etc. [
6,
7,
8]. It was first reported in 1996 during a food poisoning incident that occurred in Ireland in 1995, where several crew members developed acute gastrointestinal symptoms after consuming the blue mussels they had captured [
9]. The toxin was initially named Killary Toxin (named after its discovery site) or KT-3, and later renamed Azaspiracids to better reflect its structural formula [
10]. The physicochemical properties of AZAs toxins are significantly different from other nitrogen-containing biotoxins. As with other marine toxin groups, the toxins are relatively stable and cannot be eliminated by conventional cooking and processing. After several hours of high-temperature treatment with various organic reagents, the structure and toxicity of the azaspiracids remain unchanged, and they can be stored for a long time under refrigeration conditions [
11]. At present, more than 60 types of AZAs and their derivatives have been discovered, and most of which are derived from the secondary metabolites of mussels [
12,
13].
There is currently no definitive experimental study on its pathogenic mechanism that can demonstrate it as a blocking agent for a specific target. However, some studies have shown that it can influence the electrical activity of cells by affecting the activity of various ion channels, including potassium ion channels [
14], sodium ion channels [
15], chloride ion channels [
16], calcium ion channels [
17], and so on. For the impact on the individual digestive system, toxins can enter the bloodstream through the human intestinal barrier, causing damage to epithelial cells [
18]. It also has certain effects on inducing tumors [
19], altering the cytoskeleton, and reducing human cell metabolism [
20]. Experiments have shown that repeated exposure to azaspiracids can induce the expression of Jun B proto-oncogene, Jun D proto-oncogene, c-Jun proto-oncogene, FBJ murine osteosarcoma viral oncogene homolog B, and Fos-related antigen 1 in mice, leading to a significant increase in lung tumors and lymphatic necrosis of digestive system tissues [
21]. In addition, there have been reports on the impact to the nervous system [
22].
The European Food Safety Authority (EFSA) established an acute reference dose (ARfD) at 0.2 μg/kg body weight (b.w.) based on data from human poisoning incidents [
23] and recommended a guideline limit of 30 µg/kg maximum concentration in shellfish meat [
24]. However, European Law states that the maximum permitted threshold for total azaspiracids is 0.16 mg/kg AZA-1 equivalents [
25].
The main detection methods currently include the following aspects:
Mouse bioassay (MBA) involves injecting toxins or extracts into the abdominal cavity of mice and recording the survival time of mice at different concentrations to calculate the toxin content of the sample. This method is the simplest and most commonly used method for detecting shellfish toxins, with a detection sensitivity of 1 μg level [
26]. The minimum lethal doses for purified AZA-1, AZA-2, and AZA-3 are 200 μg/kg, 110 μg/kg, and 140 μg/kg, respectively [
27]. This method has been listed as a routine method for toxin detection by the American Society of Analytical Chemists. The advantage is that it does not require complex equipment and has high operability. However, there are also drawbacks such as poor sensitivity, low reproducibility, high false positive rate, inability to identify toxicity, and significant influence from individual differences in mice.
Liquid chromatography tandem mass spectrometry (LC-MS/MS) was first used in 1999 to detect protochloroalginic acid, with a detection sensitivity of up to 50 pg [
28]. This method is the European Reference Method for determination of AZAs, with advantages such as high sensitivity, high specificity, and the ability to accurately determine the composition of mixtures. However, there are still drawbacks such as expensive instruments and the need for skilled technicians to operate them.
Enzyme-linked immunosorbent assay (ELISA) has a detection sensitivity of 0.45–8.6 ng/mL [
29]. Using new plate-coating antigen, an ELISA was developed with a working range of 0.30–4.1 ng/mL and a limit of quantification at 37 μg/kg for AZA-1 in shellfish [
30]. ELISA can also be employed in conjunction with gel methods [
31]. This method may have adverse effects such as false positives and cross-reactivity. And currently, there is no commercial ELISA available for purchase and use.
Biosensors: Current monitoring methods are limited to the detection of standard samples, including polyclonal antibody recognition [
32] and monoclonal antibody recognition [
33] targeting the C28–C40 site FGHI loop. However, there are still issues with the toxicity and lethality of immune conjugates in vivo. Other biological assays, including magnetic bead based, chromatographic column based, and microsphere immunoassay [
34], have provided new detection approaches.
These detection methods each have their own advantages, but most still have many obstacles in application. As a novel molecular recognition tool, nucleic acid aptamers have multiple significant advantages, with clear origins and a wide range of applications. The word comes from the Latin word “aptus” (adaptation) and the Greek word “meros” (partial) [
35]. It is mainly composed of DNA and RNA, and aptamers form structured single-stranded nucleic acid secondary structures through hydrogen bonding interactions between bases, such as hair clips, loops, inner loops, multi-loops, protrusions, stems, pseudoknots, and non-standard base pairing. On the basis of the secondary structure, they can also form certain pocket structures by curling and folding themselves, or form guanine-rich triple- or quadruple-stranded structures through layer stacking [
36]. Small molecules can be recognized by nucleic acid aptamers through electrostatic binding, groove surface binding, insertion binding, and other methods [
37]. Electrostatic binding mainly occurs when the small molecule ligand is a positive dot molecule, and the binding occurs through charge adsorption. The electrostatic effect is very weak, and it is only meaningful for the complex to bind to the aptamer in the presence of a large distribution of positive charges. The specificity of simple small molecule binding is poor; groove surface binding mainly refers to the groove binding formed between ligands and hydrogen bond surfaces or multiple pairs of nucleic acids; The structure formed by stacking π-bonds and basal planes in compounds with smaller molecular weights using insertion binding design is the most stable [
38]. After partial screening and optimization, the equilibrium dissociation constant
KD value of some aptamers can reach between nM and pM, which makes them highly accurate in molecular recognition [
39,
40,
41]. Nucleic acid aptamers have demonstrated extremely high advantages in low immunogenicity, low production cost, stability after modification, small batch differences, small volume, convenient transport, resistance to pH and temperature-induced denaturation, ease of modification with nanomaterials or organic dye molecules, and flexibility in engineering performance manipulation [
42]. For some target nucleic acid aptamers, they exhibit higher affinity and sensitivity than proteins while also being able to recognize hidden binding sites that are inaccessible to smaller targets or protein antibodies [
43]. Nucleic acid aptamers can be combined with various signal amplification techniques to achieve high-sensitivity detection of target molecules. One of its advantages is strong adaptability, which can be applied to various monitoring scenarios such as biosensing, environmental monitoring, food safety, clinical diagnosis, and nucleic acid aptamers. After binding to the target molecule, it can be dissociated under denaturing conditions (such as high temperature, pH changes) to restore its activity, achieve reuse, and reduce detection costs. With the development of experimental technology, aptamers are expected to replace enzyme-linked immunosorbent assays as an effective method for the detection of various chemical molecules.
Azaspiracids exhibit significant cytotoxicity and cardiotoxicity, posing a direct threat to consumer health [
21]. With global climate change and deterioration in the marine environment, harmful algal bloom (HAB) incidents occur frequently [
44], leading to an expansion of the contamination range of AZA-2. The European Food Safety Authority (EFSA) has reported multiple shellfish recall incidents caused by AZA-2 contamination, highlighting the necessity for strengthening detection efforts [
45]. Furthermore, AZA-2 is highly stable in the environment and difficult to remove through conventional water treatment processes. Its bioaccumulative properties may result in the gradual amplification of toxin concentrations within marine ecosystems [
46]. The establishment of environmental benchmark values and ecological risk assessment models for AZA-2 relies on large-scale and high-frequency monitoring data, which current monitoring capabilities struggle to meet. Therefore, more novel and efficient detection methods are urgently needed.
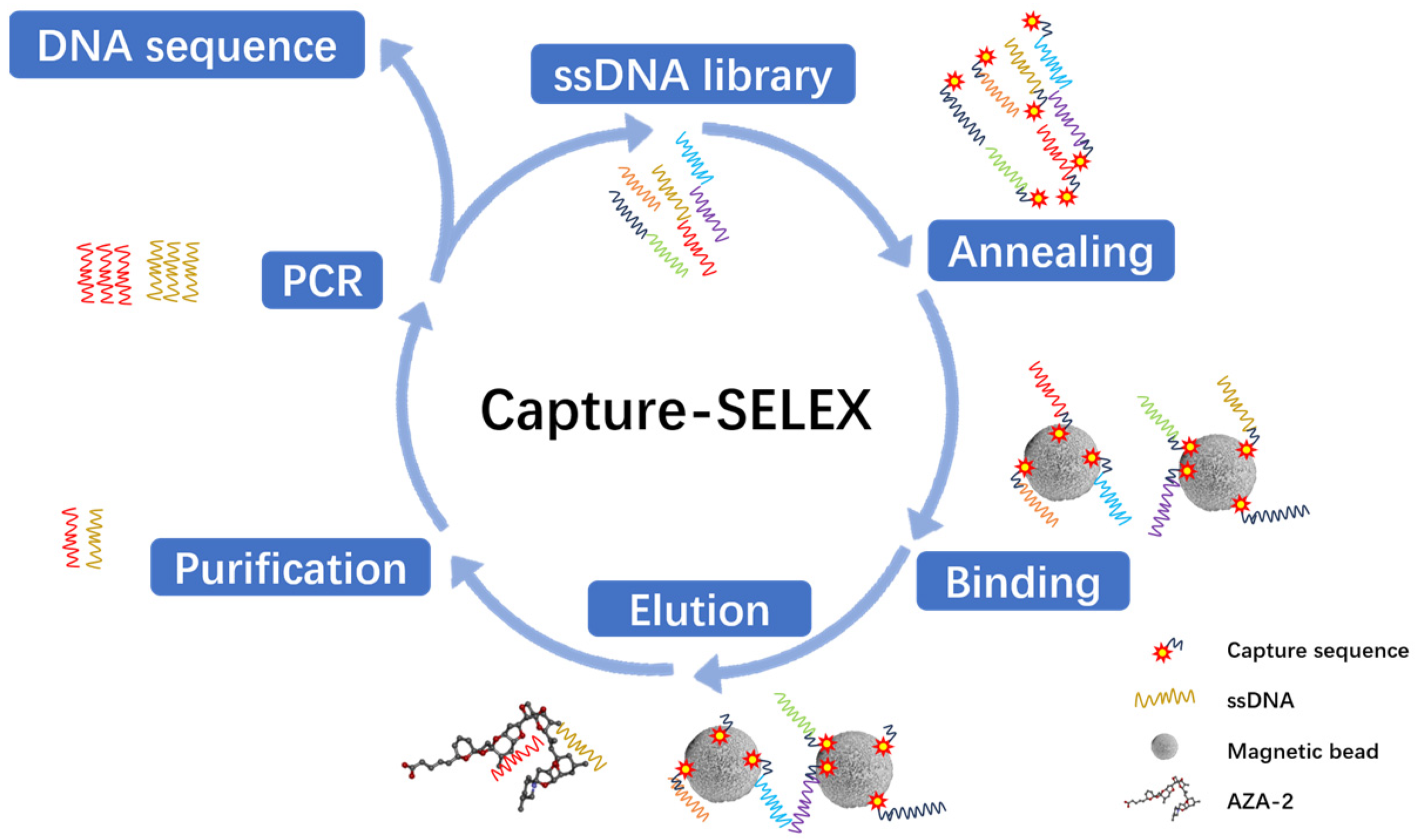
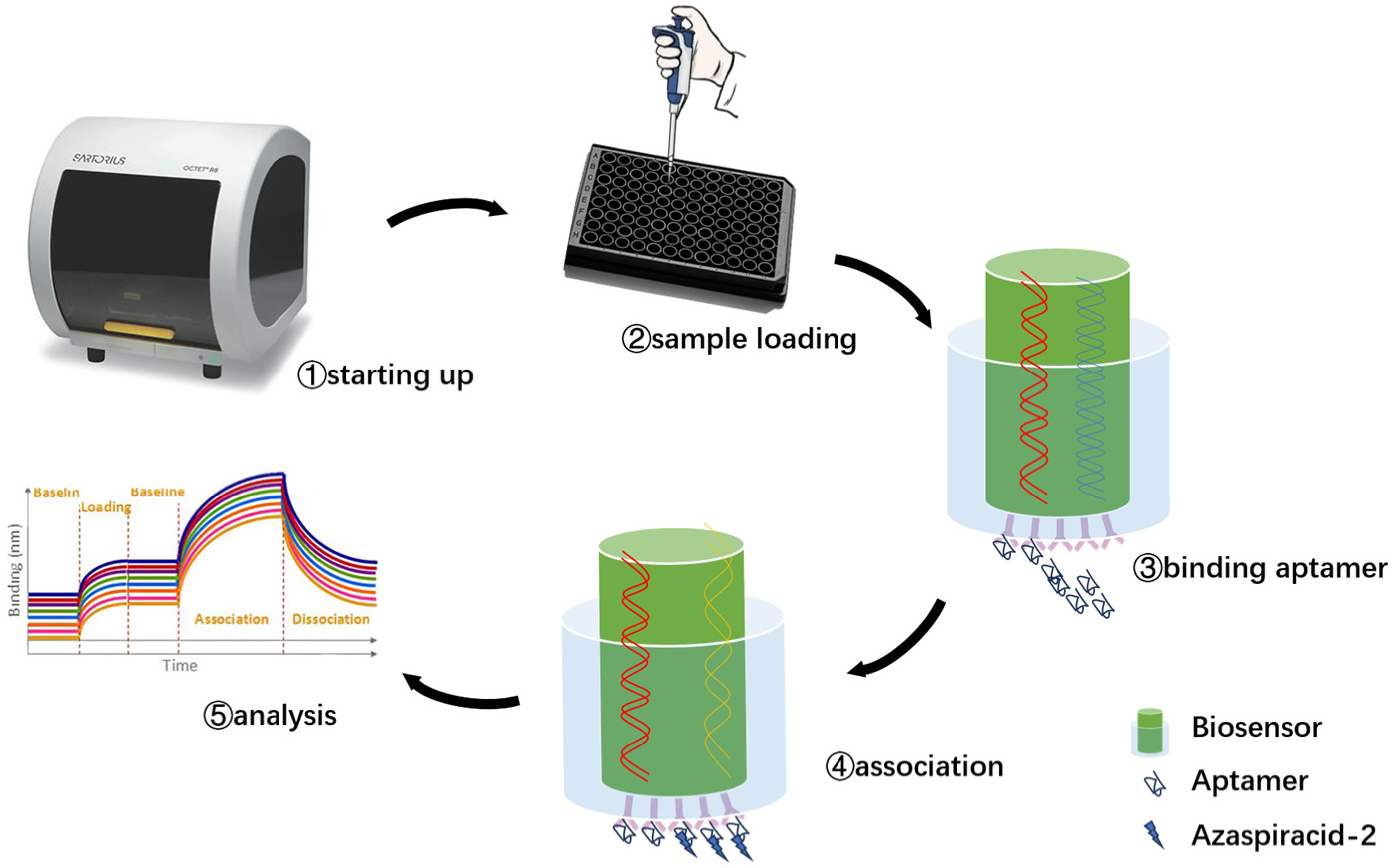
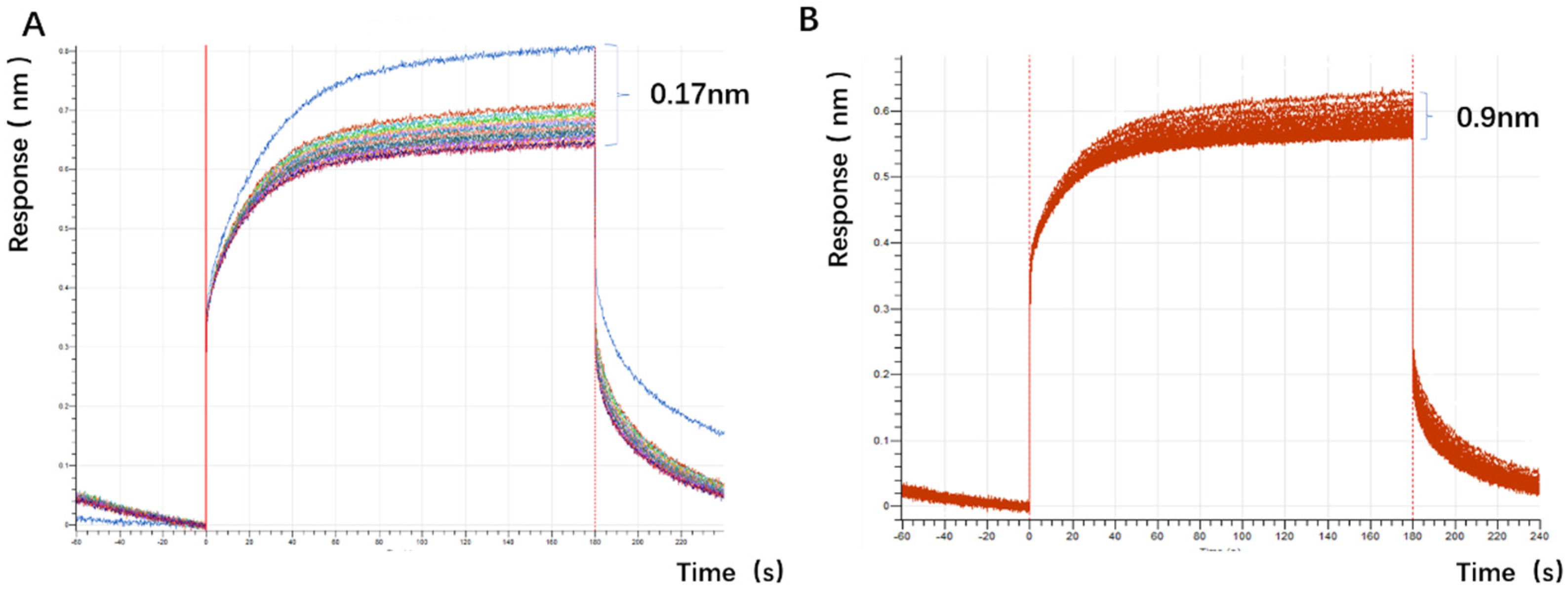
The experimental plan is to screen for aptamers of Azaspiracid-2 using the Capture-SELEX method. To find one or more high-affinity aptamers for toxin detection, BLI technology is used to verify the affinity of the toxin. Additionally, computer simulation is used to investigate the stability of the aptamer docking site and molecular dynamics simulation to improve the affinity of the aptamer for the toxin. The results ultimately achieve specific detection of Azaspiracid-2 in the sample.
3. Discussion
The choice of the Capture-SELEX method is justified as it avoids direct chemical modification of the azaspiracid (AZA-2), thereby preserving the toxin’s natural structure and activity. This is crucial for ensuring that the selected aptamers possess high specificity and affinity.
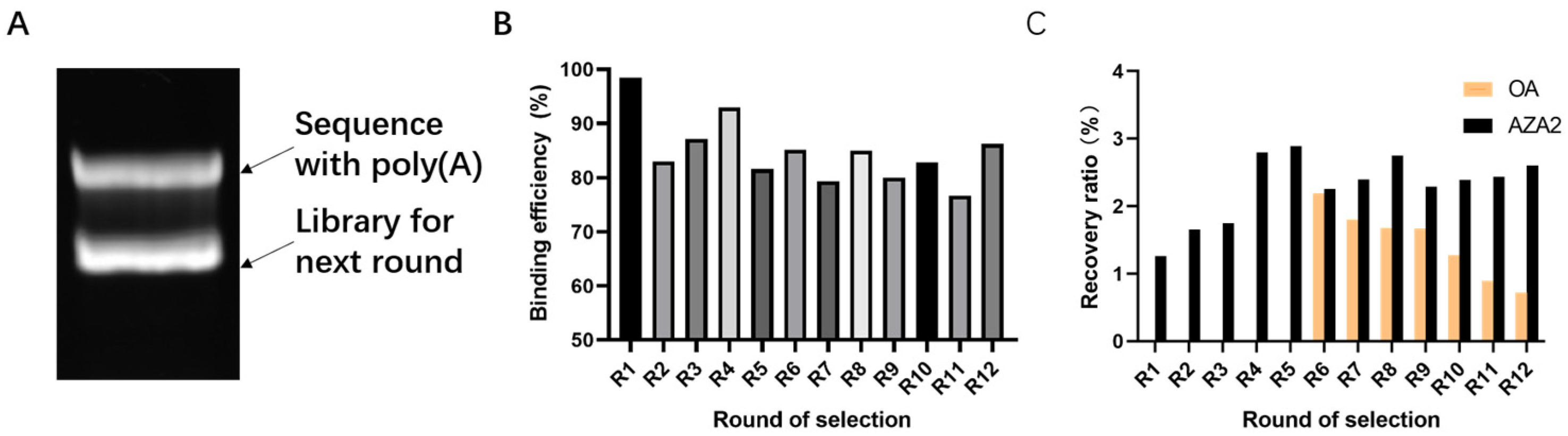
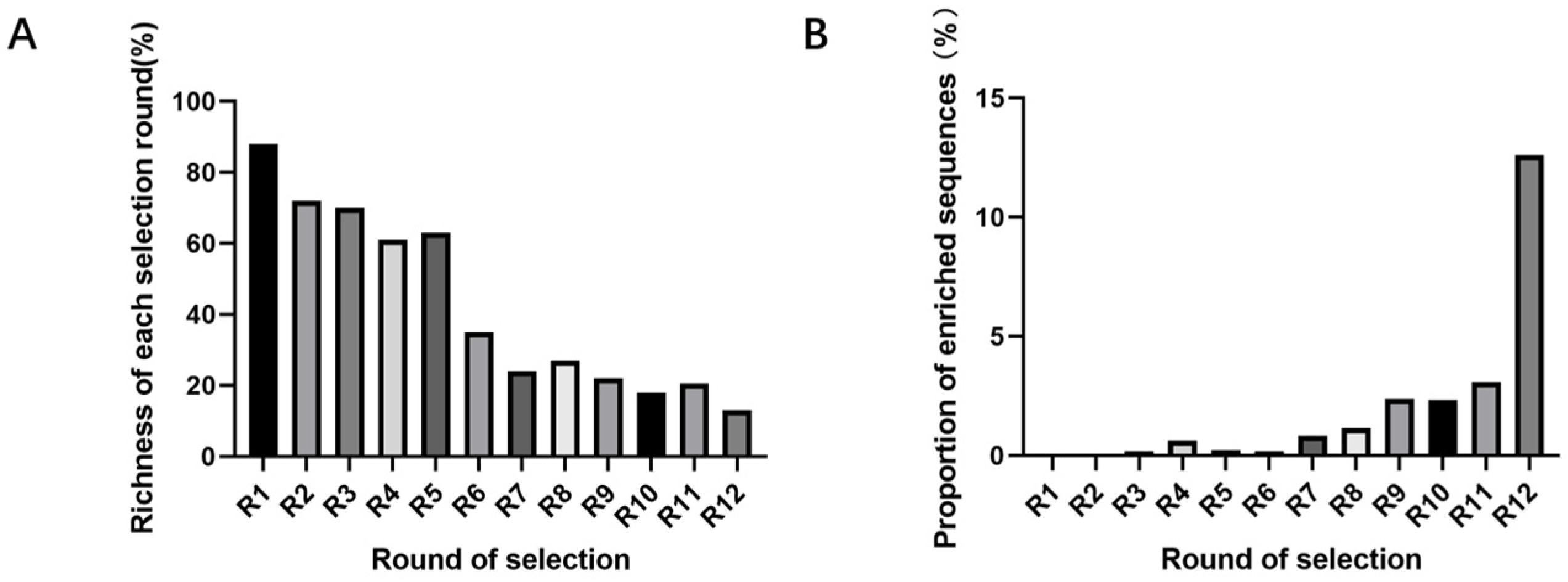
This experiment experiences library loss at multiple steps. Factors such as the richness of library synthesis, the immobilization rate of the library not reaching 100%, base bias in PCR, inefficient recovery methods, qPCR prior to high-throughput sequencing, and decomposition during library collection and sample submission can all reduce library richness. These steps offer significant potential and importance for enhancing library richness during the screening process. At the same time, applying reasonable screening pressure to reduce non-specific binding is also necessary. During the screening process, methods such as reducing the number of magnetic beads, lowering library concentration, and shortening incubation time are used to increase screening pressure, which helps improve the specificity of aptamers [
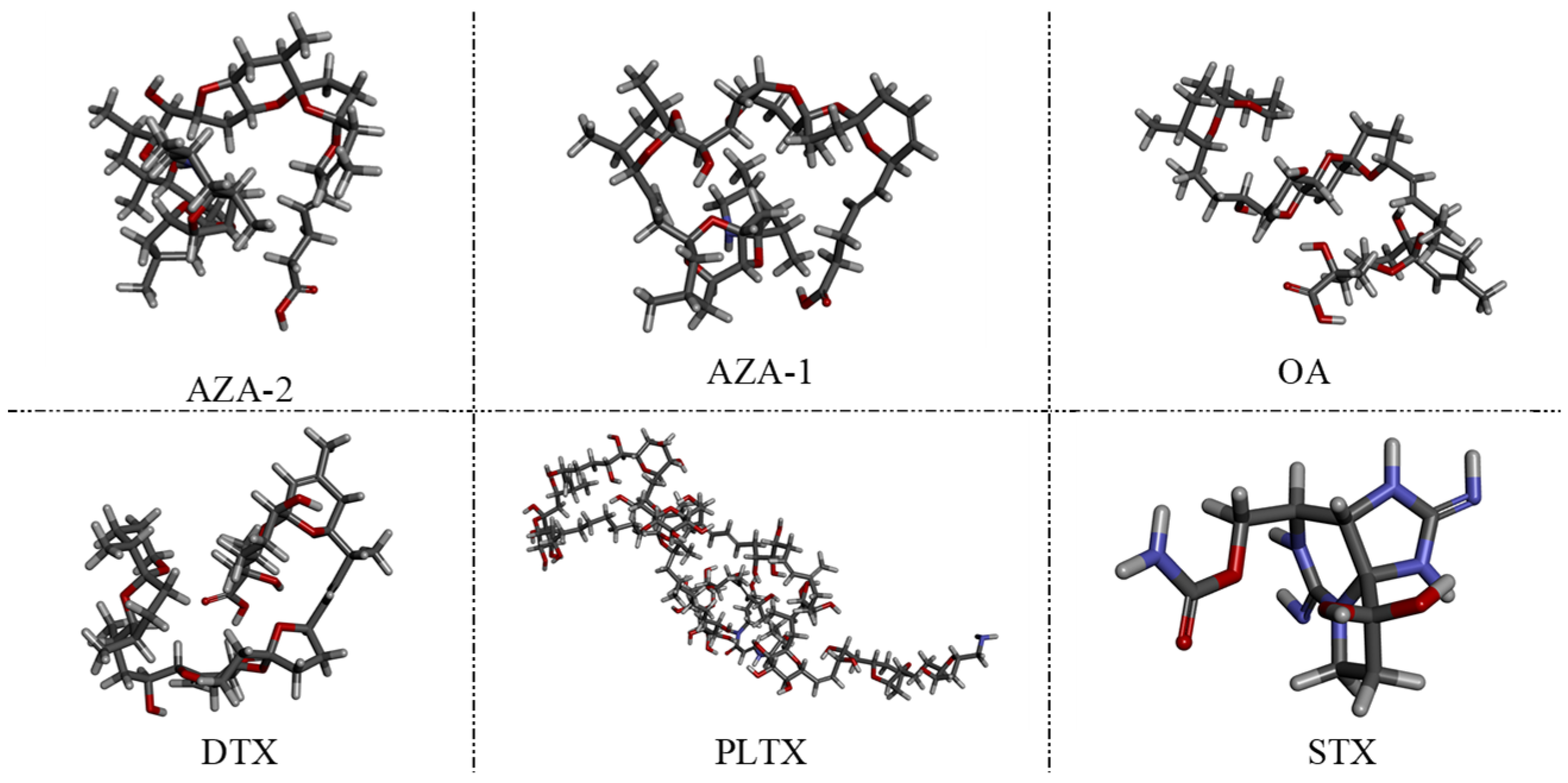
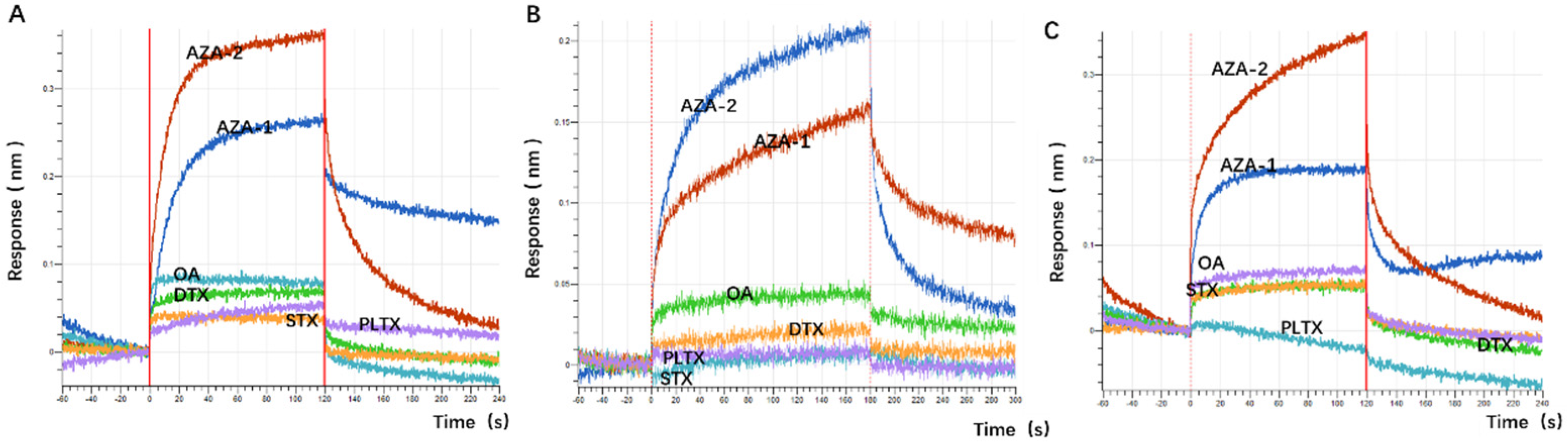
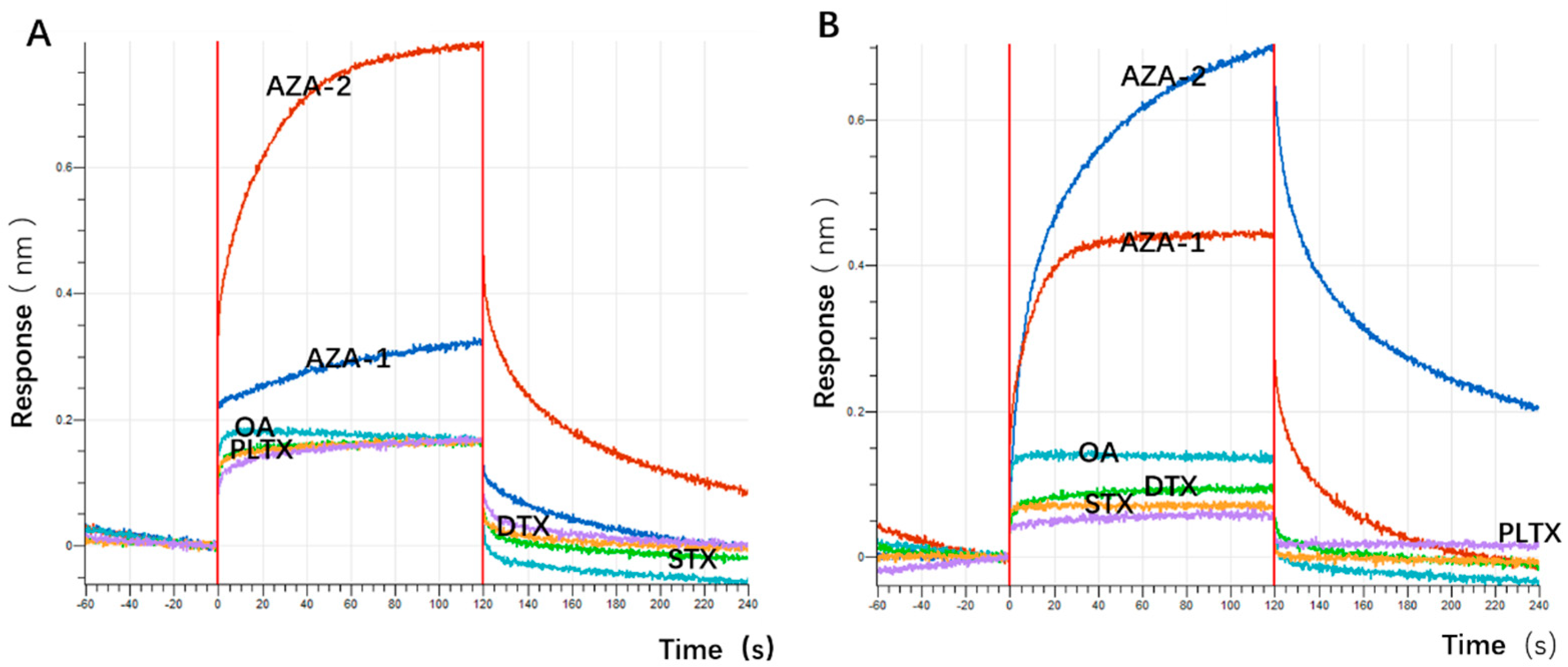
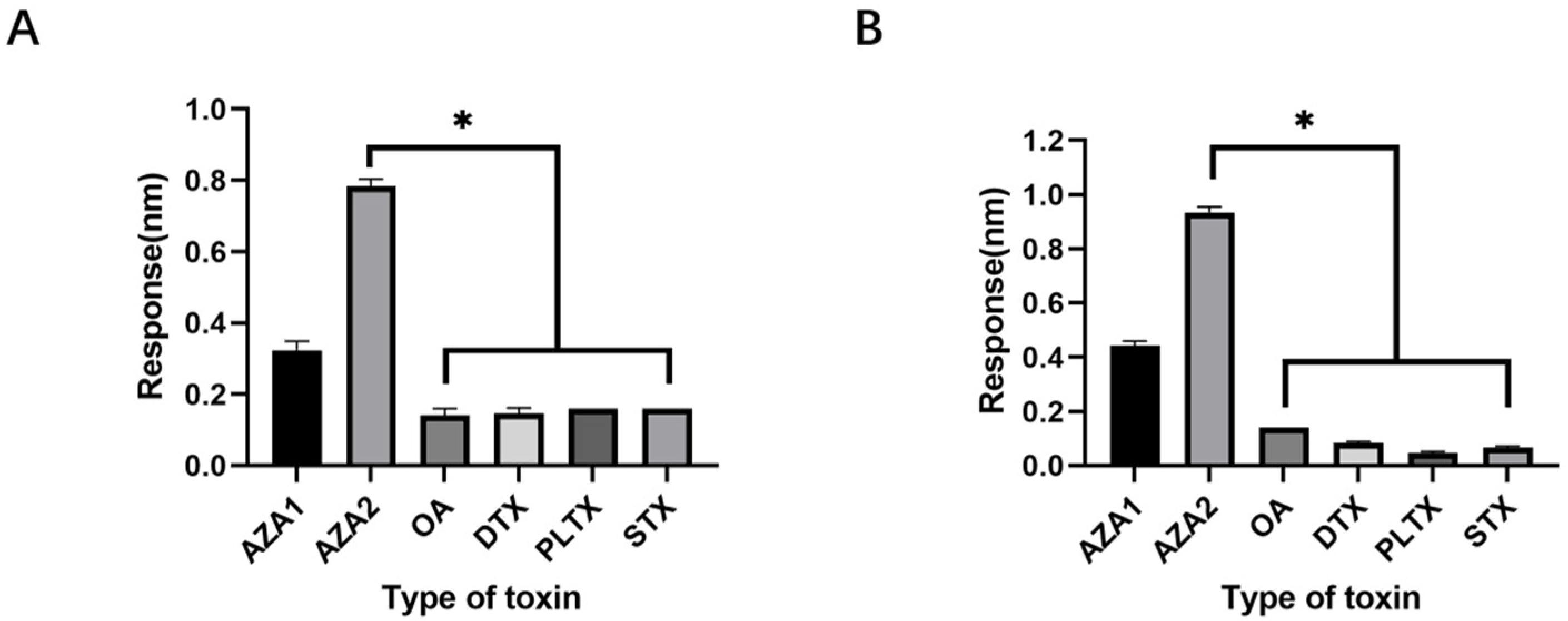
48]. The choice of okadaic acid (OA) as a counter-selection target is reasonable because it belongs to the same class of polyether marine toxins as AZA-2 and shares similar chemical structures and molecular weights. The impact of counter-selection target choice on aptamer specificity is discussed below.
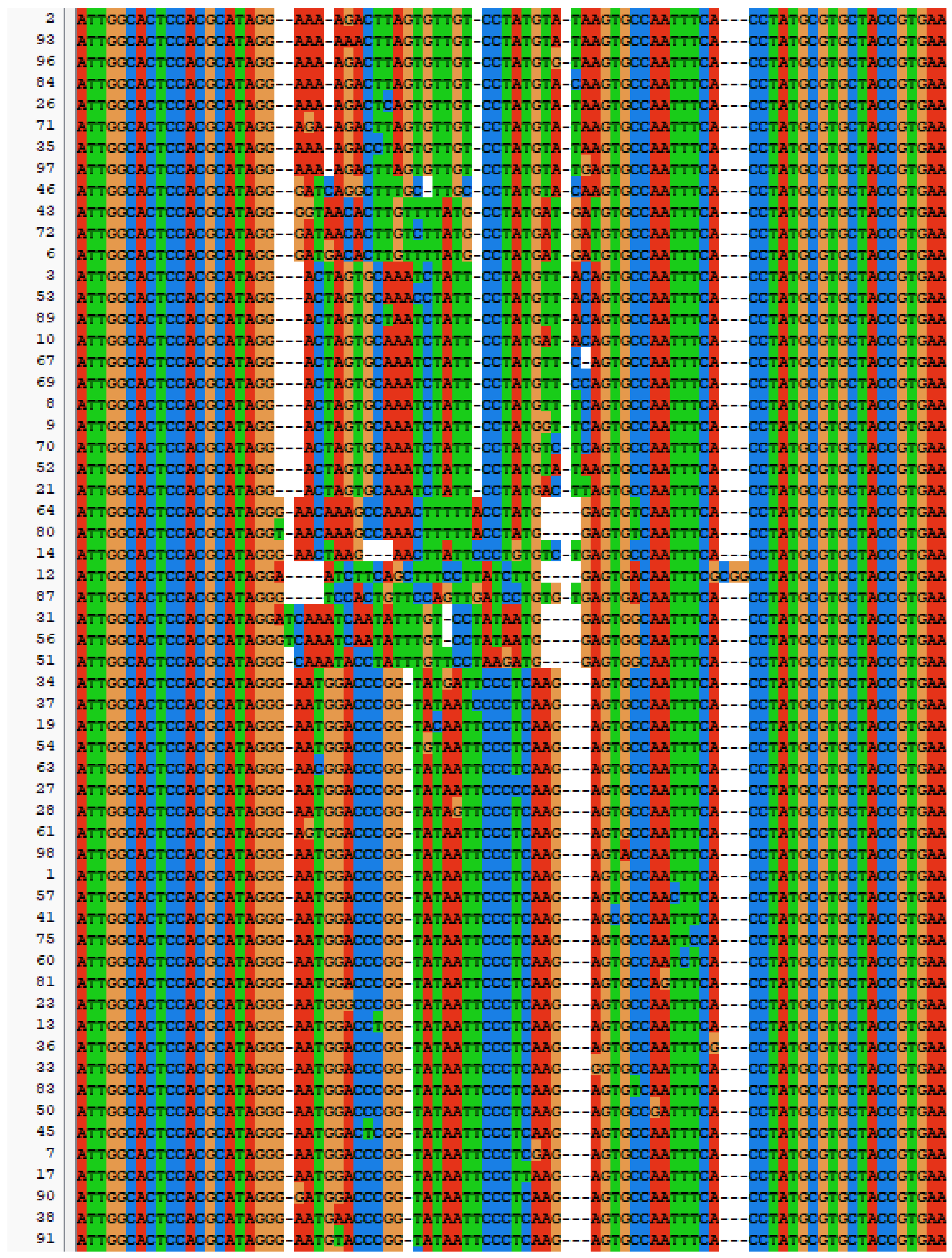
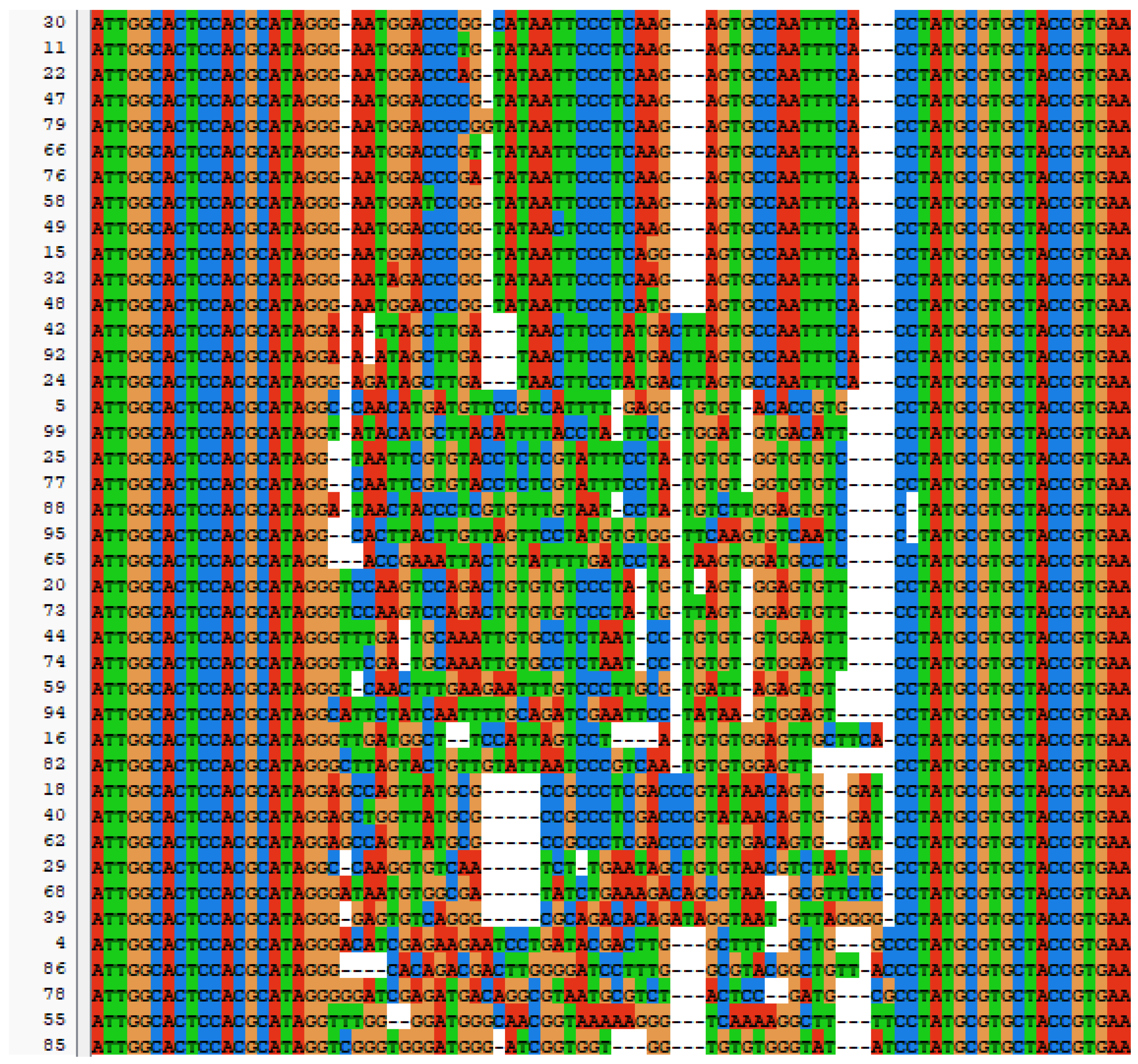
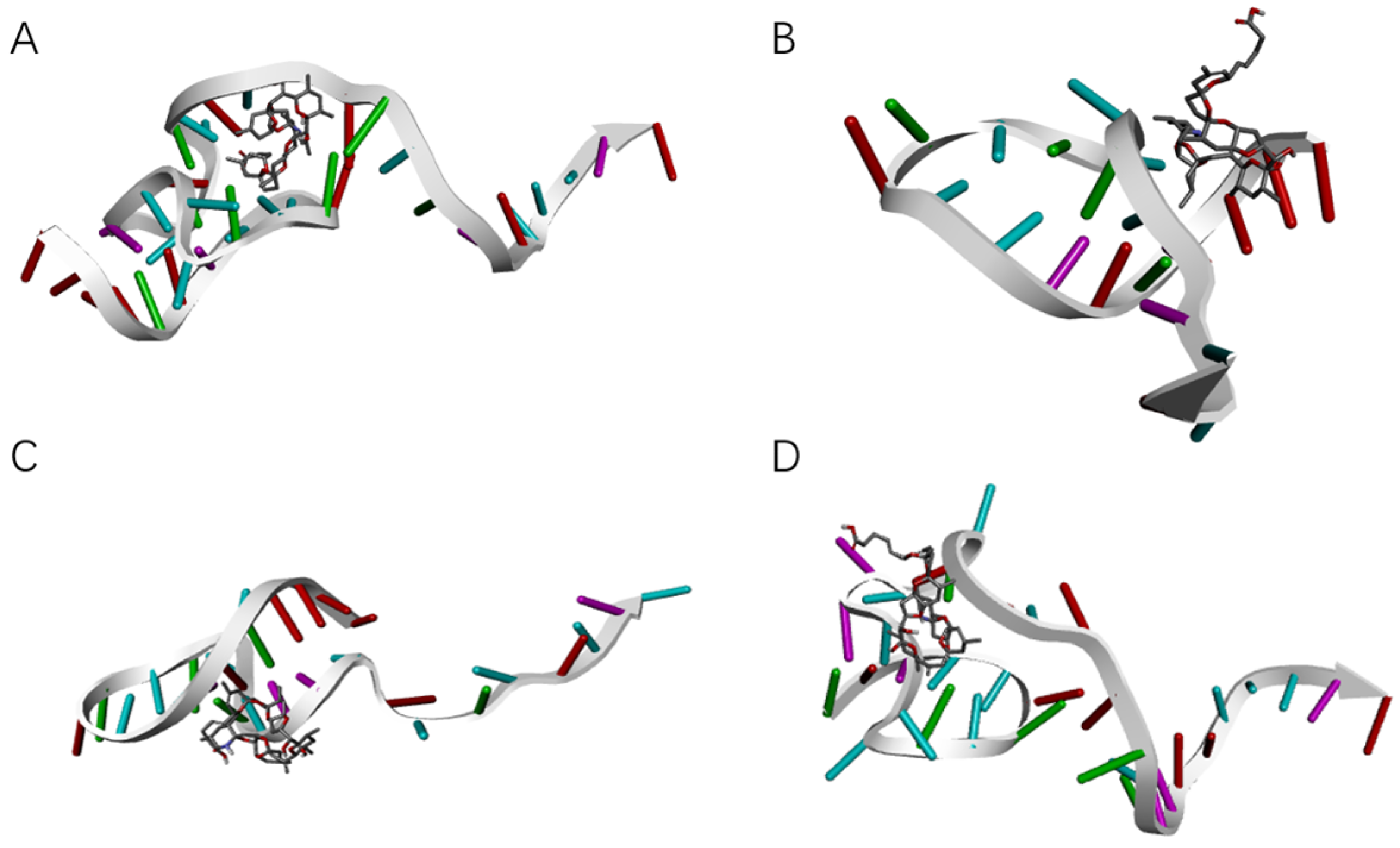
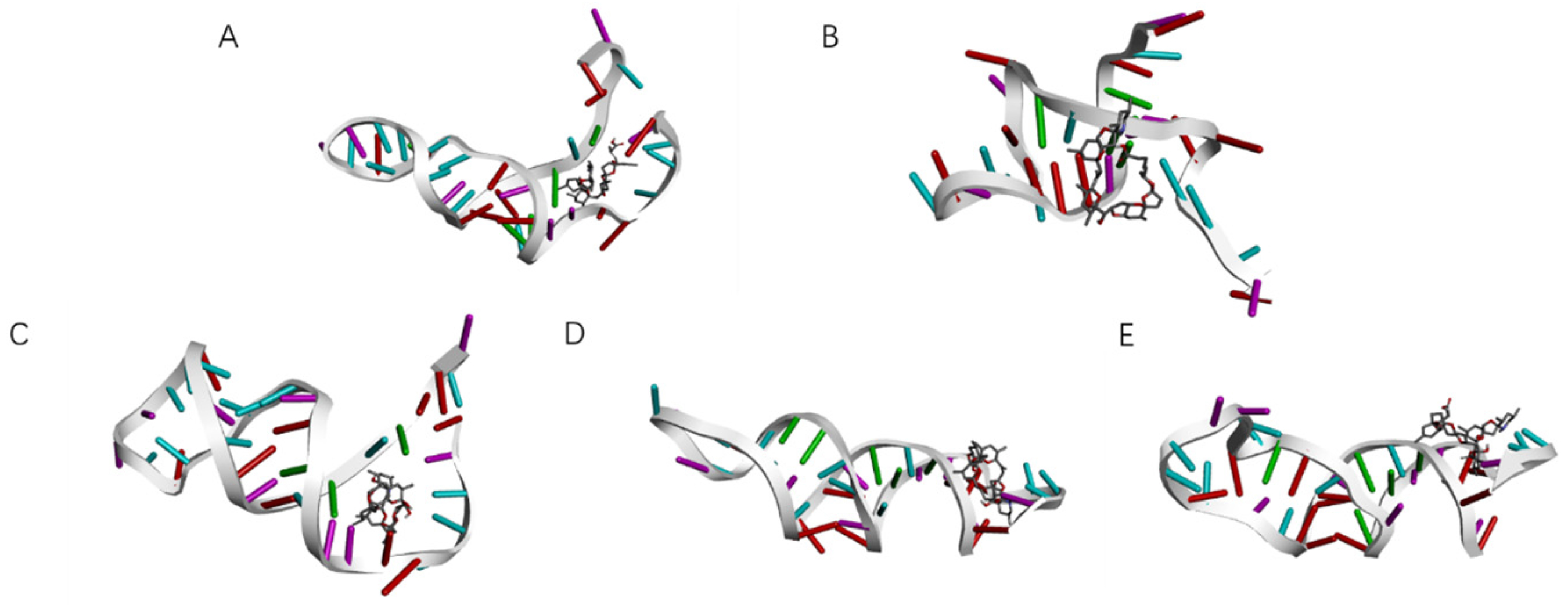
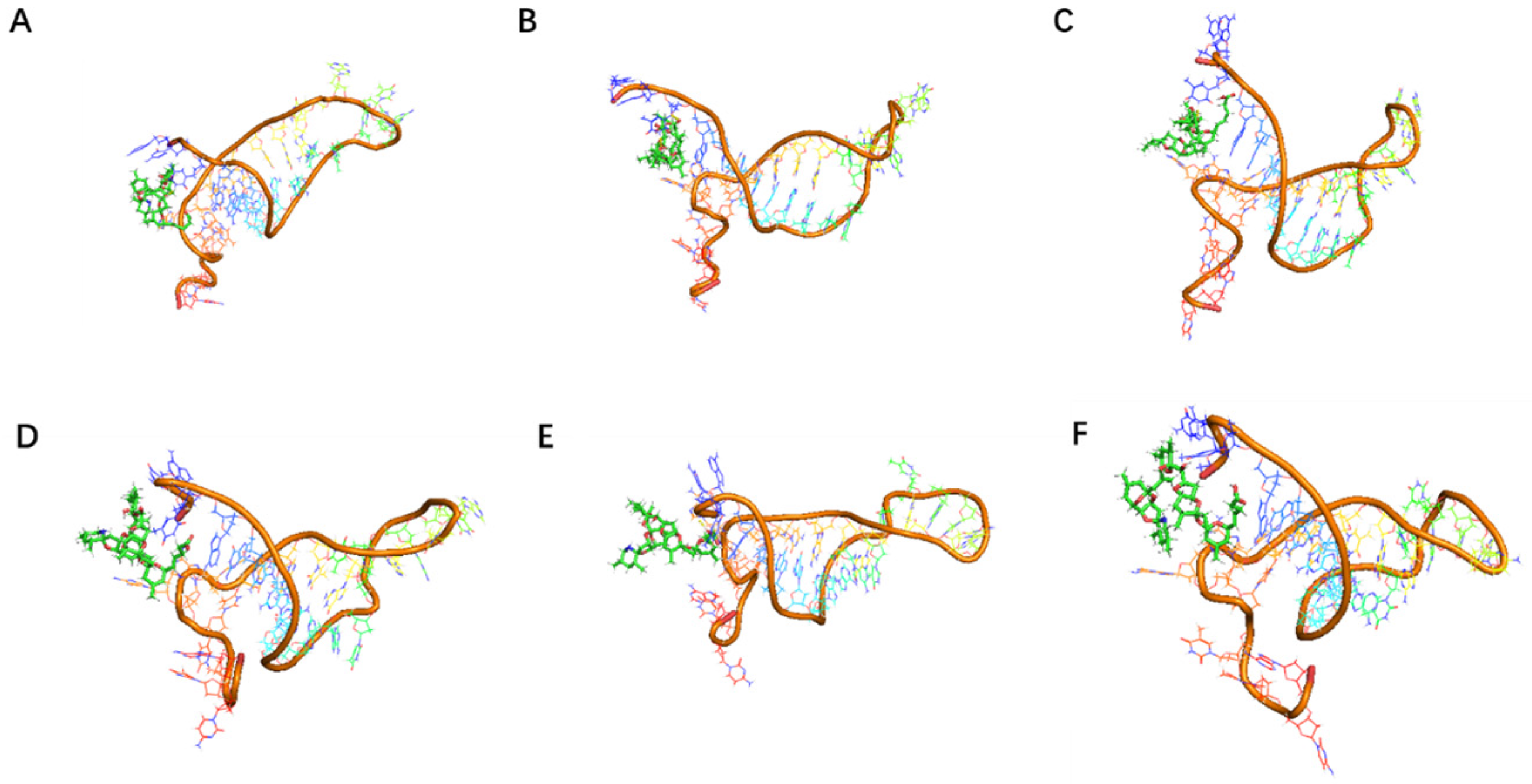
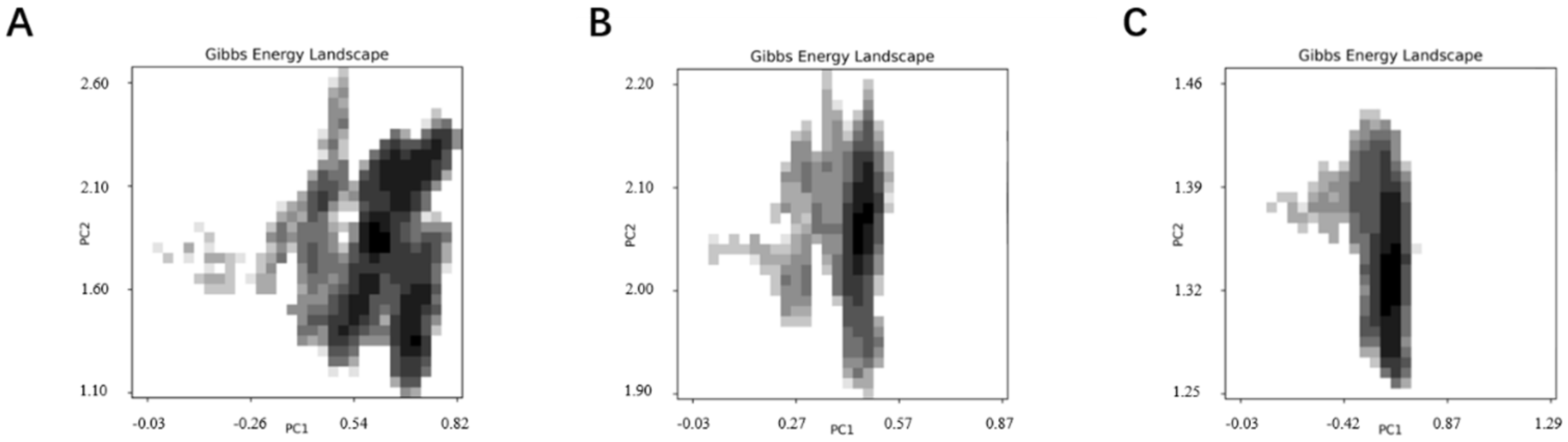
SELEX identifies the top 10 enriched sequences from the final round of sequence analysis. In the initial rounds, some sequences already rank high in read counts. However, this does not mean that we can directly perform one-round SELEX, as there are still many complex structures that require further competitive elimination. Moreover, due to the similar structures generated by similar sequences, there are subtle differences in affinity and specificity that still require additional rounds of elimination to distinguish.
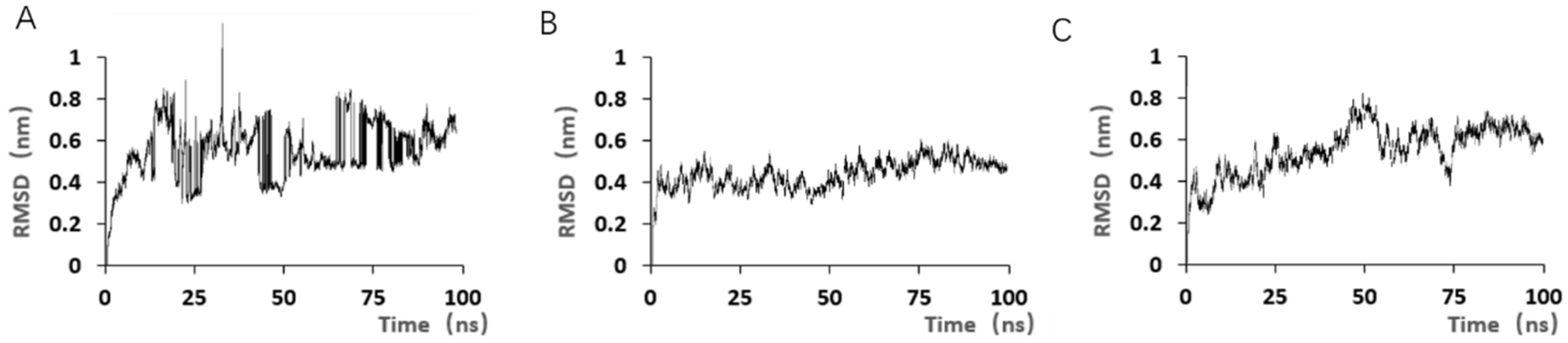
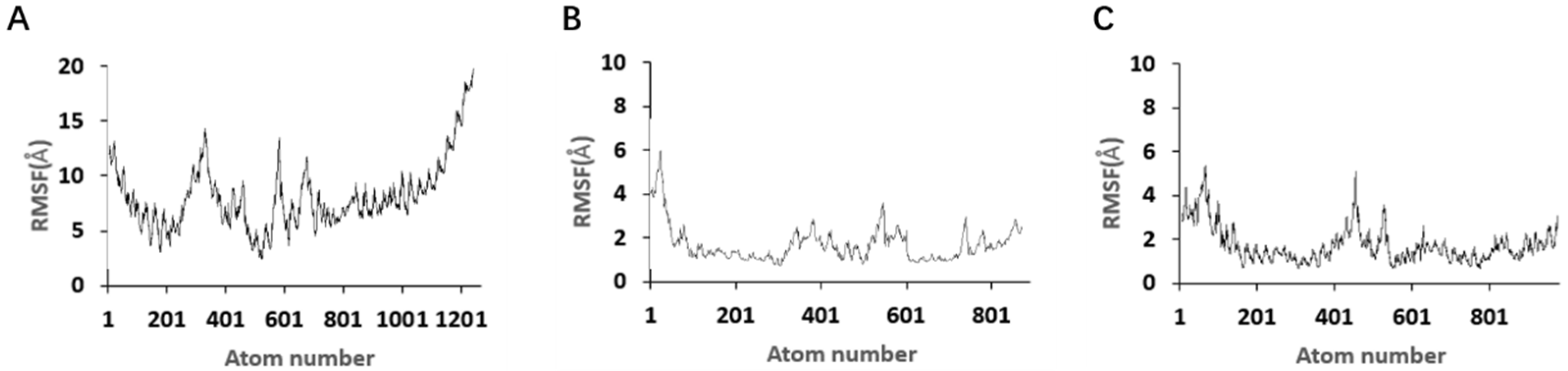
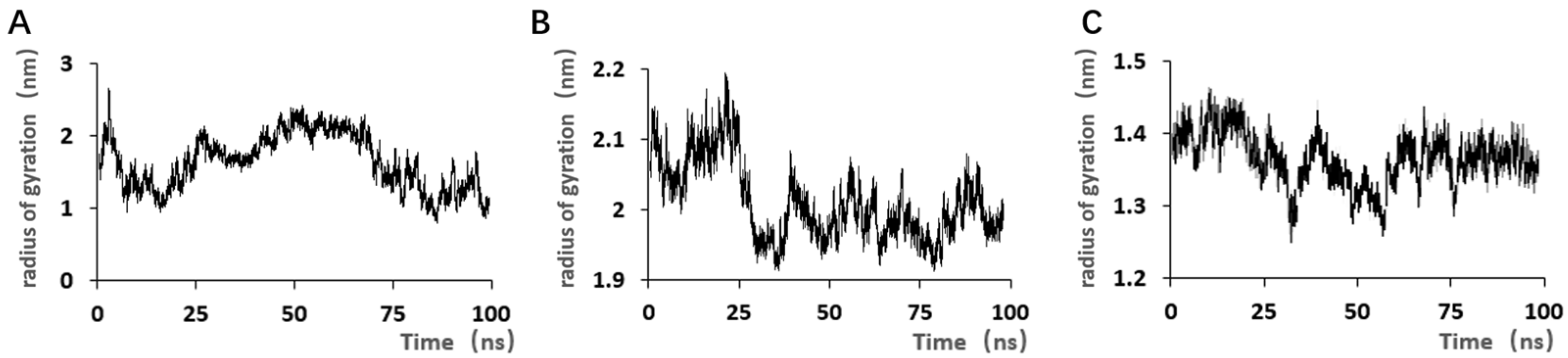
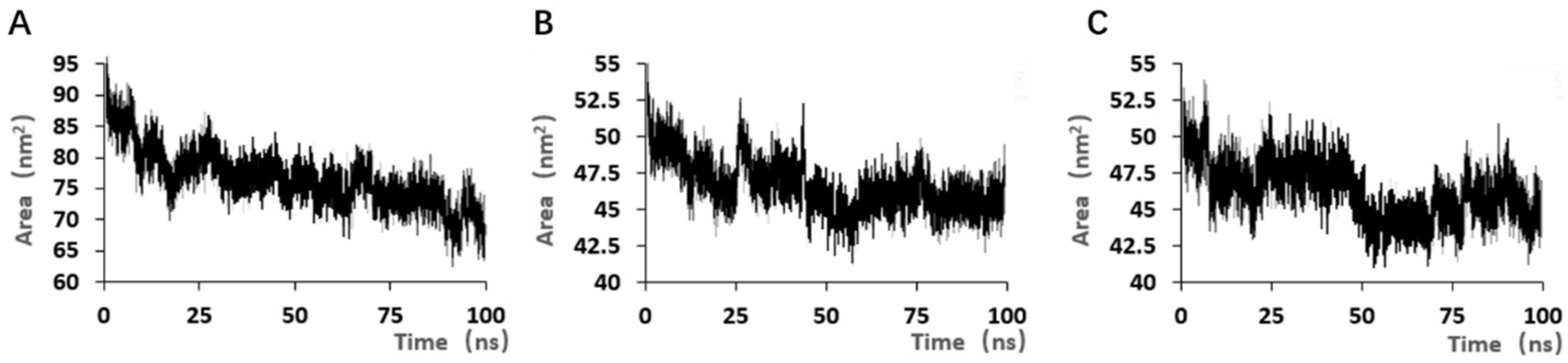
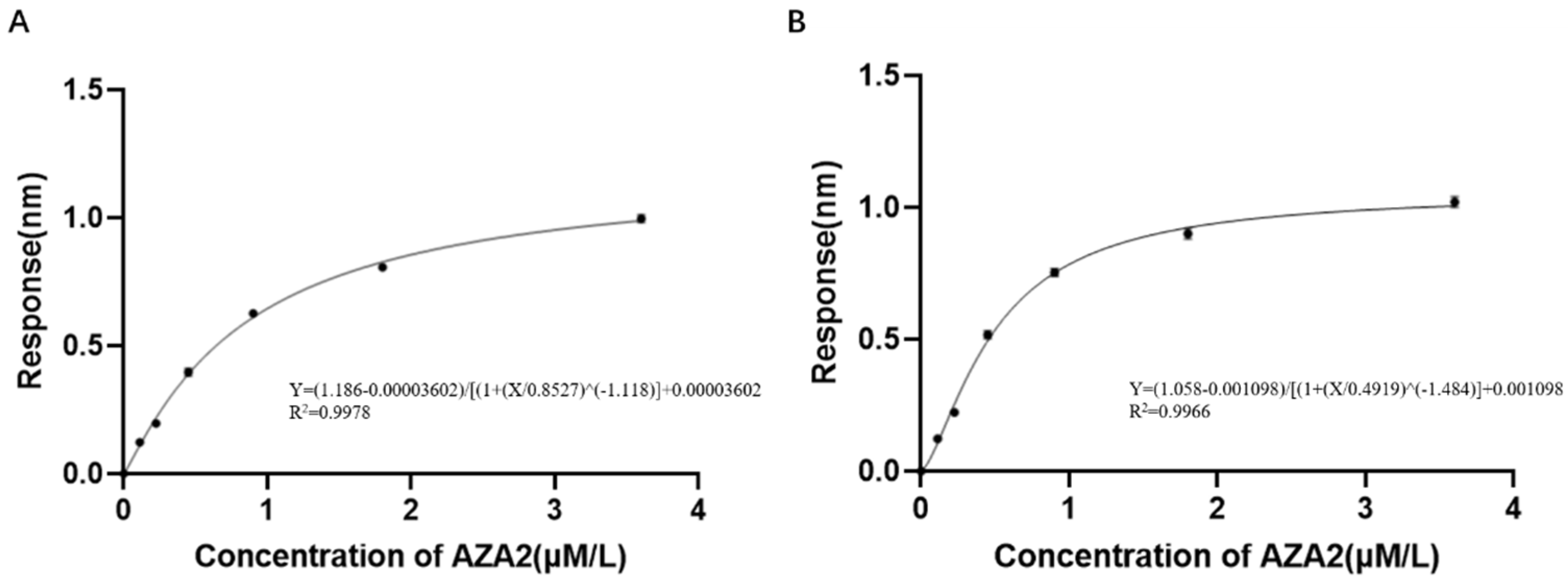
Although the selected aptamers exhibit high affinity and specificity, they may contain some non-essential nucleotides. These nucleotides not only increase the length and complexity of the aptamers but may also reduce their binding efficiency to the target [
49]. Therefore, optimizing the aptamers is necessary. In this study, we truncated the aptamers by shortening the primers at both ends and further validated the effectiveness of the optimization strategy through molecular docking and dynamics simulations. The aptamer optimization in this study primarily focused on truncation. During truncation, as many existing sequences as possible were removed without disrupting existing base pairings. If changes in the docking site or a significant decrease in affinity occurred, we referred to the sequences in the selected library for backfilling or designed by adding or reducing paired base sequences. This process did not involve large-scale affinity measurements of aptamers, which may have resulted in the loss of high-affinity aptamers. Additionally, the optimization process did not employ other methods, which also reduced the likelihood of obtaining aptamers with higher affinity. Methods such as phosphate backbone modification, sugar ring modification, optimization of the binding environment, and combining aptamers with other functional molecules (such as antibody fragments, nanomaterials) can further enhance the affinity of aptamers for their targets by improving nuclease resistance and thermal stability [
50].
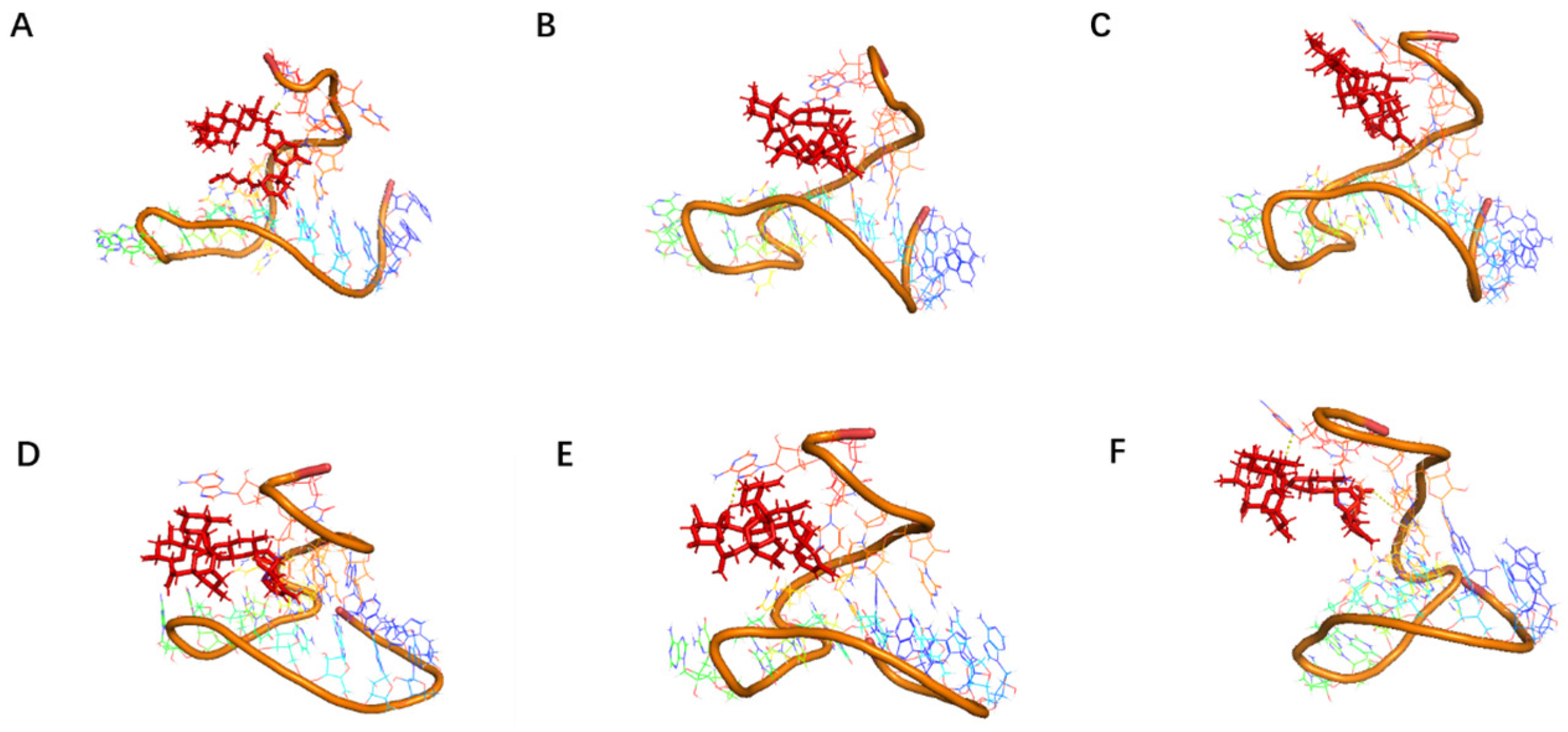
Docking results can only provide a reference for truncation optimization. Sometimes, using several random aptamer sequences for docking can yield docking results, but these may not have practical research applications. Ultimately, it is necessary to revert to affinity detection methods such as biolayer interferometry (BLI), microscale thermophoresis (MST) [
51], surface plasmon resonance (SPR) [
52], isothermal titration calorimetry (ITC) [
53], and fluorescence resonance energy transfer (FRET) [
54] for validation. Different affinity characterization methods are not universally applicable. This experiment only used a single molecular detection method, and incorporating more affinity characterization methods could further enhance credibility.
Although the study preliminarily explored the binding mechanism between aptamers and AZA-2 using computer simulations, the research exploring the mechanism is not yet in-depth. In the future, multiple technical means, such as X-ray crystallography and nuclear magnetic resonance, should be further employed to deeply analyze the interaction mechanism between aptamers and their targets [
55,
56].
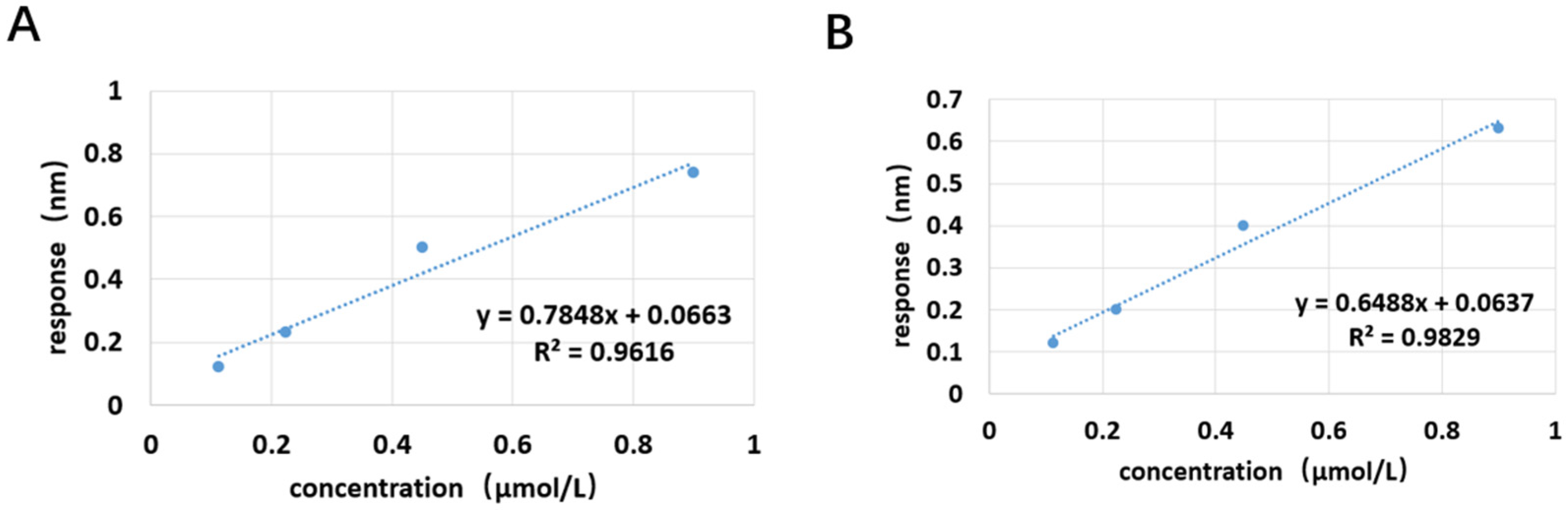
The application of spike detection in this experiment is highly persuasive. The sensitivity of the sensor designed in this experiment is comparable to that of ELISA, but its sensitivity cannot match that of the LC-MS detection method (
Table 4). However, the detection results in environmental samples may be less convincing. 1. The distribution of toxins, especially marine biotoxins, in the environment is inherently sparse, and the distribution and occurrence of azaspiracids in China are relatively rare, reducing the likelihood of toxin detection. 2. The lack of positive control detection results based on existing detection methods such as LC-MS further reduces persuasiveness. In the future, it is necessary to learn relevant technologies and achieve controlled detection. 3. Sampling during algal blooms or harmful algal outbreaks may help improve the detection significance and expected detection rate. Additionally, the sensor developed in this experiment has not been tested in replicate environmental samples. In the future, the detection performance of the sensor under complex environments can be evaluated to further improve the affinity and specificity of aptamers, optimize the detection sensitivity and stability of the sensor, and expand its application scope.