Optimization and Preparation of Polysaccharide–Protamine Microspheres with Enhanced Hemostatic and Antibacterial Properties for Wound Healing
Abstract
1. Introduction
2. Results
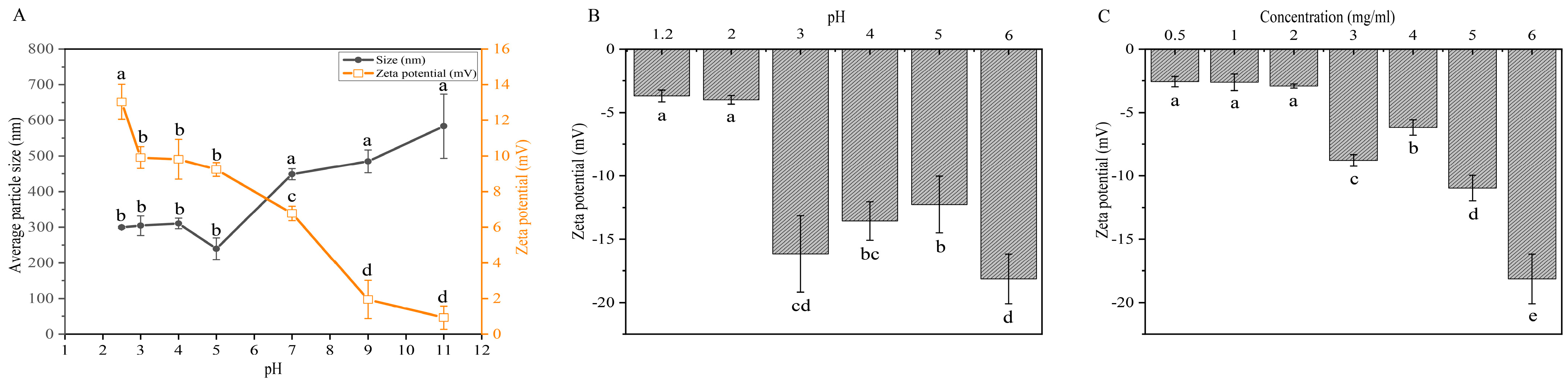
2.1. Effects of pH and Concentration on Electrostatic Properties of Protamine and CMS for Monolayer Microspheres Assembly
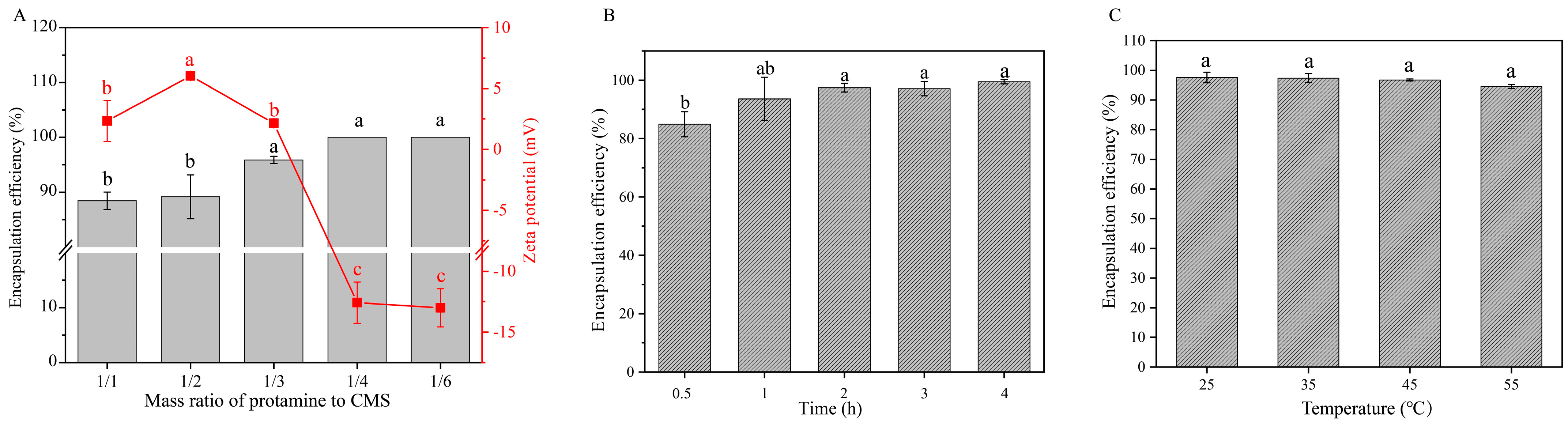
2.2. Effects of Mass Ratio, Assembly Time, and Temperature on Encapsulation Efficiency of Protamine Monolayer Assembly
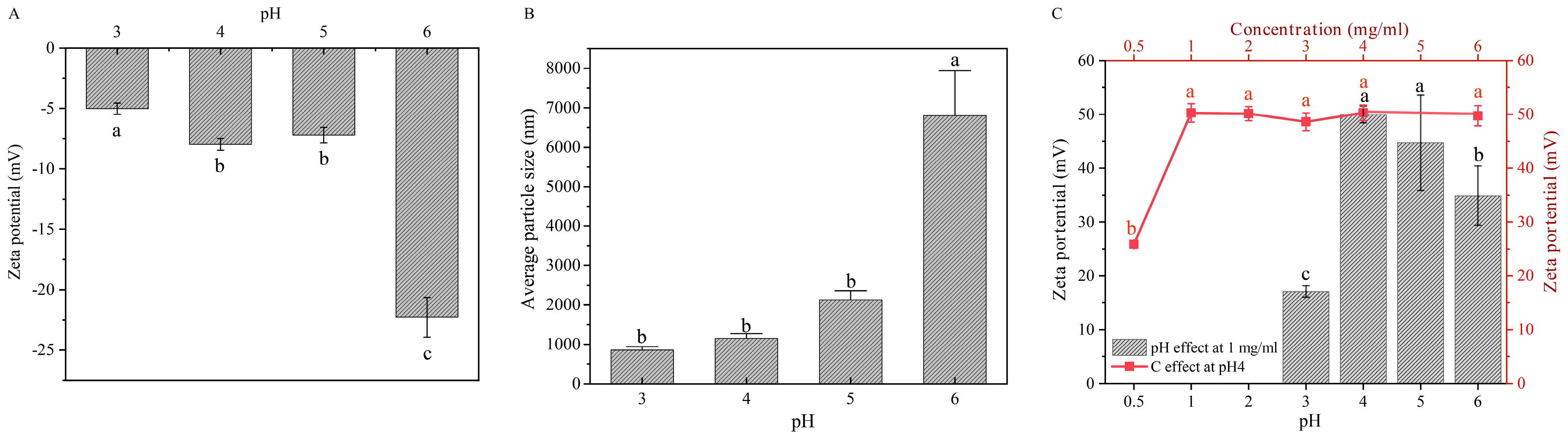
2.3. Effects of pH and HACC Concentration on the Preparation of Bilayer Assemblies
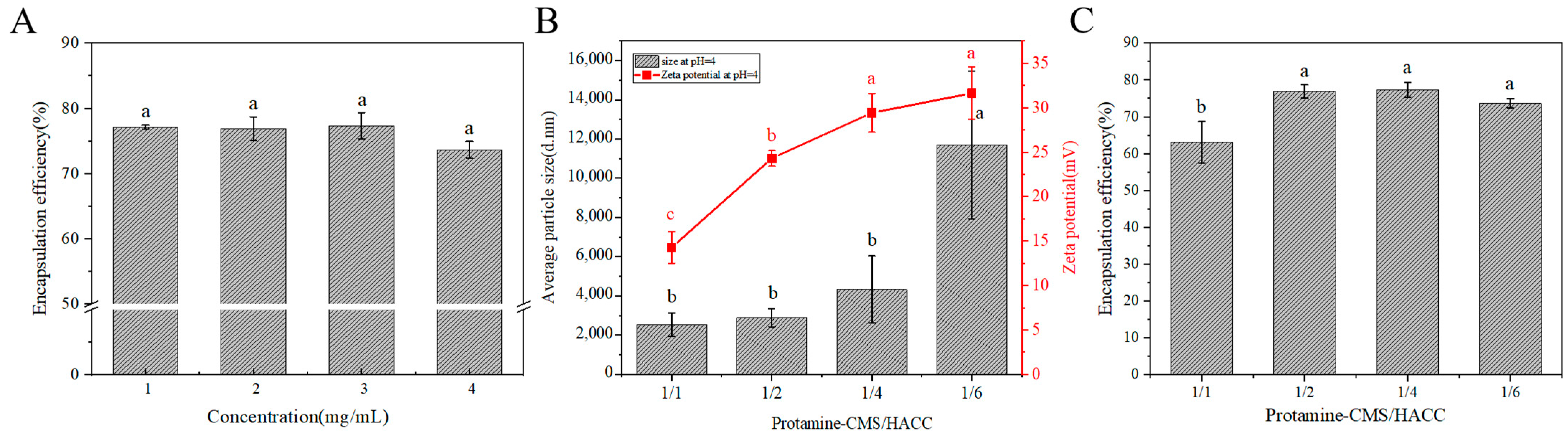
2.4. Effects of HACC Concentration and Mass Ratio of (Protamine-CMS)/HACC on Encapsulation Efficiency of Protamine Bilayer Assembly
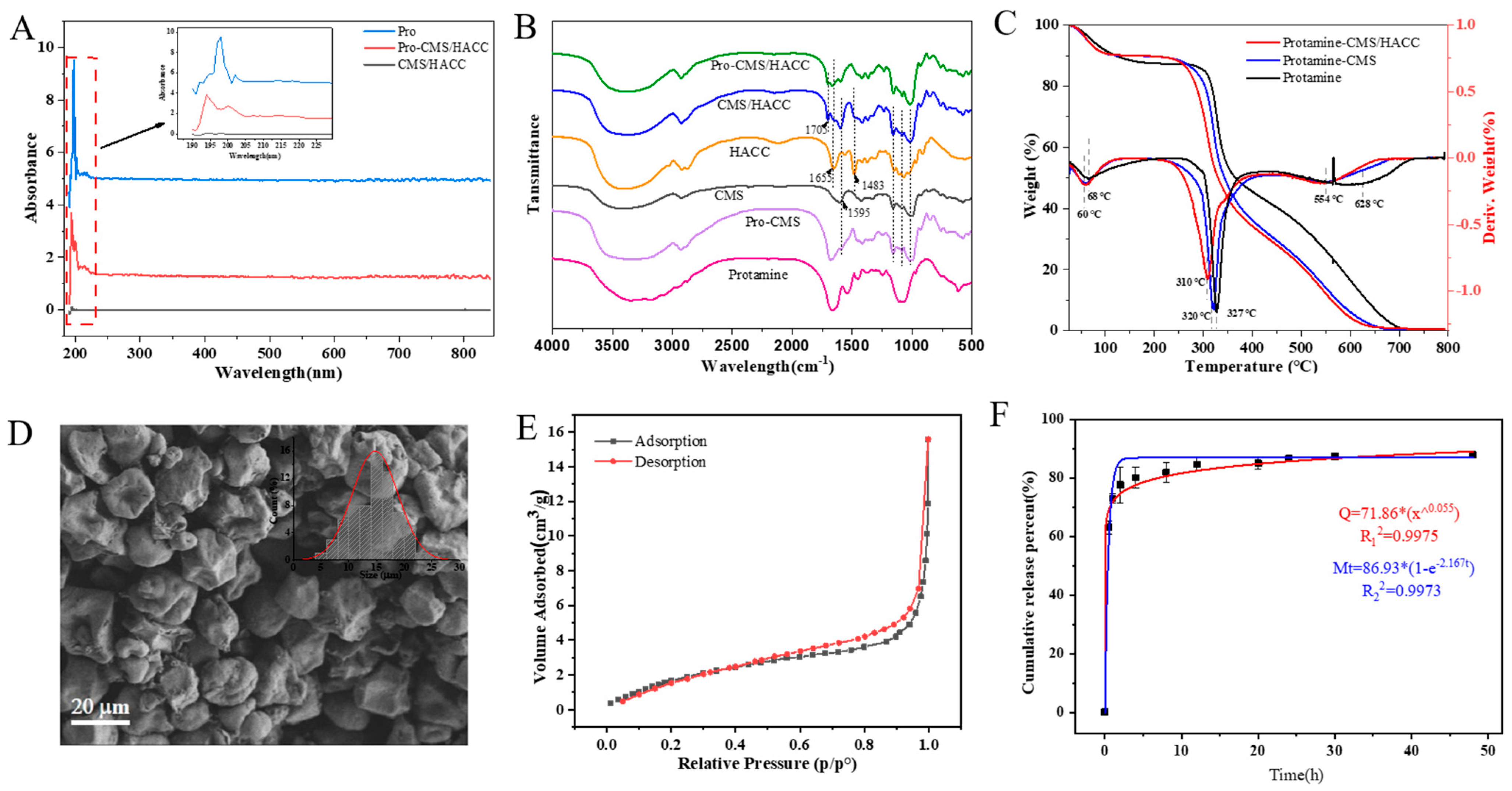
2.5. Characterization of Assembled Bilayer Microspheres
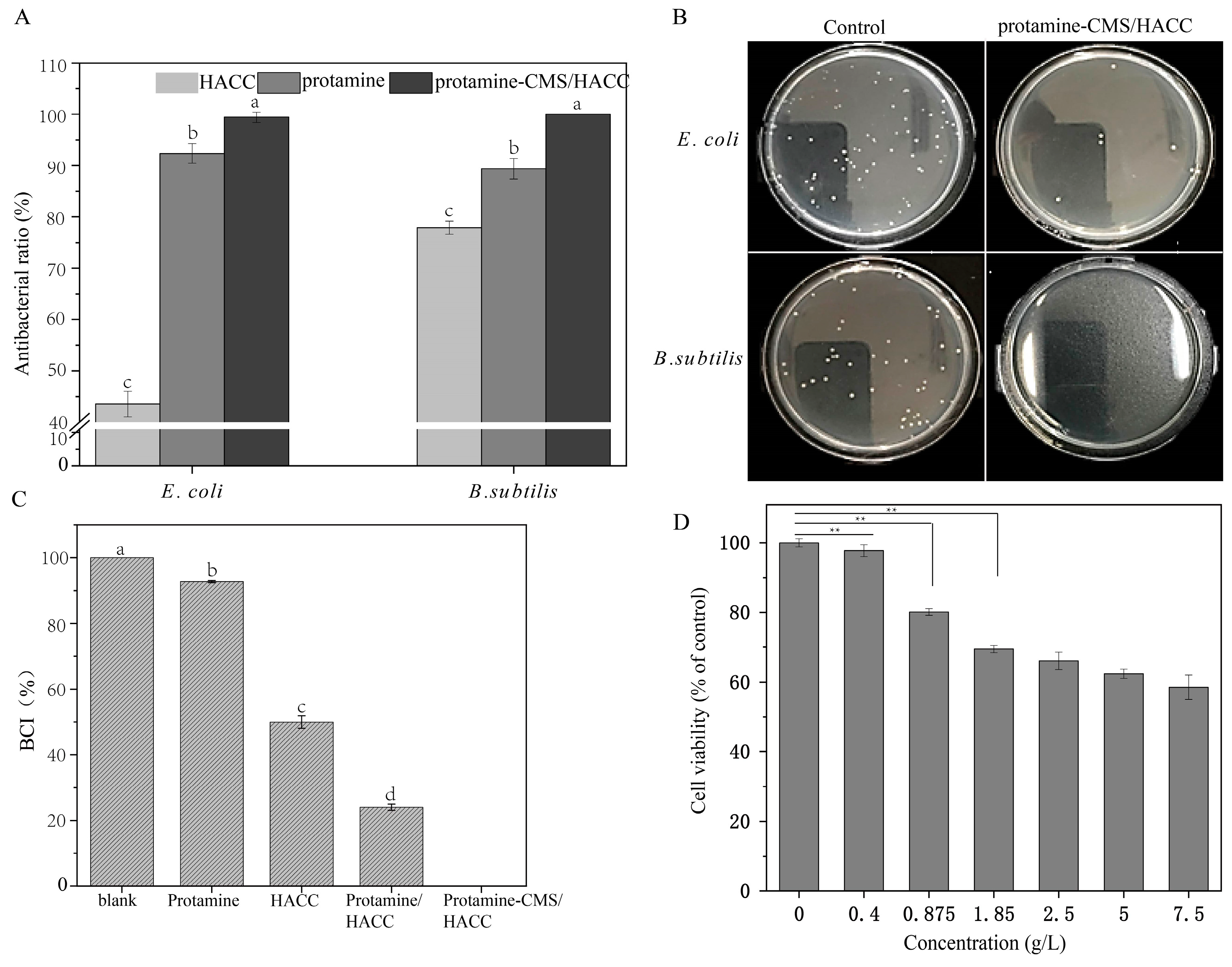
2.6. Antibacterial Efficacy and Coagulation Indices of the Bilayer Assemblies
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Crosslinker-Free Bilayer Assembly Microspheres
4.3. Microsphere Characterization
4.4. Antibacterial Test
4.5. In Vitro Cytotoxicity and Compatibility
4.6. In Vitro Hemostatic Evaluation
4.7. Release Characteristics Characterization
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zeibi Shirejini, S.; Carberry, J.; Alt, K.; Gregory, S.D.; Hagemeyer, C.E. Shear-Responsive Drug Delivery Systems in Medical Devices: Focus on Thrombosis and Bleeding. Adv. Funct. Mater. 2023, 33, 2303717. [Google Scholar] [CrossRef]
- Mecwan, M.; Li, J.; Falcone, N.; Ermis, M.; Torres, E.; Morales, R.; Hassani, A.; Haghniaz, R.; Mandal, K.; Sharma, S.; et al. Recent advances in biopolymer-based hemostatic materials. Regen. Biomater. 2022, 9, rbac063. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, M.; Liu, Q.; Liu, G.; Wang, S.; Li, J. Recent advances in the medical applications of hemostatic materials. Theranostics 2023, 13, 161–196. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Verma, C.; Singh, P.; Mukhopadhyay, S.; Gupta, A.; Gupta, B. Alginate based biomaterials for hemostatic applications: Innovations and developments. Int. J. Biol. Macromol. 2024, 264 Pt 2, 130771. [Google Scholar]
- Zhou, L.; Zheng, H.; Liu, Z.; Wang, S.; Liu, Z.; Chen, F.; Zhang, H.; Kong, J.; Zhou, F.; Zhang, Q. Conductive Antibacterial Hemostatic Multifunctional Scaffolds Based on Ti(3)C(2)T(x) MXene Nanosheets for Promoting Multidrug-Resistant Bacteria-Infected Wound Healing. ACS Nano 2021, 15, 2468–2480. [Google Scholar] [CrossRef] [PubMed]
- Verbanic, S.; Shen, Y.; Lee, J.; Deacon, J.M.; Chen, I.A. Microbial predictors of healing and short-term effect of debridement on the microbiome of chronic wounds. NPJ Biofilms Microbiomes 2020, 6, 21. [Google Scholar]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [PubMed]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar]
- Yoo, M.S.; Jin, H.J.; Lee, S.Y. Synergistic Antibacterial Efficacies of Chlorhexidine Digluconate or Protamine Sulfate Combined with Laminaria japonica or Rosmarinus officinalis Extracts against Streptococcus mutans. Biocontrol Sci. 2020, 25, 41–44. [Google Scholar]
- Törnudd, M.; Ramström, S.; Kvitting, J.P.; Alfredsson, J.; Pihl, R.; Berg, S. Protamine stimulates platelet aggregation in vitro with activation of the fibrinogen receptor and alpha-granule release, but impairs secondary activation via ADP and thrombin receptors. Platelets 2021, 32, 90–96. [Google Scholar] [CrossRef]
- Olsson, A.; Alfredsson, J.; Thelander, M.; Svedjeholm, R.; Berglund, J.S.; Berg, S. Activated platelet aggregation is transiently impaired also by a reduced dose of protamine. Scand. Cardiovasc. J. 2019, 53, 355–360. [Google Scholar] [CrossRef]
- Kunz, S.A.; Miles, L.F.; Ianno, D.J.; Mirowska-Allen, K.L.; Matalanis, G.; Bellomo, R.; Seevanayagam, S. The effect of protamine dosing variation on bleeding and transfusion after heparinisation for cardiopulmonary bypass. Perfusion 2018, 33, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hakeem, M.A.; Abdel-Haseb, O.M.; Abdel-Ghany, S.E.; Cevik, E.; Sabit, H. Doxorubicin loaded on chitosan-protamine nanoparticles triggers apoptosis via downregulating Bcl-2 in breast cancer cells. J. Drug Deliv. Sci. Technol. 2020, 55, 101423. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Choudhury, P.; Das, P.K. Fabrication of Orange-Emitting Organic Nanoparticle-Protamine Conjugate: Fluorimetric Sensor of Heparin. Langmuir 2019, 35, 15180–15191. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Gao, Y.; He, L.; Ge, W.; Liu, J.; Yu, Y.; Xie, X. Multifunctional polysaccharide composited microneedle for oral ulcers healing. Mater Today Bio 2023, 22, 100782. [Google Scholar] [CrossRef]
- Zhou, P.; Xia, Y.; Wang, J.; Liang, C.; Yu, L.; Tang, W.; Gu, S.; Xu, S. Antibacterial properties and bioactivity of HACC- and HACC-Zein-modified mesoporous bioactive glass scaffolds. J. Mater. Chem. B 2013, 1, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yang, Z.; Xun, X.; Ma, L.; Chen, Z.; Hu, X.; Wu, X.; Wan, Y.; Ao, H. De novo strategy with engineering a multifunctional bacterial cellulose-based dressing for rapid healing of infected wounds. Bioact. Mater. 2022, 13, 212–222. [Google Scholar] [CrossRef]
- Xu, C.; Zeng, X.; Yang, Z.; Ji, H. Enhanced Sunscreen Effects via Layer-By-Layer Self-Assembly of Chitosan/Sodium Alginate/Calcium Chloride/EHA. Molecules 2022, 27, 1148. [Google Scholar] [CrossRef] [PubMed]
- Wågberg, L.; Erlandsson, J. The Use of Layer-by-Layer Self-Assembly and Nanocellulose to Prepare Advanced Functional Materials. Adv. Mater. 2021, 33, e2001474. [Google Scholar] [CrossRef]
- Nita, L.E.; Chiriac, A.P.; Rusu, A.G.; Ghilan, A.; Dumitriu, R.P.; Bercea, M.; Tudorachi, N. Stimuli Responsive Scaffolds Based on Carboxymethyl Starch and Poly(2-Dimethylaminoethyl Methacrylate) for Anti-Inflammatory Drug Delivery. Macromol. Biosci. 2020, 20, e1900412. [Google Scholar] [CrossRef]
- Li, X.-M.; Xie, Q.-T.; Zhu, J.; Pan, Y.; Meng, R.; Zhang, B.; Chen, H.-Q.; Jin, Z.-Y. Chitosan hydrochloride/carboxymethyl starch complex nanogels as novel Pickering stabilizers: Physical stability and rheological properties. Food Hydrocoll. 2019, 93, 215–225. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Namazi, H. Developments on carboxymethyl starch-based smart systems as promising drug carriers: A review. Carbohydr. Polym. 2021, 258, 117654. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, Y.; Shi, Y.; Zhao, L. pH-responsive calcium alginate hydrogel laden with protamine nanoparticles and hyaluronan oligosaccharide promotes diabetic wound healing by enhancing angiogenesis and antibacterial activity. Drug Deliv. Transl. Res. 2019, 9, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Tao, H.; Tan, C.; Yuan, F.; Guo, L.; Cui, B.; Gao, S.; Wu, Z.; Zou, F.; Wu, Z.; et al. Adsorption properties of corn starch modified by malt amylases and crosslinking agents: A comparison between sodium trimetaphosphate and organic acids. Int. J. Biol. Macromol. 2023, 253, 127140. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Dong, R.; Liang, Y.; Li, M. Haemostatic materials for wound healing applications. Nat. Rev. Chem. 2021, 5, 773–791. [Google Scholar] [CrossRef]
- El-Sakhawy, M.; Salama, A.; Tohamy, H.S. Applications of propolis-based materials in wound healing. Arch. Dermatol. Res. 2023, 316, 61. [Google Scholar] [CrossRef]
- Mo, Z.; Ma, Y.; Chen, W.; You, L.; Liu, W.; Zhou, Q.; Zeng, Z.; Chen, T.; Li, H.; Tang, S. Protamine-grafted carboxymethyl chitosan based hydrogel with adhesive and long-term antibacterial properties for hemostasis and skin wound healing. Carbohydr. Polym. 2024, 336, 122125. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sa, Y.; Li, P.; Guo, Y.; Du, Y.; Deng, H.; Jiang, T.; Wang, Y. A versatile and injectable poly(methyl methacrylate) cement functionalized with quaternized chitosan-glycerophosphate/nanosized hydroxyapatite hydrogels. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Panwar, V.; Sharma, A.; Thomas, J.; Chopra, V.; Kaushik, S.; Kumar, A.; Ghosh, D. In-vitro and In-vivo evaluation of biocompatible and biodegradable calcium-modified carboxymethyl starch as a topical hemostat. Materialia 2019, 7, 100373. [Google Scholar] [CrossRef]
- Tamara, F.R.; Lin, C.; Mi, F.L.; Ho, Y.C. Antibacterial Effects of Chitosan/Cationic Peptide Nanoparticles. Nanomaterials 2018, 8, 88. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yao, W.; Tian, M.; Wei, J.; Song, Q.; Qiao, W. Mussel-inspired degradable antibacterial polydopamine/silica nanoparticle for rapid hemostasis. Biomaterials 2018, 179, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Escobar, A.; Muzzio, N.; Moya, S.E. Antibacterial Layer-by-Layer Coatings for Medical Implants. Pharmaceutics 2020, 13, 16. [Google Scholar] [CrossRef]
- Liu, X.Q.; Picart, C. Layer-by-Layer Assemblies for Cancer Treatment and Diagnosis. Adv. Mater. 2016, 28, 1295–1301. [Google Scholar] [PubMed]
- Lai, W.F.; Wong, W.T.; Rogach, A.L. Molecular Design of Layer-by-Layer Functionalized Liposomes for Oral Drug Delivery. ACS Appl. Mater. Interfaces 2020, 12, 43341–43351. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Y.; Kun, Q.; Qing, G.; Weixia, S.; Ping, C.; Wei, S.; Wang, Y. Balance of disinfection and cytotoxicity of hydroxypropyltrimethyl ammonium chloride chitosan with polyhexamethylene biguanide at low concentrations. J. Macromol. Sci. Part A 2017, 54, 923–930. [Google Scholar] [CrossRef]
- Peng, Z.-X.; Wang, L.; Du, L.; Guo, S.-R.; Wang, X.-Q.; Tang, T.-T. Adjustment of the antibacterial activity and biocompatibility of hydroxypropyltrimethyl ammonium chloride chitosan by varying the degree of substitution of quaternary ammonium. Carbohydr. Polym. 2010, 81, 275–283. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, L.; Gu, X.; Wei, L.; Wang, J.; Wang, Y. Biological Synergy and Antimicrobial Mechanism of Hydroxypropyltrimethyl Ammonium Chloride Chitosan with Benzalkonium Chloride. Chem. Pharm. Bull. 2021, 69, 612–619. [Google Scholar]
- Zhu, M.; Hu, X.; Liu, H.; Tian, J.; Yang, J.; Li, L.; Luo, B.; Zhou, C.; Lu, L. Antibacterial peptide encapsulation and sustained release from chitosan-based delivery system. Eur. Polym. J. 2022, 181, 111640. [Google Scholar] [CrossRef]
- Jahanizadeh, S.; Yazdian, F.; Marjani, A.; Omidi, M.; Rashedi, H. Curcumin-loaded chitosan/carboxymethyl starch/montmorillonite bio-nanocomposite for reduction of dental bacterial biofilm formation. Int. J. Biol. Macromol. 2017, 105, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-W.; Ho, Y.-C.; Tsai, T.-N.; Tseng, C.-L.; Lin, C.; Mi, F.-L. Enhancement of the permeability and activities of epigallocatechin gallate by quaternary ammonium chitosan/fucoidan nanoparticles. Carbohydr. Polym. 2020, 242, 116312. [Google Scholar] [CrossRef]
- Zhao, Z.; Han, J.; Xu, S.; Jin, Z.; Yin, T.H.; Zhao, K. Amoxicillin encapsulated in the N-2-hydroxypropyl trimethyl ammonium chloride chitosan and N,O-carboxymethyl chitosan nanoparticles: Preparation, characterization, and antibacterial activity. Int. J. Biol. Macromol. 2022, 221, 613–622. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, D.; Cheng, F.; Li, Y.; Zhang, S.; Zhao, X.; Zhao, Y. Optimization and Preparation of Polysaccharide–Protamine Microspheres with Enhanced Hemostatic and Antibacterial Properties for Wound Healing. Mar. Drugs 2025, 23, 160. https://doi.org/10.3390/md23040160
Mei D, Cheng F, Li Y, Zhang S, Zhao X, Zhao Y. Optimization and Preparation of Polysaccharide–Protamine Microspheres with Enhanced Hemostatic and Antibacterial Properties for Wound Healing. Marine Drugs. 2025; 23(4):160. https://doi.org/10.3390/md23040160
Chicago/Turabian StyleMei, Danling, Feifan Cheng, Yifan Li, Suzhen Zhang, Xueqin Zhao, and Yanyan Zhao. 2025. "Optimization and Preparation of Polysaccharide–Protamine Microspheres with Enhanced Hemostatic and Antibacterial Properties for Wound Healing" Marine Drugs 23, no. 4: 160. https://doi.org/10.3390/md23040160
APA StyleMei, D., Cheng, F., Li, Y., Zhang, S., Zhao, X., & Zhao, Y. (2025). Optimization and Preparation of Polysaccharide–Protamine Microspheres with Enhanced Hemostatic and Antibacterial Properties for Wound Healing. Marine Drugs, 23(4), 160. https://doi.org/10.3390/md23040160




