Sustainable and Integral Valorization of Dosidicus gigas Pen Waste: Combined Production of Chitosan with Antibacterial Properties and Human and Marine Probiotics
Abstract
1. Introduction
2. Results and Discussion
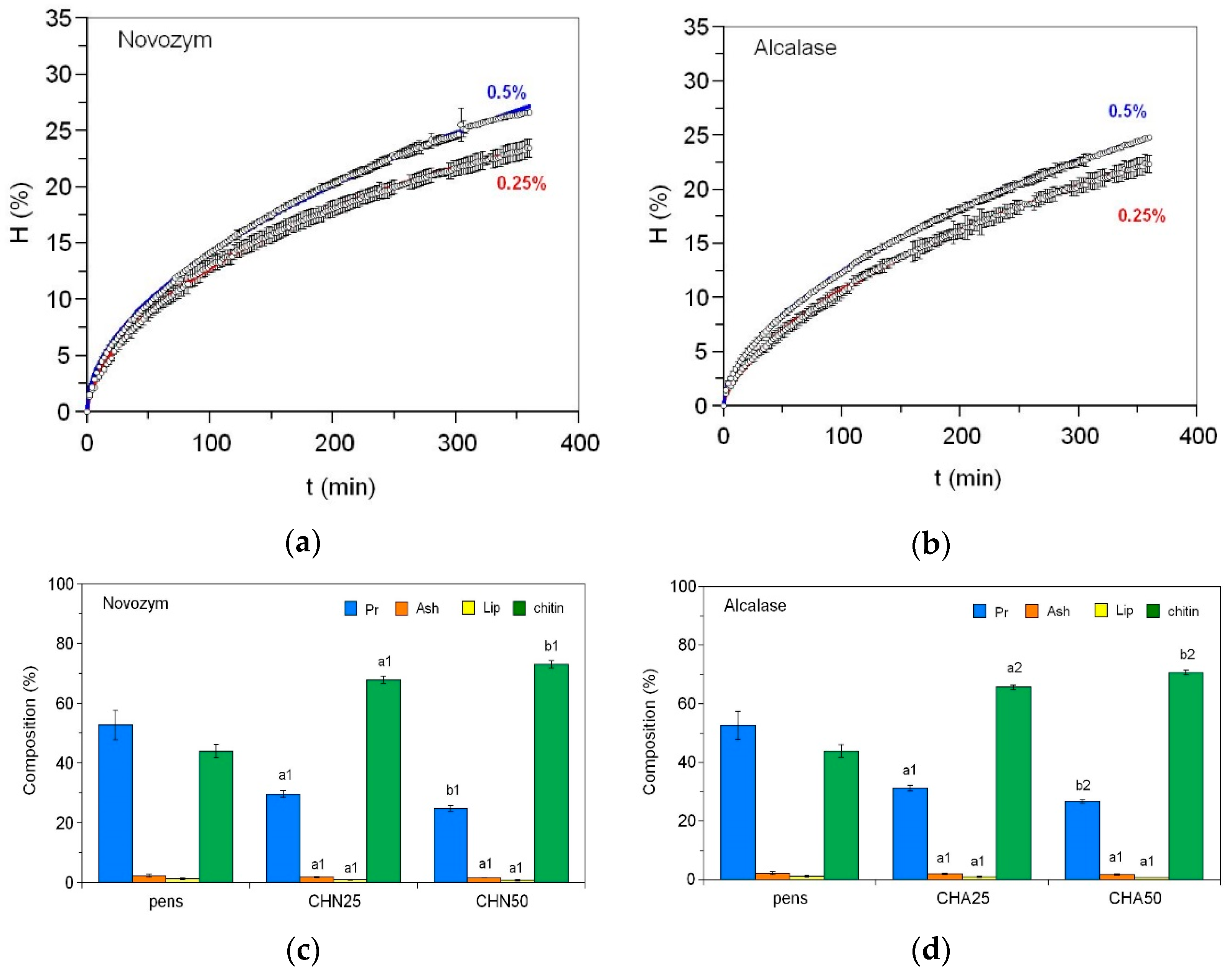
2.1. Pen Deproteinization for Chitin Production
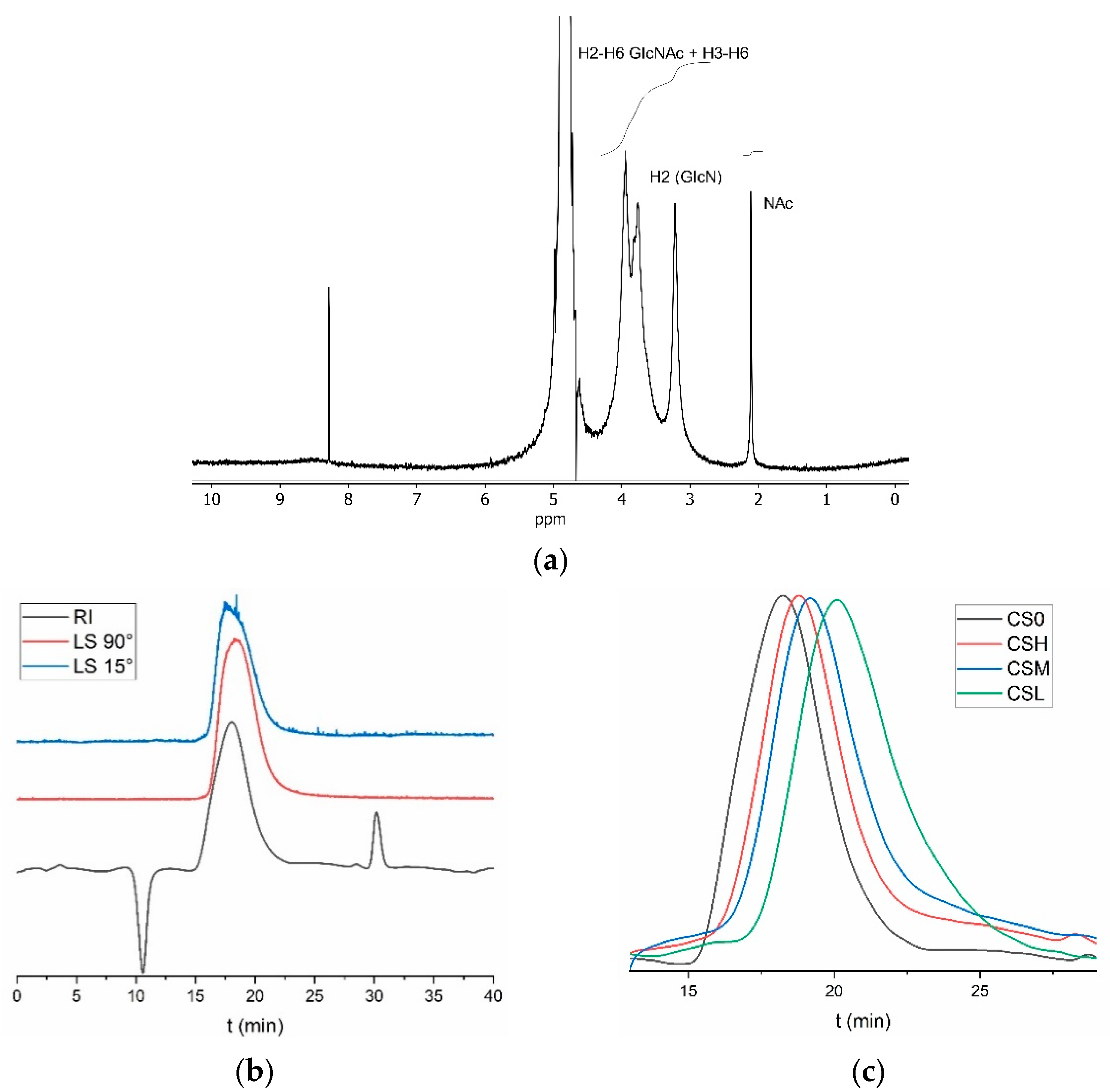
2.2. Chitin Deacetylation for Chitosan Production
2.3. Chitosan Depolymerization
2.4. Antimicrobial Activity
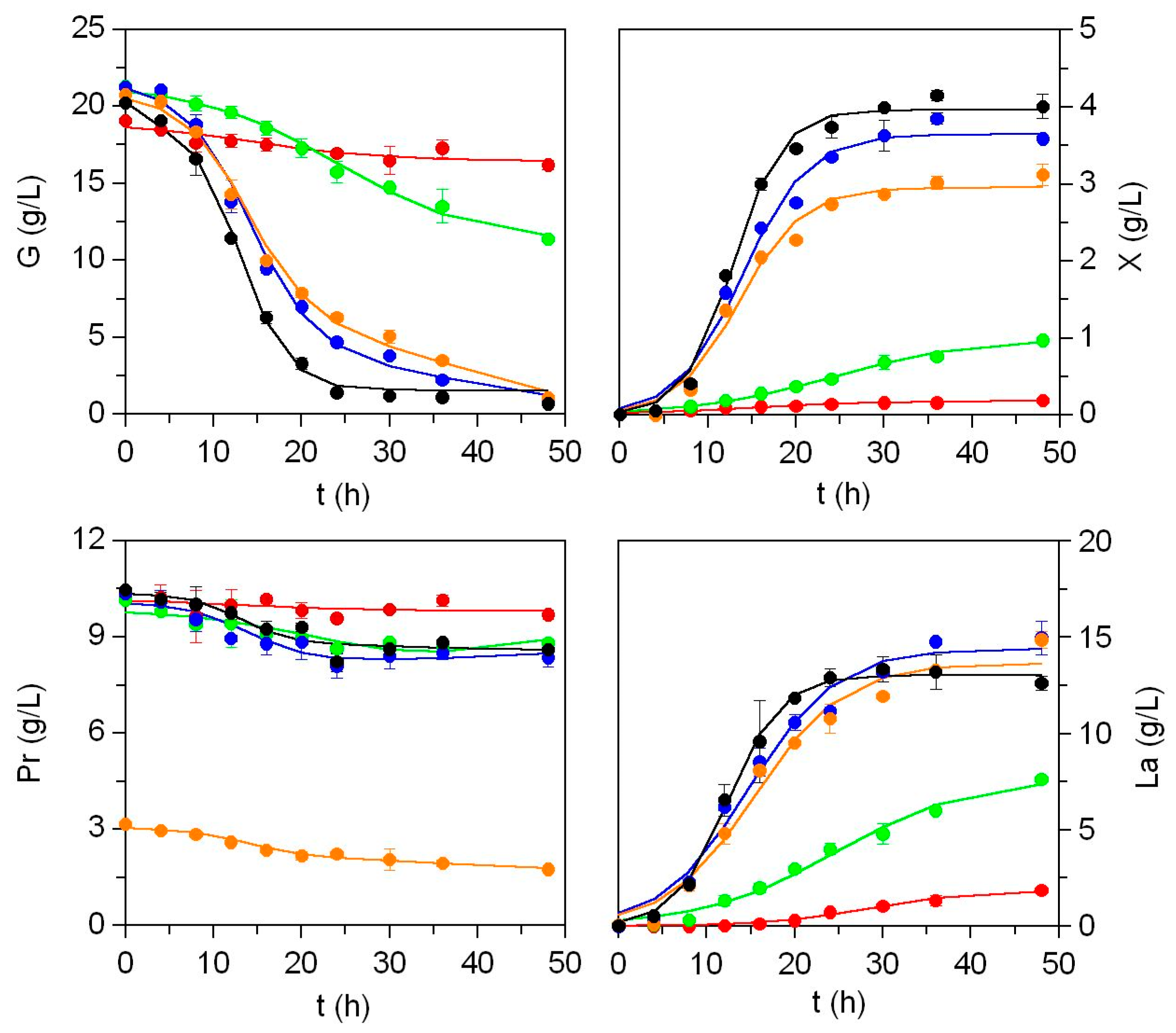
2.5. Bioconversion of Residual Effluents
2.5.1. Production of Human Probiotics
2.5.2. Production of Marine Probiotics
3. Materials and Methods
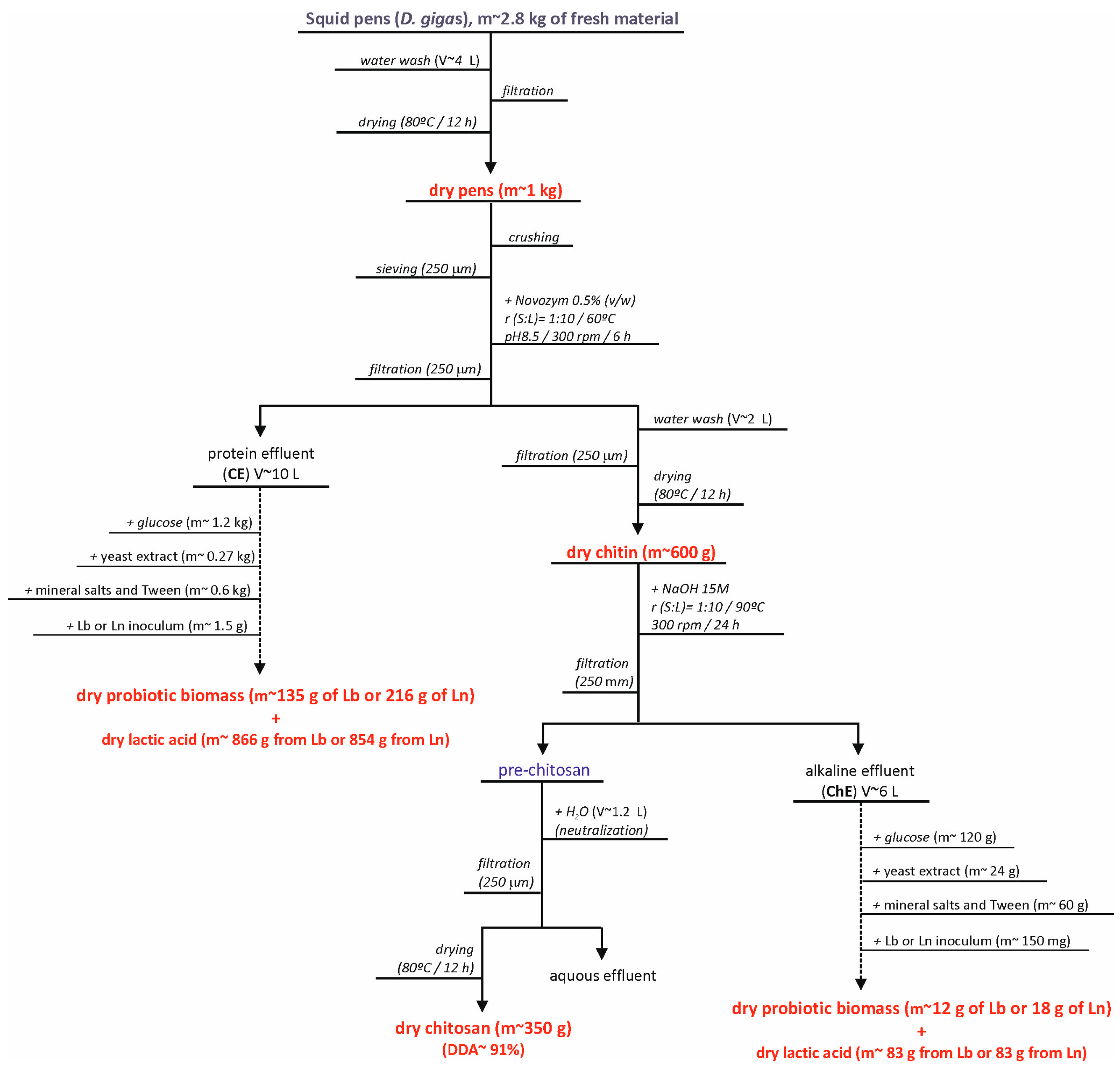
3.1. Squid Pen By-Products
3.2. Chitin Extraction
3.3. Chitin Deacetylation to Chitosan
3.4. Chitosan Purification and Depolymerization
3.5. Polymer Characterization
3.6. Chemical Analysis of Residual Effluents
3.7. Antimicrobial Activity
3.8. Residual Effluents Bioconversion by Probiotic Bacteria
3.9. Numerical Fitting and Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibáñez, C.M.; Sepúlveda, R.D.; Ulloa, P.; Keyl, F.; Pardo-Gandarillas, M.C. The biology and ecology of the jumbo squid Dosidicus gigas (Cephalopoda) in Chilean waters: A review. Lat. Am. J. Aquat. Res. 2015, 43, 402–414. [Google Scholar] [CrossRef]
- Zeidberg, L.D.; Robison, B.H. Invasive range expansion by the Humboldt squid, Dosidicus gigas, in the eastern North Pacific. Proc. Natl. Acad. Sci. USA 2007, 104, 12948–12950. [Google Scholar] [CrossRef]
- Arkhipkin, A.I.; Rodhouse, P.G.K.; Pierce, G.J.; Sauer, W.; Sakai, M.; Allcock, L.; Arguelles, J.; Bower, J.R.; Castillo, G.; Ceriola, L.; et al. World Squid Fisheries; Taylor & Francis: Abingdon, UK, 2015; Volume 23. [Google Scholar]
- FAO. Fishery and Aquaculture Statistics—Yearbook 2021; FAO Yearbook of Fishery and Aquaculture Statistics; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Liu, B.L.; Chen, X.J.; Li, J.H.; Fang, Z. Statolith microstructure, age, and maturity of jumbo squid (Dosidicus gigas) in equatorial waters of the eastern tropical Pacific Ocean. Bull. Mar. Sci. 2017, 93, 943–957. [Google Scholar] [CrossRef]
- Rodhouse, P.G.K.; Yamashiro, C.; Arguelles, J. Jumbo squid in the eastern Pacific Ocean: A quarter century of challenges and change. Fish. Res. 2016, 173, 109–112. [Google Scholar] [CrossRef]
- Zeidberg, L. Advances in Squid Biology, Ecology and Fisheries. In Part I–Myopsid Squids; Nova Science Publishers Inc.: New York, NY, USA, 2013; pp. 159–204. [Google Scholar]
- Yan, N.; Chen, X. Sustainability: Don’t waste seafood waste. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef]
- Hülsey, M.J. Shell biorefinery: A comprehensive introduction. Green Energy Environ. 2018, 3, 318–327. [Google Scholar] [CrossRef]
- Oyatogun, G.M.; Esan, T.A.; Akpan, E.I.; Adeosun, S.O.; Popoola, A.P.I.; Imasogie, B.I.; Soboyejo, W.O.; Afonja, A.A.; Ibitoye, S.A.; Abere, V.D. Chitin, chitosan, marine to market. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. 335–376. [Google Scholar]
- Pellis, A.; Guebitz, G.M.; Nyanhongo, G.S. Chitosan: Sources, Processing and Modification Techniques. Gels 2022, 8, 393. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Ramos, P.; Mirón, J.; Valcarcel, J.; Sotelo, C.G.; Pérez-Martín, R.I. Production of chitin from penaeus vannamei by-products to pilot plant scale using a combination of enzymatic and chemical processes and subsequent optimization of the chemical production of chitosan by response surface methodology. Mar. Drugs 2017, 15, 180. [Google Scholar] [CrossRef] [PubMed]
- Domard, A. A perspective on 30 years research on chitin and chitosan. Carbohydr. Polym. 2011, 84, 696–703. [Google Scholar] [CrossRef]
- Srinivasan, H.; Kanayairam, V.; Ravichandran, R. Chitin and chitosan preparation from shrimp shells Penaeus monodon and its human ovarian cancer cell line, PA-1. Int. J. Biol. Macromol. 2018, 107, 662–667. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Ramos, P.; Valcarcel, J.; Antelo, L.T.; Novoa-Carballal, R.; Reis, R.L.; Pérez-Martín, R.I. An integral and sustainable valorisation strategy of squid pen by-products. J. Clean. Prod. 2018, 201, 207–218. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Noriega, D.; Ramos, P.; Valcarcel, J.; Novoa-Carballal, R.; Pastrana, L.; Reis, R.L.; Pérez-Martín, R.I. Optimization of high purity chitin and chitosan production from Illex argentinus pens by a combination of enzymatic and chemical processes. Carbohydr. Polym. 2017, 174, 262–272. [Google Scholar] [CrossRef]
- Goy, R.C.; Britto, D.d.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Cheung, R.; Ng, T.; Wong, J.; Chan, W. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156. [Google Scholar] [CrossRef] [PubMed]
- D’Almeida, M.; Attik, N.; Amalric, J.; Brunon, C.; Renaud, F.; Abouelleil, H.; Toury, B.; Grosgogeat, B. Chitosan coating as an antibacterial surface for biomedical applications. PLoS ONE 2017, 12, e0189537. [Google Scholar] [CrossRef] [PubMed]
- Tikhonov, V.E.; Stepnova, E.A.; Babak, V.G.; Yamskov, I.A.; Palma-Guerrero, J.; Jansson, H.-B.; Lopez-Llorca, L.V.; Salinas, J.; Gerasimenko, D.V.; Avdienko, I.D.; et al. Bactericidal and antifungal activities of a low molecular weight chitosan and its N-/2(3)-(dodec-2-enyl)succinoyl/-derivatives. Carbohydr. Polym. 2006, 64, 66–72. [Google Scholar] [CrossRef]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Shavandi, A.; Hu, Z.; Teh, S.; Zhao, J.; Carne, A.; Bekhit, A.; Bekhit, A.E.-D.A. Antioxidant and functional properties of protein hydrolysates obtained from squid pen chitosan extraction effluent. Food Chem. 2017, 227, 194–201. [Google Scholar] [CrossRef]
- Casero-Díaz, T.; Castro-Barros, C.; Taboada-Santos, A.; Rodríguez-Hernández, L.; Mauricio-Iglesias, M.; Carballa, M. Turning fish canning wastewater into resources: Effluents and operational conditions selection for volatile fatty acids production. J. Water Process Eng. 2024, 64, 105738. [Google Scholar] [CrossRef]
- Huang, L.P.; Dong, T.; Chen, J.W.; Li, N. Biotechnological production of lactic acid integrated with fishmeal wastewater treatment by Rhizopus oryzae. Bioprocess Biosyst. Eng. 2007, 30, 135–140. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Menduíña, A.; Durán, A.I.; Nogueira, M.; Fraguas, J.; Pedreira, A.; Valcarcel, J. Microbial bioconversion of chemical waste effluents from marine gelatin isolation: Production of probiotics under circular economy philosophy. J. Clean. Prod. 2023, 416, 137952. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Pedreira, A.; Salmerón, I.; Wardhani, D.H.; Valcarcel, J. The Fermentation of a Marine Probiotic Bacterium on Low-Cost Media Formulated with Industrial Fish Gelatin Waterstreams and Collagen Hydrolysates. Processes 2023, 11, 2397. [Google Scholar] [CrossRef]
- Kozma, M.; Acharya, B.; Bissessur, R. Chitin, Chitosan, and Nanochitin: Extraction, Synthesis, and Applications. Polymers 2022, 14, 3989. [Google Scholar] [CrossRef]
- Kou, S.G.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.-T.; Chen, R.-L.; Wu, P.-Y.; Chiang, T.-Y.; Chen, S.-C. A Study on the Effects of the Degree of Deacetylation of Chitosan Films on Physical and Antibacterial Properties. Polym. Plast. Technol. Eng. 2007, 46, 1121–1127. [Google Scholar] [CrossRef]
- Jung, E.J.; Youn, D.K.; Lee, S.H.; No, H.K.; Ha, J.G.; Prinyawiwatkul, W. Antibacterial activity of chitosans with different degrees of deacetylation and viscosities. Int. J. Food Sci. Technol. 2010, 45, 676–682. [Google Scholar] [CrossRef]
- Jung, J.; Zhao, Y. Characteristics of deacetylation and depolymerization of β-chitin from jumbo squid (Dosidicus gigas) pens. Carbohydr. Res. 2011, 346, 1876–1884. [Google Scholar] [CrossRef]
- Jung, J.; Zhao, Y. Alkali- or acid-induced changes in structure, moisture absorption ability and deacetylating reaction of β-chitin extracted from jumbo squid (Dosidicus gigas) pens. Food Chem. 2014, 152, 355–362. [Google Scholar] [CrossRef]
- Carvalho, D.N.; Gonçalves, C.; Oliveira, J.M.; Williams, D.S.; Mearns-Spragg, A.; Reis, R.L.; Silva, T.H. Innovative methodology for marine collagen–chitosan–fucoidan hydrogels production, tailoring rheological properties towards biomedical application. Green Chem. 2021, 23, 7016–7029. [Google Scholar] [CrossRef]
- Reys, L.L.; Silva, S.S.; Pirraco, R.P.; Marques, A.P.; Mano, J.F.; Silva, T.H.; Reis, R.L. Influence of freezing temperature and deacetylation degree on the performance of freeze-dried chitosan scaffolds towards cartilage tissue engineering. Eur. Polym. J. 2017, 95, 232–240. [Google Scholar] [CrossRef]
- Zegarra-Urquia, C.L.; Santiago, J.; Bumgardner, J.D.; Vega-Baudrit, J.; Hernández-Escobar, C.A.; Zaragoza-Contreras, E.A. Synthesis of nanoparticles of the chitosan-poly((α,β)-DL-aspartic acid) polyelectrolite complex as hydrophilic drug carrier. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 497–506. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems BT-Functional Chitosan: Drug Delivery and Biomedical Applications; Jana, S., Jana, S., Eds.; Springer: Singapore, 2019; pp. 457–489. ISBN 978-981-15-0263-7. [Google Scholar]
- McDonnell, G.; Burke, P. Disinfection: Is it time to reconsider Spaulding? J. Hosp. Infect. 2011, 78, 163–170. [Google Scholar] [CrossRef]
- Tamara, F.R.; Lin, C.; Mi, F.-L.; Ho, Y.-C. Antibacterial Effects of Chitosan/Cationic Peptide Nanoparticles. Nanomaterials 2018, 8, 88. [Google Scholar] [CrossRef]
- Vishu Kumar, A.B.; Varadaraj, M.C.; Gowda, L.R.; Tharanathan, R.N. Low molecular weight chitosans—Preparation with the aid of pronase, characterization and their bactericidal activity towards Bacillus cereus and Escherichia coli. Biochim. Biophys. Acta-Gen. Subj. 2007, 1770, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Tachaboonyakiat, W. 9—Antimicrobial Applications of Chitosan; Jennings, J.A., Bumgardner, Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 245–274. ISBN 978-0-08-100228-5. [Google Scholar]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef] [PubMed]
- Blanch, H.W.; Clark, D.S. Biochemical Engineering; Marcel Dekker, Inc.: New York, NY, USA, 1996. [Google Scholar]
- Vázquez, J.A.; Caprioni, R.; Nogueira, M.; Menduiña, A.; Ramos, P.; Pérez-Martín, R.I. Valorisation of effluents obtained from chemical and enzymatic chitin production of Illex argentinus pen by-products as nutrient supplements for various bacterial fermentations. Biochem. Eng. J. 2016, 116, 34–44. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Durán, A.; Nogueira, M.; Menduíña, A.; Antunes, J.; Freitas, A.C.; Gomes, A.M. Production of Marine Probiotic Bacteria in a Cost-Effective Marine Media Based on Peptones Obtained from Discarded Fish By-Products. Microorganisms 2020, 8, 1121. [Google Scholar] [CrossRef]
- Horn, S.J.; Aspmo, S.I.; Eijsink, V.G.H. Growth of Lactobacillus plantarum in media containing hydrolysates of fish viscera. J. Appl. Microbiol. 2005, 99, 1082–1089. [Google Scholar] [CrossRef]
- Dufossé, L.; De La Broise, D.; Guerard, F. Evaluation of Nitrogenous Substrates Such as Peptones from Fish:A New Method Based on Gompertz Modeling of Microbial Growth. Curr. Microbiol. 2001, 42, 32–38. [Google Scholar] [CrossRef]
- AOAC Association of Official Analytical Chemistry. Methods of Analysis, 15th ed.; AOAC International: Washington, DC, USA, 1997. [Google Scholar]
- Moore, S.; Spackman, D.H.; Stein, W.H. Chromatography of Amino Acids on Sulfonated Polystyrene Resins. An Improved System. Anal. Chem. 1958, 30, 1185–1190. [Google Scholar] [CrossRef]
- Pissard, A.; Gofflot, S.; Baeten, V.; Lecler, B.; Lorrette, B.; Morin, J.-F.; Debode, F. Chitin Assessment in Insect-Based Products from Reference Methods to Near-Infrared Models. Insects 2025, 16, 924. [Google Scholar] [CrossRef]
- Kaya, M.; Baran, T.; Mentes, A.; Asaroglu, M.; Sezen, G.; Tozak, K.O. Extraction and Characterization of α-Chitin and Chitosan from Six Different Aquatic Invertebrates. Food Biophys. 2014, 9, 145–157. [Google Scholar] [CrossRef]
- Allan, G.G.; Peyron, M. Molecular weight manipulation of chitosan I: Kinetics of depolymerization by nitrous acid. Carbohydr. Res. 1995, 277, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Sorlier, P.; Rochas, C.; Morfin, I.; Viton, C.; Domard, A. Light scattering studies of the solution properties of chitosans of varying degrees of acetylation. Biomacromolecules 2003, 4, 1034–1040. [Google Scholar] [CrossRef]
- Fernandez-Megia, E.; Novoa-Carballal, R.; Quiñoá, E.; Riguera, R. Optimal routine conditions for the determination of the degree of acetylation of chitosan by 1 H-NMR. Carbohydr. Polym. 2005, 61, 155–161. [Google Scholar] [CrossRef]
- Novoa-Carballal, R.; Fernandez-Megia, E.; Riguera, R. Dynamics of Chitosan by 1H NMR Relaxation. Biomacromolecules 2010, 11, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bernfeld, P.L.B.-B. Enzymes of starch degradation and synthesis. Adv Enzym. 1951, 12, 87–123. [Google Scholar]
- CLSI M07; Methods for Dilution Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015.
- Simões, M.; Simões, L.C.; Pereira, M.O.; Vieira, M.J. Antagonism between Bacillus cereus and Pseudomonas fluorescens in planktonic systems and in biofilms. Biofouling 2008, 24, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Rial, D. Inhibition of selected bacterial growth by three hydrocarbons: Mathematical evaluation of toxicity using a toxicodynamic equation. Chemosphere 2014, 112, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Durán, A.I.; Menduíña, A.; Nogueira, M. Biotechnological Valorization of Food Marine Wastes: Microbial Productions on Peptones Obtained from Aquaculture By-Products. Biomolecules 2020, 10, 1184. [Google Scholar] [CrossRef] [PubMed]


| DD% | DD% | |||
|---|---|---|---|---|
| 12.5 M NaOH 12 h | CSN1 | 85.9 | CSA1 | 87.0 |
| 15 M NaOH 6 h | CSN2 | 87.2 | CSA2 | 86.2 |
| 15 M NaOH 24 h | CSN3 | 91.5 | CSA3 | 90.7 |
| Mn (kDa) | Mw (kDa) | PDI | Retention Time (min) | |
|---|---|---|---|---|
| CS0 | 251 ± 18 | 389 ± 4 | 1.549 | 18.26 |
| CSH | 137 ± 9 | 196 ± 5 | 1.432 | 18.81 |
| CSM | 94 ± 11 | 139 ± 7 | 1.484 | 19.19 |
| CSL | 50 ± 6 | 82 ± 6 | 1.649 | 20.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, M.; Pedreira, A.; Sanz, N.; Vázquez, J.A.; García, M.R.; Mergulhão, F.; Valcarcel, J. Sustainable and Integral Valorization of Dosidicus gigas Pen Waste: Combined Production of Chitosan with Antibacterial Properties and Human and Marine Probiotics. Mar. Drugs 2025, 23, 382. https://doi.org/10.3390/md23100382
Lima M, Pedreira A, Sanz N, Vázquez JA, García MR, Mergulhão F, Valcarcel J. Sustainable and Integral Valorization of Dosidicus gigas Pen Waste: Combined Production of Chitosan with Antibacterial Properties and Human and Marine Probiotics. Marine Drugs. 2025; 23(10):382. https://doi.org/10.3390/md23100382
Chicago/Turabian StyleLima, Marta, Adrián Pedreira, Noelia Sanz, José Antonio Vázquez, Míriam R. García, Filipe Mergulhão, and Jesus Valcarcel. 2025. "Sustainable and Integral Valorization of Dosidicus gigas Pen Waste: Combined Production of Chitosan with Antibacterial Properties and Human and Marine Probiotics" Marine Drugs 23, no. 10: 382. https://doi.org/10.3390/md23100382
APA StyleLima, M., Pedreira, A., Sanz, N., Vázquez, J. A., García, M. R., Mergulhão, F., & Valcarcel, J. (2025). Sustainable and Integral Valorization of Dosidicus gigas Pen Waste: Combined Production of Chitosan with Antibacterial Properties and Human and Marine Probiotics. Marine Drugs, 23(10), 382. https://doi.org/10.3390/md23100382







