A Comparative Study of the In Vitro Intestinal Permeability of Pinnatoxins and Portimine
Abstract
1. Introduction
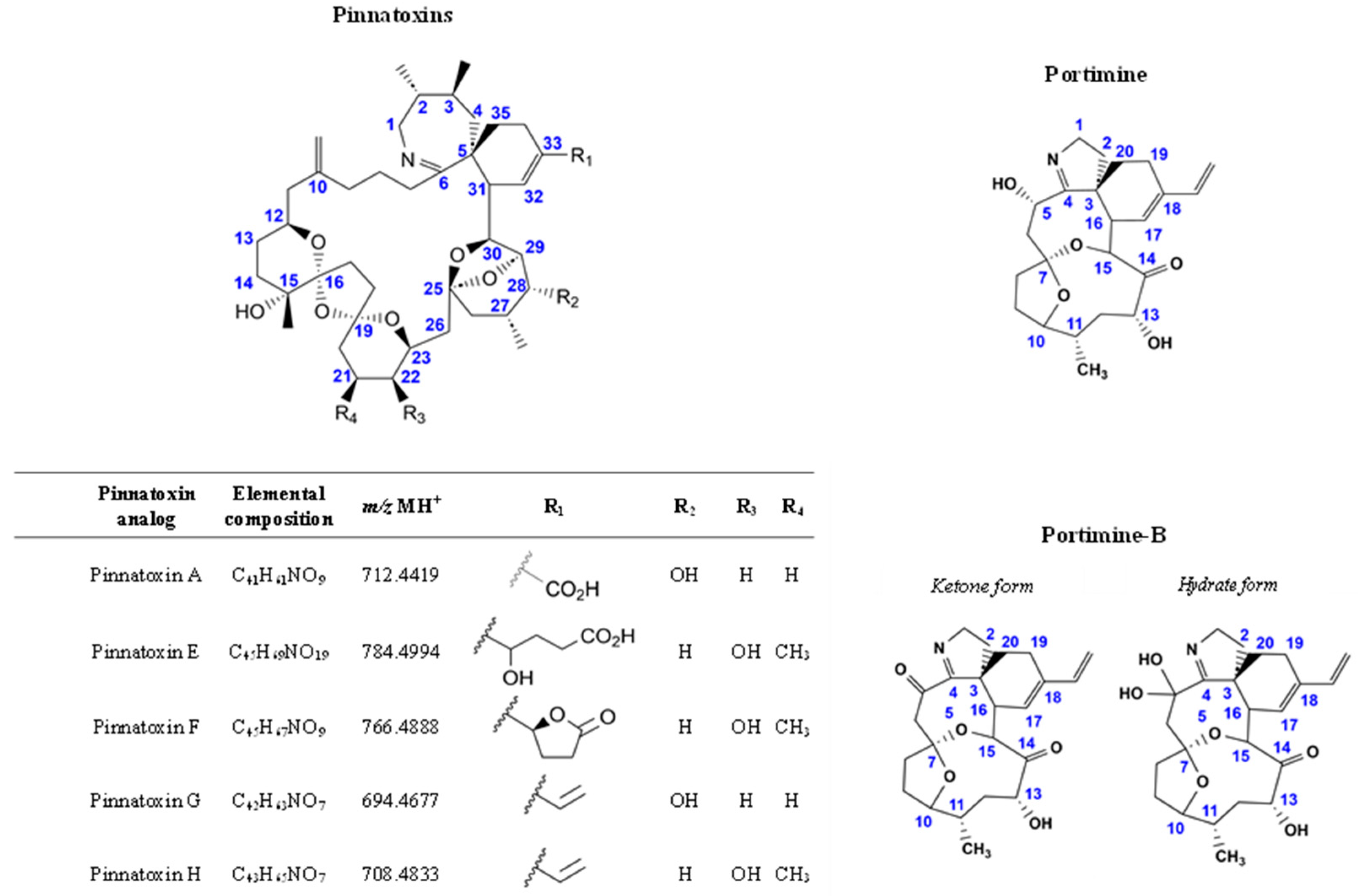
2. Results
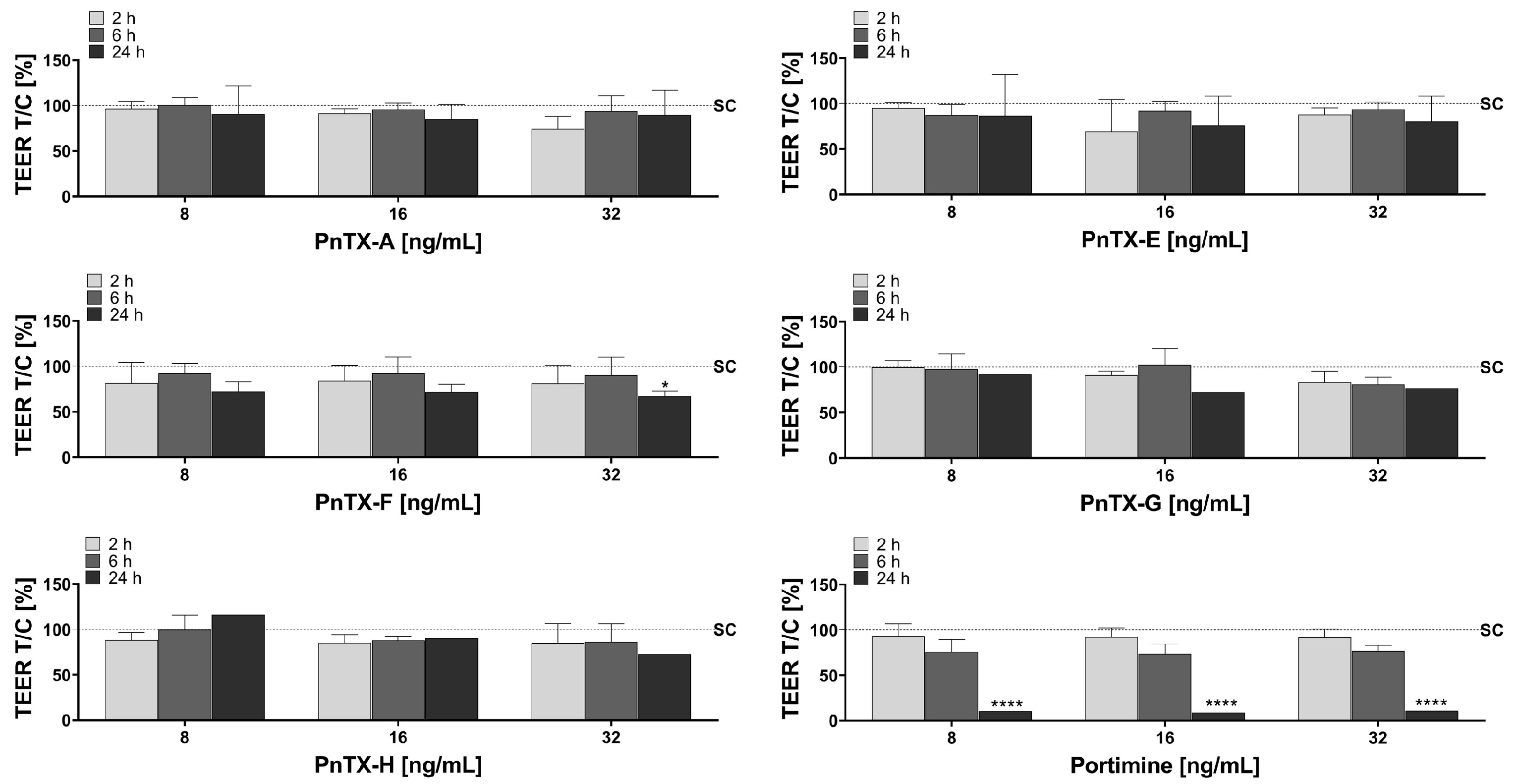
2.1. Trans-Epithelial Electrical Resistance (TEER)
2.2. Paracellular Permeability: Lucifer Yellow (LY)
2.3. Toxin Crossing
2.4. In Silico Predictions
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cells
4.2.1. Cell Culture
4.2.2. Cell Treatment
4.2.3. Barrier Integrity
- Transepithelial electrical resistance (TEER)
- Lucifer Yellow
4.2.4. Barrier Permeability of Toxins
- Liquid Chromatography coupled to tandem Mass Spectrometry (LC-MS/MS) analysis
- Calculation of the apparent permeability Papp
4.2.5. In Silico Tools
4.2.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nézan, E.; Chomérat, N. Vulcanodinium Rugosum Gen. et Sp. Nov. (Dinophyceae), Un Nouveau Dinoflagellé Marin de La Côte Méditerranéenne Française. Cryptogam. Algol. 2011, 32, 3–18. [Google Scholar] [CrossRef]
- Zeng, N.; Gu, H.; Smith, K.; Rhodes, L.; Selwood, A.; Yang, W. The First Report of Vulcanodinium rugosum (Dinophyceae) from the South China Sea with a Focus on the Life Cycle. N. Z. J. Mar. Freshw. Res. 2012, 46, 511–521. [Google Scholar] [CrossRef]
- Selwood, A.I.; Wilkins, A.L.; Munday, R.; Gu, H.; Smith, K.F.; Rhodes, L.L.; Rise, F. Pinnatoxin H: A New Pinnatoxin Analogue from a South China Sea Vulcanodinium rugosum Isolate. Tetrahedron Lett. 2014, 55, 5508–5510. [Google Scholar] [CrossRef]
- Al Muftah, A.; Selwood, A.I.; Foss, A.J.; Al-Jabri, H.M.S.J.; Potts, M.; Yilmaz, M. Algal Toxins and Producers in the Marine Waters of Qatar, Arabian Gulf. Toxicon 2016, 122, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.; Smith, K.; Selwood, A.; McNabb, P.; Munday, R.; Suda, S.; Molenaar, S.; Hallegraeff, G. Dinoflagellate Vulcanodinium Rugosum Identified as the Causative Organism of Pinnatoxins in Australia, New Zealand and Japan. Phycologia 2011, 50, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Moreira-González, A.R.; Comas-González, A.; Valle-Pombrol, A.; Seisdedo-Losa, M.; Hernández-Leyva, O.; Fernandes, L.F.; Chomérat, N.; Bilien, G.; Hervé, F.; Rovillon, G.A.; et al. Summer Bloom of Vulcanodinium rugosum in Cienfuegos Bay (Cuba) Associated to Dermatitis in Swimmers. Sci. Total Environ. 2020, 757, 143782. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.F.; Rhodes, L.L.; Suda, S.; Selwood, A.I. A Dinoflagellate Producer of Pinnatoxin G, Isolated from Sub-Tropical Japanese Waters. Harmful Algae 2011, 10, 702–705. [Google Scholar] [CrossRef]
- Mondeguer, F.; Abadie, E.; Herve, F.; Bardouil, M.; Sechet, V.; Raimbault, V.; Berteaux, T.; Zendong, S.Z.; Palvadeau, H.; Amzil, Z.; et al. Pinnatoxines en Lien Avec l’espèce Vulcanodinium Rugosum (II). 2015. Available online: https://archimer.ifremer.fr/doc/00285/39635/ (accessed on 6 December 2024).
- Rundberget, T.; Aasen, J.A.B.; Selwood, A.I.; Miles, C.O. Pinnatoxins and Spirolides in Norwegian Blue Mussels and Seawater. Toxicon 2011, 58, 700–711. [Google Scholar] [CrossRef]
- McCarron, P.; Rourke, W.A.; Hardstaff, W.; Pooley, B.; Quilliam, M.A. Identification of Pinnatoxins and Discovery of Their Fatty Acid Ester Metabolites in Mussels (Mytilus edulis) from Eastern Canada. J. Agric. Food Chem. 2012, 60, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- García-Altares, M.; Casanova, A.; Bane, V.; Diogène, J.; Furey, A.; de la Iglesia, P. Confirmation of Pinnatoxins and Spirolides in Shellfish and Passive Samplers from Catalonia (Spain) by Liquid Chromatography Coupled with Triple Quadrupole and High-Resolution Hybrid Tandem Mass Spectrometry. Mar. Drugs 2014, 12, 3706–3732. [Google Scholar] [CrossRef] [PubMed]
- Rambla-Alegre, M.; Miles, C.O.; de la Iglesia, P.; Fernandez-Tejedor, M.; Jacobs, S.; Sioen, I.; Verbeke, W.; Samdal, I.A.; Sandvik, M.; Barbosa, V.; et al. Occurrence of Cyclic Imines in European Commercial Seafood and Consumers Risk Assessment. Environ. Res. 2018, 161, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Lamas, J.P.; Arévalo, F.; Moroño, Á.; Correa, J.; Muñíz, S.; Blanco, J. Detection and Spatio-Temporal Distribution of Pinnatoxins in Shellfish from the Atlantic and Cantabrian Coasts of Spain. Toxins 2019, 11, 340. [Google Scholar] [CrossRef]
- Otero, P.; Miguéns, N.; Rodríguez, I.; Botana, L.M. LC–MS/MS Analysis of the Emerging Toxin Pinnatoxin-G and High Levels of Esterified OA Group Toxins in Galician Commercial Mussels. Toxins 2019, 11, 394. [Google Scholar] [CrossRef] [PubMed]
- Tamele, I.J.; Timba, I.; Vasconcelos, V.; Costa, P.R. First Report of Pinnatoxins in Bivalve Molluscs from Inhaca Island (South of Mozambique)—South of the Indian Ocean. J. Mar. Sci. Eng. 2022, 10, 1215. [Google Scholar] [CrossRef]
- Norambuena, L.; Mardones, J.I. Emerging Phycotoxins in the Chilean Coast: First Localized Detection of the Neurotoxic Cyclic Imine Pinnatoxin-G in Shellfish Banks. Mar. Pollut. Bull. 2023, 190, 114878. [Google Scholar] [CrossRef] [PubMed]
- Hess, P.; Abadie, E.; Hervé, F.; Berteaux, T.; Séchet, V.; Aráoz, R.; Molgó, J.; Zakarian, A.; Sibat, M.; Rundberget, T.; et al. Pinnatoxin G Is Responsible for Atypical Toxicity in Mussels (Mytilus galloprovincialis) and Clams (Venerupis decussata) from Ingril, a French Mediterranean Lagoon. Toxicon 2013, 75, 16–26. [Google Scholar] [CrossRef]
- Arnich, N.; Abadie, E.; Delcourt, N.; Fessard, V.; Fremy, J.-M.; Hort, V.; Lagrange, E.; Maignien, T.; Molgó, J.; Peyrat, M.-B.; et al. Health Risk Assessment Related to Pinnatoxins in French Shellfish. Toxicon 2020, 180, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Finch, S.C.; Harwood, D.T.; Boundy, M.J.; Selwood, A.I. A Review of Cyclic Imines in Shellfish: Worldwide Occurrence, Toxicity and Assessment of the Risk to Consumers. Mar. Drugs 2024, 22, 129. [Google Scholar] [CrossRef] [PubMed]
- Hellyer, S.D.; Indurthi, D.; Balle, T.; Runder-Varga, V.; Selwood, A.I.; Tyndall, J.D.A.; Chebib, M.; Rhodes, L.; Kerr, D.S. Pinnatoxins E, F and G Target Multiple Nicotinic Receptor Subtypes. J. Neurochem. 2015, 135, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Delcourt, N.; Lagrange, E.; Abadie, E.; Fessard, V.; Frémy, J.-M.; Vernoux, J.-P.; Peyrat, M.-B.; Maignien, T.; Arnich, N.; Molgó, J.; et al. Pinnatoxins’ Deleterious Effects on Cholinergic Networks: From Experimental Models to Human Health. Mar. Drugs 2019, 17, 425. [Google Scholar] [CrossRef] [PubMed]
- Selwood, A.I.; Miles, C.O.; Wilkins, A.L.; Van Ginkel, R.; Munday, R.; Rise, F.; McNabb, P. Isolation, Structural Determination and Acute Toxicity of Pinnatoxins E, F and G. J. Agric. Food Chem. 2010, 58, 6532–6542. [Google Scholar] [CrossRef]
- Munday, R.; Selwood, A.I.; Rhodes, L. Acute Toxicity of Pinnatoxins E, F and G to Mice. Toxicon 2012, 60, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Hellyer, S.D.; Selwood, A.I.; Rhodes, L.; Kerr, D.S. Neuromuscular Blocking Activity of Pinnatoxins E, F and G. Toxicon 2013, 76, 214–220. [Google Scholar] [CrossRef]
- Servent, D.; Malgorn, C.; Bernes, M.; Gil, S.; Simasotchi, C.; Hérard, A.-S.; Delzescaux, T.; Thai, R.; Barbe, P.; Keck, M.; et al. First Evidence That Emerging Pinnatoxin-G, a Contaminant of Shellfish, Reaches the Brain and Crosses the Placental Barrier. Sci. Total Environ. 2021, 790, 148125. [Google Scholar] [CrossRef] [PubMed]
- Selwood, A.I.; Wilkins, A.L.; Munday, R.; Shi, F.; Rhodes, L.L.; Holland, P.T. Portimine: A Bioactive Metabolite from the Benthic Dinoflagellate Vulcanodinium Rugosum. Tetrahedron Lett. 2013, 54, 4705–4707. [Google Scholar] [CrossRef]
- Fribley, A.M.; Xi, Y.; Makris, C.; Alves-de-Souza, C.; York, R.; Tomas, C.; Wright, J.L.C.; Strangman, W.K. Identification of Portimine B, a New Cell Permeable Spiroimine That Induces Apoptosis in Oral Squamous Cell Carcinoma. ACS Med. Chem. Lett. 2019, 10, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Li, W.; Chiu, T.-Y.; Martínez-Peña, F.; Luo, Z.; Chong, C.T.; Wei, Q.; Gazaniga, N.; West, T.J.; See, Y.Y.; et al. Synthesis of Portimines Reveals the Basis of Their Anti-Cancer Activity. Nature 2023, 622, 507–513. [Google Scholar] [CrossRef]
- Wood, J.S.; Tang, J.; Strangman, W.K.; Williamson, R.T. Advanced Structure Analysis Reveals a Transient Portimine B Hydrate. J. Nat. Prod. 2024, 87, 2120–2125. [Google Scholar] [CrossRef] [PubMed]
- Hort, V.; Bourcier, S. Discovery of a Series of Portimine-A Fatty Acid Esters in Mussels. Harmful Algae 2024, 134, 102621. [Google Scholar] [CrossRef] [PubMed]
- Aráoz, R.; Barnes, P.; Séchet, V.; Delepierre, M.; Zinn-Justin, S.; Molgó, J.; Zakarian, A.; Hess, P.; Servent, D. Cyclic Imine Toxins Survey in Coastal European Shellfish Samples: Bioaccumulation and Mode of Action of 28-O-Palmitoyl Ester of Pinnatoxin-G. First Report of Portimine-A Bioaccumulation. Harmful Algae 2020, 98, 101887. [Google Scholar] [CrossRef] [PubMed]
- Hort, V.; Bastardo-Fernández, I.; Nicolas, M. Exploration of Vulcanodinium Rugosum Toxins and Their Metabolism Products in Mussels from the Ingril Lagoon Hotspot in France. Mar. Drugs 2023, 21, 429. [Google Scholar] [CrossRef] [PubMed]
- Cuddihy, S.L.; Drake, S.; Harwood, D.T.; Selwood, A.I.; McNabb, P.S.; Hampton, M.B. The Marine Cytotoxin Portimine Is a Potent and Selective Inducer of Apoptosis. Apoptosis 2016, 21, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Hogeveen, K.; Huet, S.; Besnard, C.; Murray, J.S.; Harwood, D.T.; Selwood, A.I.; Fessard, V. In Vitro Investigation of the Genotoxicity of Portimine, a Cyclic Imine Toxin Produced by the Dinoflagellate Vulcanodinium rugosum, on Human Hepatic HepaRG Cells. Toxicol. In Vitro 2021, 73, 105125. [Google Scholar] [CrossRef] [PubMed]
- European Centre for the Validation of Methods (ECVAM) DB-ALM Protocol n° 142: Permeability Assay on Caco-2 Cells. 2013. Available online: https://jeodpp.jrc.ec.europa.eu/ftp/jrc-opendata/EURL-ECVAM/datasets/DBALM/LATEST/online/DBALM_docs/142_P_Permeability%20Assay%20on%20Caco2%20Cells.pdf (accessed on 6 December 2024).
- Prieto, P.; Hoffmann, S.; Tirelli, V.; Tancredi, F.; González, I.; Bermejo, M.; De Angelis, I. An Exploratory Study of Two Caco-2 Cell Models for Oral Absorption: A Report on Their Within-Laboratory and Between-Laboratory Variability, and Their Predictive Capacity. Altern. Lab. Anim. 2010, 38, 367–386. [Google Scholar] [CrossRef]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the Human Colon Carcinoma Cell Line (Caco-2) as a Model System for Intestinal Epithelial Permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Natoli, M.; Leoni, B.D.; D’Agnano, I.; Zucco, F.; Felsani, A. Good Caco-2 Cell Culture Practices. Toxicol. In Vitro 2012, 26, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ema.europa.eu/en/ich-m9-biopharmaceutics-classification-system-based-biowaivers-scientific-guideline (accessed on 6 December 2024).
- U.S. Food and Drug Administration (FDA). Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System Guidance for Industry; U.S. Food and Drug Administration (FDA): Silver Spring, MD, USA, 2017. [Google Scholar]
- Turco, L.; Catone, T.; Caloni, F.; Consiglio, E.D.; Testai, E.; Stammati, A. Caco-2/TC7 Cell Line Characterization for Intestinal Absorption: How Reliable Is This in Vitro Model for the Prediction of the Oral Dose Fraction Absorbed in Human? Toxicol. In Vitro 2011, 25, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Zgair, A.; Taha, D.A.; Zang, X.; Kagan, L.; Kim, T.H.; Kim, M.G.; Yun, H.; Fischer, P.M.; Gershkovich, P. Quantitative Analysis of Lab-to-Lab Variability in Caco-2 Permeability Assays. Eur. J. Pharm. Biopharm. 2017, 114, 38–42. [Google Scholar] [CrossRef]
- Kamiya, Y.; Takaku, H.; Yamada, R.; Akase, C.; Abe, Y.; Sekiguchi, Y.; Murayama, N.; Shimizu, M.; Kitajima, M.; Shono, F.; et al. Determination and Prediction of Permeability across Intestinal Epithelial Cell Monolayer of a Diverse Range of Industrial Chemicals/Drugs for Estimation of Oral Absorption as a Putative Marker of Hepatotoxicity. Toxicol. Rep. 2020, 7, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Jarc, T.; Novak, M.; Hevir, N.; Rižner, T.L.; Kreft, M.E.; Kristan, K. Demonstrating Suitability of the Caco-2 Cell Model for BCS-Based Biowaiver According to the Recent FDA and ICH Harmonised Guidelines. J. Pharm. Pharmacol. 2019, 71, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. iLOGP: A Simple, Robust, and Efficient Description of n -Octanol/Water Partition Coefficient for Drug Design Using the GB/SA Approach. J. Chem. Inf. Model. 2014, 54, 3284–3301. [Google Scholar] [CrossRef]
- Wildman, S.A.; Crippen, G.M. Prediction of Physicochemical Parameters by Atomic Contributions. J. Chem. Inf. Comput. Sci. 1999, 39, 868–873. [Google Scholar] [CrossRef]
- Moriguchi, I.; Hirono, S.; Liu, Q.; Nakagome, I.; Matsushita, Y. Simple Method of Calculating Octanol/Water Partition Coefficient. Chem. Pharm. Bull. 1992, 40, 127–130. [Google Scholar] [CrossRef]
- Moriguchi, I.; Hirono, S.; Nakagome, I.; Hirano, H. Comparison of Reliability of Log P Values for Drugs Calculated by Several Methods. Chem. Pharm. Bull. 1994, 42, 976–978. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings 1. Adv. Drug Delivery Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Sosa, S.; Pelin, M.; Cavion, F.; Hervé, F.; Hess, P.; Tubaro, A. Acute Oral Toxicity of Pinnatoxin G in Mice. Toxins 2020, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Takada, N.; Umemura, N.; Suenaga, K.; Chou, T.; Nagatsu, A.; Haino, T.; Yamada, K.; Uemura, D. Pinnatoxins B and C, the Most Toxic Components in the Pinnatoxin Series from the Okinawan Bivalve Pinna Muricata. Tetrahedron Lett. 2001, 42, 3491–3494. [Google Scholar] [CrossRef]
- Dahlgren, D.; Lennernäs, H. Intestinal Permeability and Drug Absorption: Predictive Experimental, Computational and In Vivo Approaches. Pharmaceutics 2019, 11, 411. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.P.; Augustijns, P.; Brandl, M.; Brayden, D.J.; Brouwers, J.; Griffin, B.T.; Holm, R.; Jacobsen, A.-C.; Lennernäs, H.; Vinarov, Z.; et al. Best Practices in Current Models Mimicking Drug Permeability in the Gastrointestinal Tract—An UNGAP Review. Eur. J. Pharm. Sci. 2022, 170, 106098. [Google Scholar] [CrossRef] [PubMed]
- Press, B.; Di Grandi, D. Permeability for Intestinal Absorption: Caco-2 Assay and Related Issues. Curr. Drug Metab. 2008, 9, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Kus, M.; Ibragimow, I.; Piotrowska-Kempisty, H. Caco-2 Cell Line Standardization with Pharmaceutical Requirements and In Vitro Model Suitability for Permeability Assays. Pharmaceutics 2023, 15, 2523. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chow, E.C.; Liu, S.; Du, Y.; Pang, K.S. The Caco-2 Cell Monolayer: Usefulness and Limitations. Expert Opin. Drug Metab. Toxicol. 2008, 4, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Linnankoski, J.; Mäkelä, J.; Palmgren, J.; Mauriala, T.; Vedin, C.; Ungell, A.; Lazorova, L.; Artursson, P.; Urtti, A.; Yliperttula, M. Paracellular Porosity and Pore Size of the Human Intestinal Epithelium in Tissue and Cell Culture Models. J. Pharm. Sci. 2010, 99, 2166–2175. [Google Scholar] [CrossRef] [PubMed]
- Huth, F.; Domange, N.; Poller, B.; Vapurcuyan, A.; Durrwell, A.; Hanna, I.D.; Faller, B. Predicting Oral Absorption for Compounds Outside the Rule of Five Property Space. J. Pharm. Sci. 2021, 110, 2562–2569. [Google Scholar] [CrossRef]
- Artursson, P.; Ungell, A.; Löfroth, J. Selective paracellular permeability in two models of intestinal absorption: Cultured monolayers of human intestinal epithelial cells and rat intestinal segments. Pharm. Res. 1993, 10, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Volpe, D.A. Advances in Cell-Based Permeability Assays to Screen Drugs for Intestinal Absorption. Expert Opin. Drug Discov. 2020, 15, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.; Vieira, A.C.F.; Teixeira, J.; Gomes, M.J.; Barrocas, P.; Vasconcelos, T.; Sarmento, B. Cell-Based Intestinal In Vitro Models for Drug Absorption Screening. In Drug Discovery and Evaluation: Safety and Pharmacokinetic Assays; Hock, F.J., Gralinski, M.R., Pugsley, M.K., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–22. ISBN 978-3-030-73317-9. [Google Scholar]
- Alves, R.N.; Rambla-Alegre, M.; Braga, A.C.; Maulvault, A.L.; Barbosa, V.; Campàs, M.; Reverté, L.; Flores, C.; Caixach, J.; Kilcoyne, J.; et al. Bioaccessibility of Lipophilic and Hydrophilic Marine Biotoxins in Seafood: An in Vitro Digestion Approach. Food Chem. Toxicol. 2019, 129, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Spenkelink, A.; Beekmann, K.; Baccaro, M.; Xing, F.; Rietjens, I.M.C.M. Species Differences in in Vitro and Estimated in Vivo Kinetics for Intestinal Microbiota Mediated Metabolism of Acetyl-deoxynivalenols. Mol. Nutr. Food Res. 2021, 65, 2001085. [Google Scholar] [CrossRef] [PubMed]
- Crudo, F.; Aichinger, G.; Mihajlovic, J.; Varga, E.; Dellafiora, L.; Warth, B.; Dall’Asta, C.; Berry, D.; Marko, D. In Vitro Interactions of Alternaria Mycotoxins, an Emerging Class of Food Contaminants, with the Gut Microbiota: A Bidirectional Relationship. Arch. Toxicol. 2021, 95, 2533–2549. [Google Scholar] [CrossRef]
- Fedi, A.; Vitale, C.; Ponschin, G.; Ayehunie, S.; Fato, M.; Scaglione, S. In Vitro Models Replicating the Human Intestinal Epithelium for Absorption and Metabolism Studies: A Systematic Review. J. Control. Release 2021, 335, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, D.; Roos, C.; Sjögren, E.; Lennernäs, H. Direct In Vivo Human Intestinal Permeability (Peff) Determined with Different Clinical Perfusion and Intubation Methods. J. Pharm. Sci. 2015, 104, 2702–2726. [Google Scholar] [CrossRef]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An Integrated Online Platform for Accurate and Comprehensive Predictions of ADMET Properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lou, C.; Sun, L.; Li, J.; Cai, Y.; Wang, Z.; Li, W.; Liu, G.; Tang, Y. admetSAR 2.0: Web-service for prediction and optimization of chemical ADMET properties. Bioinformatics 2019, 35, 1067–1069. [Google Scholar] [CrossRef]
- Pires, D.E.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]

| Toxin | Time (h) | Loaded Concentration (ng/mL) | ||
|---|---|---|---|---|
| 8 | 16 | 32 | ||
| PnTX-A | 2 | 2.35 ± 1.66 | 2.26 ± 0.34 | 2.01 ± 0.63 |
| 6 | 3.44 ± 0.62 | 3.10 ± 1.53 | 2.39 ± 0.65 | |
| PnTX-E | 2 | 6.05 * | 2.41 ± 0.65 | 2.92 ± 0.45 |
| 6 | 3.29 ± 0.64 | 2.40 ± 1.06 | 3.85 ± 1.15 | |
| PnTX-F | 2 | 14.43 ± 2.73 | 10.12 ± 3.82 | 11.20 ± 3.27 |
| 6 | 26.00 ± 0.52 | 18.36 ± 4.64 | 22.09 ± 1.76 | |
| PnTX-G | 2 | 15.62 ± 5.24 | 23.32 ± 4.36 | 23.07 ± 5.56 |
| 6 | 34.43 ± 9.39 | 36.83 ± 5.26 | 43.41 ± 6.15 | |
| PnTX-H | 2 | 31.52 ± 5.07 | 22.05 ± 7.80 | 29.07 ± 3.54 |
| 6 | 58.06 ± 8.71 | 48.83 ± 11.22 | 54.22 ± 3.91 | |
| Portimine | 2 | 9.23 ± 1.46 | 15.48 ± 0.89 | 23.25 ± 6.88 |
| 6 | 24.23 ± 3.69 | 30.63 ± 5.62 | 39.45 ± 4.86 | |
| Toxin | Time (h) | Loaded Concentration (ng/mL) | ||
|---|---|---|---|---|
| 8 | 16 | 32 | ||
| PnTX-A | 2 | 1.45 ± 1.03 | 1.40 ± 0.21 | 1.25 ± 0.39 |
| 6 | 2.13 ± 0.38 | 1.92 ± 0.95 | 1.48 ± 0.41 | |
| PnTX-E | 2 | 3.75 * | 1.50 ± 0.40 | 1.81 ± 0.28 |
| 6 | 2.04 ± 0.40 | 1.49 ± 0.65 | 2.38 ± 0.71 | |
| PnTX-F | 2 | 8.95 ± 1.69 | 6.28 ± 2.37 | 6.94 ± 2.03 |
| 6 | 16.12 ± 0.24 | 11.38 ± 2.88 | 13.70 ± 1.09 | |
| PnTX-G | 2 | 9.69 ± 3.25 | 14.45 ± 2.70 | 14.31 ± 3.45 |
| 6 | 21.35 ± 5.82 | 22.84 ± 3.26 | 26.92 ± 3.82 | |
| PnTX-H | 2 | 19.54 ± 3.14 | 13.67 ± 4.83 | 18.03 ± 2.19 |
| 6 | 35.99 ± 5.40 | 30.28 ± 6.69 | 33.62 ± 2.43 | |
| Portimine | 2 | 5.72 ± 0.91 | 9.60 ± 0.55 | 14.42 ± 5.27 |
| 6 | 15.02 ± 2.29 | 18.99 ± 349 | 24.46 ± 3.01 | |
| Toxin | PnTX-A | PnTX-E | PnTX-F | PnTX-G | PnTX-H | Portimine | |
|---|---|---|---|---|---|---|---|
| Canonical SMILE | CC1CC23CCC(=CC2C4C5C(C(CC(O4)(O5)CC6CCCC7(O6)CCC8(O7)C(CCC(O8)CC(=C)CCCC3=NCC1C)(C)O)C)O)C(=O)O | CC1CC2C3C4C=C(CCC45CC(C(CN=C5CCCC(=C)CC6CCC(C7(O6)CCC8(O7)CC(C(C(O8)CC(C1)(O2)O3)O)C)(C)O)C)C)C(CCC(=O)O)O | CC1CC2C3C4C=C(CCC45CC(C(CN=C5CCCC(=C)CC6CCC(C7(O6)CCC8(O7)CC(C(C(O8)CC(C1)(O2)O3)O)C)(C)O)C)C)C9CCC(=O)O9 | CC1CC23CCC(=CC2C4C5C(C(CC(O4)(O5)CC6CCCC7(O6)CCC8(O7)C(CCC(O8)CC(=C)CCCC3=NCC1C)(C)O)C)O)C=C | CC1CC2C3C4C=C(CCC45CC(C(CN=C5CCCC(=C)CC6CCC(C7(O6)CCC8(O7)CC(C(C(O8)CC(C1)(O2)O3)O)C)(C)O)C)C)C=C | CC1CC(C(=O)C2C3C=C(CCC34CCN=C4C(CC5(O2)CCC1O5)O)C=C)O | |
| Solubility (logP) | ADMETlab 3.0 | 3.62 | 3.47 | 3.78 | 4.88 | 5.16 | 0.62 |
| AdmetSAR3.0 | 3.22 | 4.20 | 4.91 | 5.47 | 6.12 | 2.19 | |
| SwissADME/iLogP | 4.41 | 4.80 | 5.25 | 5.63 | 5.52 | 2.73 | |
| SwissADME/XLogP3 | 4.36 | 4.10 | 4.89 | 5.44 | 5.88 | 1.44 | |
| SwissADME/WLOGP | 6.23 | 6.62 | 7.10 | 7.33 | 7.58 | 1.95 | |
| SwissADME/MLogP | 3.20 | 3.10 | 3.86 | 4.10 | 4.27 | 1.32 | |
| SwissADME/Silicos-IT | 4.69 | 5.29 | 5.78 | 6.15 | 6.28 | 2.89 | |
| Lipinski | SwissADME | 1 | 2 | 1 | 1 | 2 | 0 |
| Toxin | PnTX-A | PnTX-E | PnTX-F | PnTX-G | PnTX-H | Portimine | |
|---|---|---|---|---|---|---|---|
| Caco2 Papp (10−6 cm·s−1) | PKCSM | 1.89 | 0.30 | 2.08 | 2.1 | 2.17 | 1.09 |
| ADMETlab 3.0 | 5.2 | 5.18 | 9.39 | 8.83 | 8.77 | 9.54 | |
| AdmetSAR3.0 | 1.04 | 0.72 | 2.31 | 3.71 | 3.73 | 1.24 | |
| MDCK | ADMETlab 3.0 (10−6 cm·s−1) | 11.34 | 10.58 | 17.56 | 15.46 | 16.49 | 18.74 |
| AdmetSAR3.0 | low | low | low | low | low | high | |
| PAMPA (logPeff) | ADMETlab 3.0 | 0.954 | 0.256 | 0.017 | 0.023 | 0.001 | 0.415 |
| Intestinal absorption | AdmetSAR3.0 | low | low | low | low | high | high |
| ADMETlab3.0 | low | low | low | low | low | low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanceleur, R.; Hort, V.; Peyrat, M.; Habauzit, D.; Selwood, A.I.; Fessard, V. A Comparative Study of the In Vitro Intestinal Permeability of Pinnatoxins and Portimine. Mar. Drugs 2025, 23, 26. https://doi.org/10.3390/md23010026
Lanceleur R, Hort V, Peyrat M, Habauzit D, Selwood AI, Fessard V. A Comparative Study of the In Vitro Intestinal Permeability of Pinnatoxins and Portimine. Marine Drugs. 2025; 23(1):26. https://doi.org/10.3390/md23010026
Chicago/Turabian StyleLanceleur, Rachelle, Vincent Hort, Marion Peyrat, Denis Habauzit, Andrew I. Selwood, and Valérie Fessard. 2025. "A Comparative Study of the In Vitro Intestinal Permeability of Pinnatoxins and Portimine" Marine Drugs 23, no. 1: 26. https://doi.org/10.3390/md23010026
APA StyleLanceleur, R., Hort, V., Peyrat, M., Habauzit, D., Selwood, A. I., & Fessard, V. (2025). A Comparative Study of the In Vitro Intestinal Permeability of Pinnatoxins and Portimine. Marine Drugs, 23(1), 26. https://doi.org/10.3390/md23010026







