Cell-Death Metabolites from Cocconeis scutellum var. parva Identified by Integrating Bioactivity-Based Fractionation and Non-Targeted Metabolomic Approaches
Abstract
1. Introduction
2. Results
2.1. Interaction of Sex-Reversal Activity from C. scutellum parva with Anion-Exchanger
2.2. Fractionation of Sex-Reversal Activity from C. scutellum var. parva by HPLC
2.3. Molecular Features Present in Active Fraction F2 and Anion-Exchanger Extract
2.4. Metabolic Profiles of C. scutellum parva Differ When Cultivated under Different pH Conditions
2.5. Active Molecular Features Up-Modulated under pH 8.2
3. Discussion
4. Materials and Methods
4.1. Collection and Isolation of the Diatom Cocconeis scutellum var. parva
4.2. Collection of Hippolyte inermis and Larval Production
4.3. Bioactivity-Guided Fractionation and Identification of Molecular Features from LC-MS Data
4.3.1. C. scutellum var. parva Culture and Harvest of Biomass for Fractionations
4.3.2. Extraction, Purification and Fractionation of C. scutellum var. parva
Extraction
Lipophilic Solid-Phase Extraction
Anion-Exchange Solid-Phase Extraction
Chromatography and Fractionation by HPLC
4.3.3. Bioassay with Hippolyte inermis
4.3.4. Acquisition of Fractions LC-MS Metabolic Profiles
4.3.5. LC-MS Data Preprocessing
4.3.6. Data Processing and Identification of Molecular Features from LC-MS Data
4.4. Non-Targeted Metabolomic Analysis of C. scutellum var. parva under Two Different pH Conditions
4.4.1. C. scutellum var. parva Culture and Harvest of Biomass for Metabolomics
4.4.2. Set-Up of pH-Controlled Photobioreactors for Metabolomics
4.4.3. Carbonate Measurements
4.4.4. Endometabolome Extraction of C. scutellum var. parva Cells under Two Different pH Conditions
4.4.5. Acquisition of LC-MS Profiles and Date Preprocessing
4.4.6. Data Processing and Statistical Analyses of LC-MS Metabolomics Data
4.4.7. Compound Identification
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amato, A.; Sabatino, V.; Nylund, G.M.; Bergkvist, J.; Basu, S.; Andersson, M.X.; Sanges, R.; Godhe, A.; Kiorboe, T.; Selander, E.; et al. Grazer-induced transcriptomic and metabolomic response of the chain-forming diatom Skeletonema marinoi. ISME J. 2018, 12, 1594–1604. [Google Scholar] [CrossRef] [PubMed]
- Nieri, P.; Carpi, S.; Esposito, R.; Costantini, M.; Zupo, V. Bioactive Molecules from Marine Diatoms and Their Value for the Nutraceutical Industry. Nutrients 2023, 15, 464. [Google Scholar] [CrossRef] [PubMed]
- Miralto, A.; Barone, G.; Romano, G.; Poulet, S.A.; Ianora, A.; Russo, G.L.; Buttino, I.; Mazzarella, G.; Laabir, M.; Cabrini, M.; et al. The insidious effect of diatoms on copepod reproduction. Nature 1999, 402, 173–176. [Google Scholar] [CrossRef]
- Zupo, V. Crustaceans: Endocrinology, Biology and Aquaculture; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Nuzzo, G.; Gallo, C.; D’Ippolito, G.; Manzo, E.; Ruocco, N.; Russo, E.; Carotenuto, Y.; Costantini, M.; Zupo, V.; Sardo, A.; et al. UPLC-MS/MS Identification of Sterol Sulfates in Marine Diatoms. Mar. Drugs 2019, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Berdalet, E.; Carotenuto, Y.; Fink, P.; Harder, T.; John, U.; Not, F.; Pohnert, G.; Potin, P.; Selander, E.; et al. Using chemical language to shape future marine health. Front. Ecol. Environ. 2019, 17, 530–537. [Google Scholar] [CrossRef]
- Ruocco, N.; Costantini, S.; Zupo, V.; Lauritano, C.; Caramiello, D.; Ianora, A.; Budillon, A.; Romano, G.; Nuzzo, G.; D’Ippolito, G.; et al. Toxigenic effects of two benthic diatoms upon grazing activity of the sea urchin: Morphological, metabolomic and transcriptomic analysis. Sci. Rep. 2018, 8, 5622. [Google Scholar] [CrossRef] [PubMed]
- Zupo, V. Co-Evolution of Secondary Metabolites; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 135–147. [Google Scholar] [CrossRef]
- Zupo, V. Effect of microalgal food on the sex reversal of Hippolyte inermis (Crustacea: Decapoda). Mar. Ecol. Prog. Ser. 2000, 201, 251–259. [Google Scholar] [CrossRef]
- Zupo, V.; Messina, P.; Buttino, I.; Sagi, A.; Avila, C.; Nappo, M.; Bastida, J.; Codina, C.; Zupo, S. Do benthic and planktonic diatoms produce equivalent effects in crustaceans? Mar. Freshw. Behav. Physiol. 2007, 40, 169–181. [Google Scholar] [CrossRef]
- Zupo, V.; Costantini, M.; Aflalo, E.D.; Levy, T.; Chalifa-Caspi, V.; Obayomi, O.; Mutalipassi, M.; Ruocco, N.; Glaviano, F.; Somma, E.; et al. Ferroptosis precedes apoptosis to facilitate specific death signalling by fatty acids. Proc. R. Soc. B Biol. Sci. 2023, 290, 20231327. [Google Scholar] [CrossRef]
- Levy, T.; Zupo, V.; Mutalipassi, M.; Somma, E.; Ruocco, N.; Costantini, M.; Abehsera, S.; Manor, R.; Chalifa-Caspi, V.; Sagi, A.; et al. Protandric Transcriptomes to Uncover Parts of the Crustacean Sex-Differentiation Puzzle. Front. Mar. Sci. 2021, 8, 745540. [Google Scholar] [CrossRef]
- Nappo, M.; Berkov, S.; Massucco, C.; Di Maria, V.; Bastida, J.; Codina, C.; Avila, C.; Messina, P.; Zupo, V.; Zupo, S. Apoptotic activity of the marine diatom Cocconeis scutellum and eicosapentaenoic acid in BT20 cells. Pharm. Biol. 2012, 50, 529–535. [Google Scholar] [CrossRef]
- Zupo, V.; Juttner, F.; Maibam, C.; Butera, E.; Blom, J.F. Apoptogenic metabolites in fractions of the Benthic diatom Cocconeis scutellum parva. Mar. Drugs 2014, 12, 547–567. [Google Scholar] [CrossRef] [PubMed]
- Mutalipassi, M.; Mazzella, V.; Zupo, V. Ocean acidification influences plant-animal interactions: The effect of Cocconeis scutellum parva on the sex reversal of Hippolyte inermis. PLoS ONE 2019, 14, e0218238. [Google Scholar] [CrossRef] [PubMed]
- Mutalipassi, M.; Mazzella, V.; Schott, M.; Fink, P.; Glaviano, F.; Porzio, L.; Lorenti, M.; Buia, M.C.; von Elert, E.; Zupo, V. Ocean Acidification Affects Volatile Infochemicals Production and Perception in Fauna and Flora Associated with Posidonia oceanica (L.) Delile. Front. Mar. Sci. 2022, 9, 809702. [Google Scholar] [CrossRef]
- Poulin, R.X.; Pohnert, G. Simplifying the complex: Metabolomics approaches in chemical ecology. Anal. Bioanal. Chem. 2019, 411, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Perez de Souza, L.; Fernie, A.R. Computational methods for processing and interpreting mass spectrometry-based metabolomics. Essays Biochem. 2024, 68, 5–13. [Google Scholar] [CrossRef]
- Ebbels, T.M.D.; van der Hooft, J.J.J.; Chatelaine, H.; Broeckling, C.; Zamboni, N.; Hassoun, S.; Mathe, E.A. Recent advances in mass spectrometry-based computational metabolomics. Curr. Opin. Chem. Biol. 2023, 74, 102288. [Google Scholar] [CrossRef] [PubMed]
- Djoumbou Feunang, Y.; Eisner, R.; Knox, C.; Chepelev, L.; Hastings, J.; Owen, G.; Fahy, E.; Steinbeck, C.; Subramanian, S.; Bolton, E.; et al. ClassyFire: Automated chemical classification with a comprehensive, computable taxonomy. J. Cheminform 2016, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Wang, M.; Leber, C.A.; Nothias, L.F.; Reher, R.; Kang, K.B.; van der Hooft, J.J.J.; Dorrestein, P.C.; Gerwick, W.H.; Cottrell, G.W. NPClassifier: A Deep Neural Network-Based Structural Classification Tool for Natural Products. J. Nat. Prod. 2021, 84, 2795–2807. [Google Scholar] [CrossRef]
- Duhrkop, K.; Shen, H.; Meusel, M.; Rousu, J.; Bocker, S. Searching molecular structure databases with tandem mass spectra using CSI:FingerID. Proc. Natl. Acad. Sci. USA 2015, 112, 12580–12585. [Google Scholar] [CrossRef]
- Yao, L.; Gerde, J.A.; Lee, S.L.; Wang, T.; Harrata, K.A. Microalgae lipid characterization. J. Agric. Food Chem. 2015, 63, 1773–1787. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.; Moreira, A.S.P.; Rey, F.; da Costa, E.; Melo, T.; Maciel, E.; Rego, A.; Abreu, M.H.; Domingues, P.; Calado, R.; et al. Lipidomic signature of the green macroalgae Ulva rigida farmed in a sustainable integrated multi-trophic aquaculture. J. Appl. Phycol. 2019, 31, 1369–1381. [Google Scholar] [CrossRef]
- Lopes, D.; Melo, T.; Meneses, J.; Abreu, M.H.; Pereira, R.; Domingues, P.; Lillebo, A.I.; Calado, R.; Domingues, M.R. A New Look for the Red Macroalga Palmaria palmata: A Seafood with Polar Lipids Rich in EPA and with Antioxidant Properties. Mar. Drugs 2019, 17, 533. [Google Scholar] [CrossRef] [PubMed]
- da Costa, E.; Melo, T.; Reis, M.; Domingues, P.; Calado, R.; Abreu, M.H.; Domingues, M.R. Polar Lipids Composition, Antioxidant and Anti-Inflammatory Activities of the Atlantic Red Seaweed Grateloupia turuturu. Mar. Drugs 2021, 19, 414. [Google Scholar] [CrossRef] [PubMed]
- da Costa, E.; Azevedo, V.; Melo, T.; Rego, A.M.; Evtuguin, D.V.; Domingues, P.; Calado, R.; Pereira, R.; Abreu, M.H.; Domingues, M.R. High-Resolution Lipidomics of the Early Life Stages of the Red Seaweed Porphyra dioica. Molecules 2018, 23, 187. [Google Scholar] [CrossRef] [PubMed]
- Coniglio, D.; Bianco, M.; Ventura, G.; Calvano, C.D.; Losito, I.; Cataldi, T.R.I. Lipidomics of the Edible Brown Alga Wakame (Undaria pinnatifida) by Liquid Chromatography Coupled to Electrospray Ionization and Tandem Mass Spectrometry. Molecules 2021, 26, 4480. [Google Scholar] [CrossRef] [PubMed]
- Poulaki, A.; Giannouli, S. Mitochondrial Lipids: From Membrane Organization to Apoptotic Facilitation. Int. J. Mol. Sci. 2022, 23, 3738. [Google Scholar] [CrossRef] [PubMed]
- von Haefen, C.; Wendt, J.; Semini, G.; Sifringer, M.; Belka, C.; Radetzki, S.; Reutter, W.; Daniel, P.T.; Danker, K. Synthetic glycosidated phospholipids induce apoptosis through activation of FADD, caspase-8 and the mitochondrial death pathway. Apoptosis 2011, 16, 636–651. [Google Scholar] [CrossRef] [PubMed]
- Birgbauer, E.; Chun, J. New developments in the biological functions of lysophospholipids. Cell. Mol. Life Sci. 2006, 63, 2695–2701. [Google Scholar] [CrossRef]
- Tan, S.T.; Ramesh, T.; Toh, X.R.; Nguyen, L.N. Emerging roles of lysophospholipids in health and disease. Prog. Lipid Res. 2020, 80, 101068. [Google Scholar] [CrossRef]
- Makide, K.; Kitamura, H.; Sato, Y.; Okutani, M.; Aoki, J. Emerging lysophospholipid mediators, lysophosphatidylserine, lysophosphatidylthreonine, lysophosphatidylethanolamine and lysophosphatidylglycerol. Prostaglandins Other Lipids Mediat. 2009, 89, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Rustenbeck, I.; Eibl, H.; Lenzen, S. Structural requirements of lysophospholipid-regulated mitochondrial Ca2+ transport. Biochim. Biophys. Acta 1991, 1069, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Graler, M.H.; Goetzl, E.J. Lysophospholipids and their G protein-coupled receptors in inflammation and immunity. Biochim. Biophys. Acta 2002, 1582, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Savill, J. Apoptosis in resolution of inflammation. J. Leukoc. Biol. 1997, 61, 375–380. [Google Scholar] [CrossRef] [PubMed]
- da Costa, E.; Domingues, P.; Melo, T.; Coelho, E.; Pereira, R.; Calado, R.; Abreu, M.H.; Domingues, M.R. Lipidomic Signatures Reveal Seasonal Shifts on the Relative Abundance of High-Valued Lipids from the Brown Algae Fucus vesiculosus. Mar. Drugs 2019, 17, 335. [Google Scholar] [CrossRef]
- Jin, P.; Liang, Z.; Lu, H.; Pan, J.; Li, P.; Huang, Q.; Guo, Y.; Zhong, J.; Li, F.; Wan, J.; et al. Lipid Remodeling Reveals the Adaptations of a Marine Diatom to Ocean Acidification. Front. Microbiol. 2021, 12, 748445. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Arcos, C.; Paris, D.; Mazzella, V.; Mutalipassi, M.; Costantini, M.; Buia, M.C.; von Elert, E.; Cutignano, A.; Zupo, V. Responses of the Macroalga Ulva prolifera Müller to Ocean Acidification Revealed by Complementary NMR- and MS-Based Omics Approaches. Mar. Drugs 2022, 20, 743. [Google Scholar] [CrossRef]
- Vítová, M.; Goecke, F.; Sigler, K.; Rezanka, T. Lipidomic analysis of the extremophilic red alga Galdieria sulphuraria in response to changes in pH. Algal Res. 2016, 13, 218–226. [Google Scholar] [CrossRef]
- Moreira, A.S.P.; da Costa, E.; Melo, T.; Sulpice, R.; Cardoso, S.M.; Pitarma, B.; Pereira, R.; Abreu, M.H.; Domingues, P.; Calado, R.; et al. Seasonal plasticity of the polar lipidome of Ulva rigida cultivated in a sustainable integrated multi-trophic aquaculture. Algal Res. 2020, 49, 101958. [Google Scholar] [CrossRef]
- Popko, J.; Herrfurth, C.; Feussner, K.; Ischebeck, T.; Iven, T.; Haslam, R.; Hamilton, M.; Sayanova, O.; Napier, J.; Khozin-Goldberg, I.; et al. Metabolome Analysis Reveals Betaine Lipids as Major Source for Triglyceride Formation, and the Accumulation of Sedoheptulose during Nitrogen-Starvation of Phaeodactylum tricornutum. PLoS ONE 2016, 11, e0164673. [Google Scholar] [CrossRef]
- Okazaki, Y.; Saito, K. Roles of lipids as signaling molecules and mitigators during stress response in plants. Plant J. 2014, 79, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Ruelland, E.; Kravets, V.; Derevyanchuk, M.; Martinec, J.; Zachowski, A.; Pokotylo, I. Role of phospholipid signalling in plant environmental responses. Environ. Exp. Bot. 2015, 114, 129–143. [Google Scholar] [CrossRef]
- Hou, Q.; Ufer, G.; Bartels, D. Lipid signalling in plant responses to abiotic stress. Plant Cell Environ. 2016, 39, 1029–1048. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Suh, S.; Kim, S.; Crain, R.C.; Kwak, J.M.; Nam, H.G.; Lee, Y.S. Systemic elevation of phosphatidic acid and lysophospholipid levels in wounded plants. Plant J. 1997, 12, 547–556. [Google Scholar] [CrossRef]
- Roggatz, C.C.; Saha, M.; Blanchard, S.; Schirrmacher, P.; Fink, P.; Verheggen, F.; Hardege, J.D. Becoming nose-blind—Climate change impacts on chemical communication. Glob. Change Biol. 2022, 28, 4495–4505. [Google Scholar] [CrossRef] [PubMed]
- Zupo, V.; Mutalipassi, M.; Fink, P.; Di Natale, M. Effect of Ocean Acidification on the Communications among Invertebrates Mediated by Plant-Produced Volatile Organic Compounds. Glob. J. Ecol. 2016, 1, 12–18. [Google Scholar] [CrossRef]
- Ianora, A.; Miralto, A.; Poulet, S.A.; Carotenuto, Y.; Buttino, I.; Romano, G.; Casotti, R.; Pohnert, G.; Wichard, T.; Colucci-D’Amato, L.; et al. Aldehyde suppression of copepod recruitment in blooms of a ubiquitous planktonic diatom. Nature 2004, 429, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Pohnert, G. Diatom/Copepod Interactions in Plankton: The Indirect Chemical Defense of Unicellular Algae. ChemBioChem 2005, 6, 946–959. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Mutalipassi, M.; Di Natale, M.; Mazzella, V.; Zupo, V. Automated culture of aquatic model organisms: Shrimp larvae husbandry for the needs of research and aquaculture. Animal 2018, 12, 155–163. [Google Scholar] [CrossRef]
- Zupo, V. Strategies of Sexual Inversion in Hippolyte inermis Leach (Crustacea, Decapoda) from a Mediterranean Seagrass Meadow. J. Exp. Mar. Biol. Ecol. 1994, 178, 131–145. [Google Scholar] [CrossRef]
- Mutalipassi, M.; Maibam, C.; Zupo, V. The sex change of the caridean shrimp Hippolyte inermis Leach: Temporal development of the gonopore morphology. Zoomorphology 2018, 137, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Zupo, V.; Messina, P.; Carcaterra, A.; Aflalo, E.D.; Sagi, A. Experimental evidence of a sex reversal process in the shrimp Hippolyte inermis. Invertebr. Reprod. Dev. 2008, 52, 93–100. [Google Scholar] [CrossRef]
- Kessner, D.; Chambers, M.; Burke, R.; Agus, D.; Mallick, P. ProteoWizard: Open source software for rapid proteomics tools development. Bioinformatics 2008, 24, 2534–2536. [Google Scholar] [CrossRef] [PubMed]
- Benton, H.P.; Want, E.J.; Ebbels, T.M. Correction of mass calibration gaps in liquid chromatography-mass spectrometry metabolomics data. Bioinformatics 2010, 26, 2488–2489. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Tautenhahn, R.; Bottcher, C.; Neumann, S. Highly sensitive feature detection for high resolution LC/MS. BMC Bioinform. 2008, 9, 504. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, C.; Tautenhahn, R.; Bottcher, C.; Larson, T.R.; Neumann, S. CAMERA: An integrated strategy for compound spectra extraction and annotation of liquid chromatography/mass spectrometry data sets. Anal. Chem. 2012, 84, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chong, J.; Li, S.; Xia, J. MetaboAnalystR 3.0: Toward an Optimized Workflow for Global Metabolomics. Metabolites 2020, 10, 186. [Google Scholar] [CrossRef]
- Gattuso, J.-P.; Lee, K.; Rost, B.; Schulz, K. Approaches and tools to manipulate the carbonate chemistry. In Guide to Best Practices for Ocean Acidification Research and Data Reporting; Publications Office of the European Union: Luxembourg, 2010. [Google Scholar]
- Pierrot, D.; Lewis, E.; Wallace, D. MS Excel Program Developed for CO2 System Calculations ORNL/CDIAC-105; Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy: Oak Ridge, TN, USA, 2006. [Google Scholar]
- Riebesell, U.; Fabry, V.J.; Hansson, L.; Gattuso, J.-P. Guide to Best Practices for Ocean Acidification Research and Data Reporting; Office for Official Publications of the European Communities: Luxembourg, 2011. [Google Scholar]
- Millero, F.J. Carbonate constants for estuarine waters. Mar. Freshw. Res. 2010, 61, 139–142. [Google Scholar] [CrossRef]
- Dickson, A.G. Standard potential of the reaction: AgCl(s) + 12H2(g) = Ag(s) + HCl(aq), and the standard acidity constant of the ion HSO4− in synthetic sea water from 273.15 to 318.15 K. J. Chem. Thermodyn. 1990, 22, 113–127. [Google Scholar] [CrossRef]
- Lee, K.; Kim, T.W.; Byrne, R.H.; Millero, F.J.; Feely, R.A.; Liu, Y.M. The universal ratio of boron to chlorinity for the North Pacific and North Atlantic oceans. Geochim. Cosmochim. Acta 2010, 74, 1801–1811. [Google Scholar] [CrossRef]
- Duhrkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Bocker, S. SIRIUS 4: A rapid tool for turning tandem mass spectra into metabolite structure information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef] [PubMed]
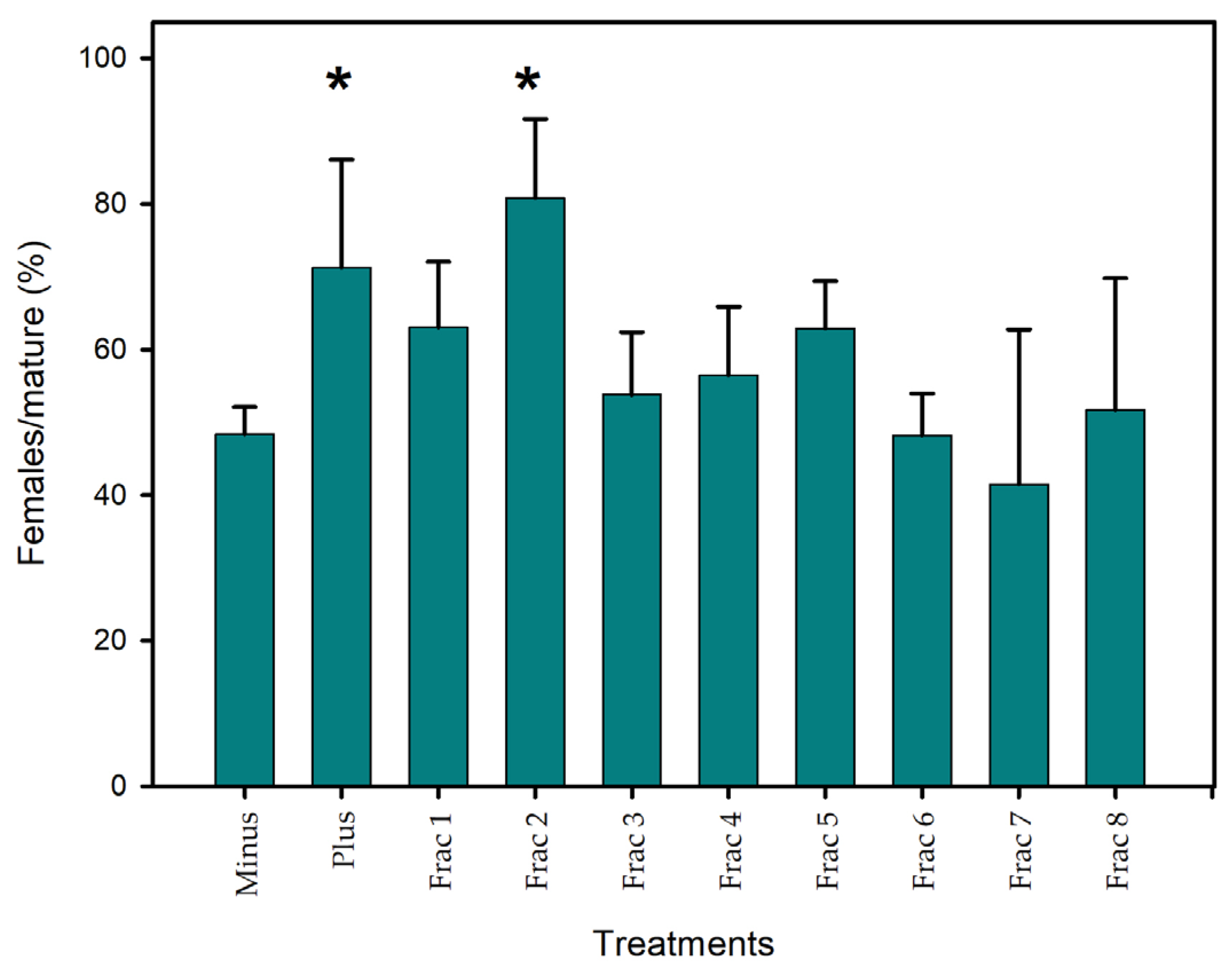


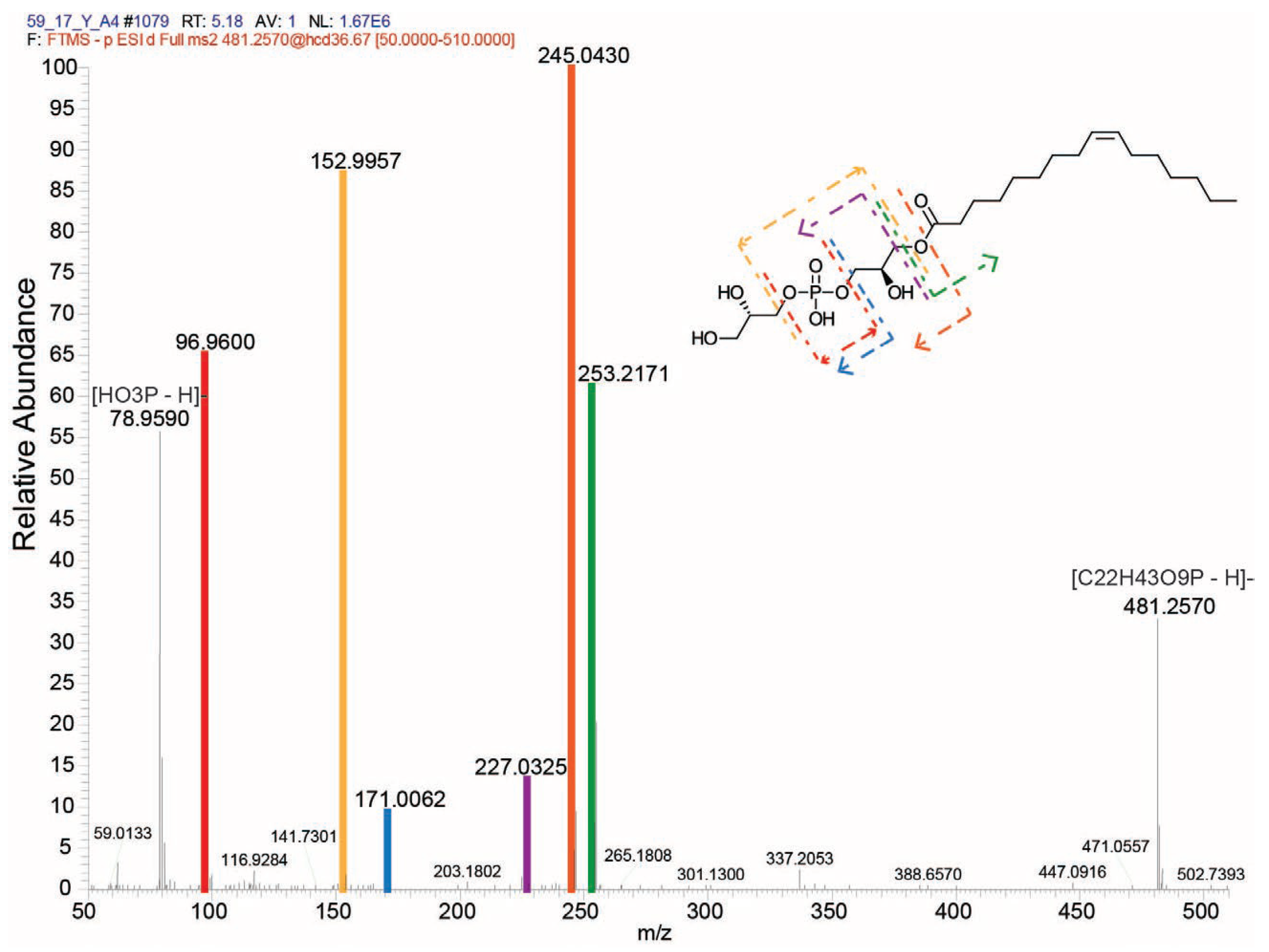
| Feature ID | Representative Feature | [M-H]− Precursor | Retention Time (min) | p-Value | FDR |
|---|---|---|---|---|---|
| 2126 | 311.1866 | 481.2572 | 3.09 | 3.83 × 10−10 | 8.71 × 10−8 |
| 2412 | 334.1272 | 521.2883 | 5.09 | 5.57 × 10−5 | 4.07 × 10−4 |
| 2470 | 339.1999 | 497.2944 | 3.49 | 4.57 × 10−4 | 1.67 × 10−3 |
| Feature ID | Fragment or Adduct | [M-H]− Precursor | Retention Time (min) | p-Value | FC |
|---|---|---|---|---|---|
| 295 | 311.1860 | 481.2570 | 5.18 | 0.0067882 | 4.638 |
| 1069 | 534.2884 | 497.3119 | 7.48 | 0.046058 | 3.957 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Arcos, C.; Mutalipassi, M.; Zupo, V.; von Elert, E. Cell-Death Metabolites from Cocconeis scutellum var. parva Identified by Integrating Bioactivity-Based Fractionation and Non-Targeted Metabolomic Approaches. Mar. Drugs 2024, 22, 320. https://doi.org/10.3390/md22070320
Sanchez-Arcos C, Mutalipassi M, Zupo V, von Elert E. Cell-Death Metabolites from Cocconeis scutellum var. parva Identified by Integrating Bioactivity-Based Fractionation and Non-Targeted Metabolomic Approaches. Marine Drugs. 2024; 22(7):320. https://doi.org/10.3390/md22070320
Chicago/Turabian StyleSanchez-Arcos, Carlos, Mirko Mutalipassi, Valerio Zupo, and Eric von Elert. 2024. "Cell-Death Metabolites from Cocconeis scutellum var. parva Identified by Integrating Bioactivity-Based Fractionation and Non-Targeted Metabolomic Approaches" Marine Drugs 22, no. 7: 320. https://doi.org/10.3390/md22070320
APA StyleSanchez-Arcos, C., Mutalipassi, M., Zupo, V., & von Elert, E. (2024). Cell-Death Metabolites from Cocconeis scutellum var. parva Identified by Integrating Bioactivity-Based Fractionation and Non-Targeted Metabolomic Approaches. Marine Drugs, 22(7), 320. https://doi.org/10.3390/md22070320








