Induction of Autophagy by Extract from Corydalis heterocarpa for Skin Anti-Aging
Abstract
1. Introduction
2. Results
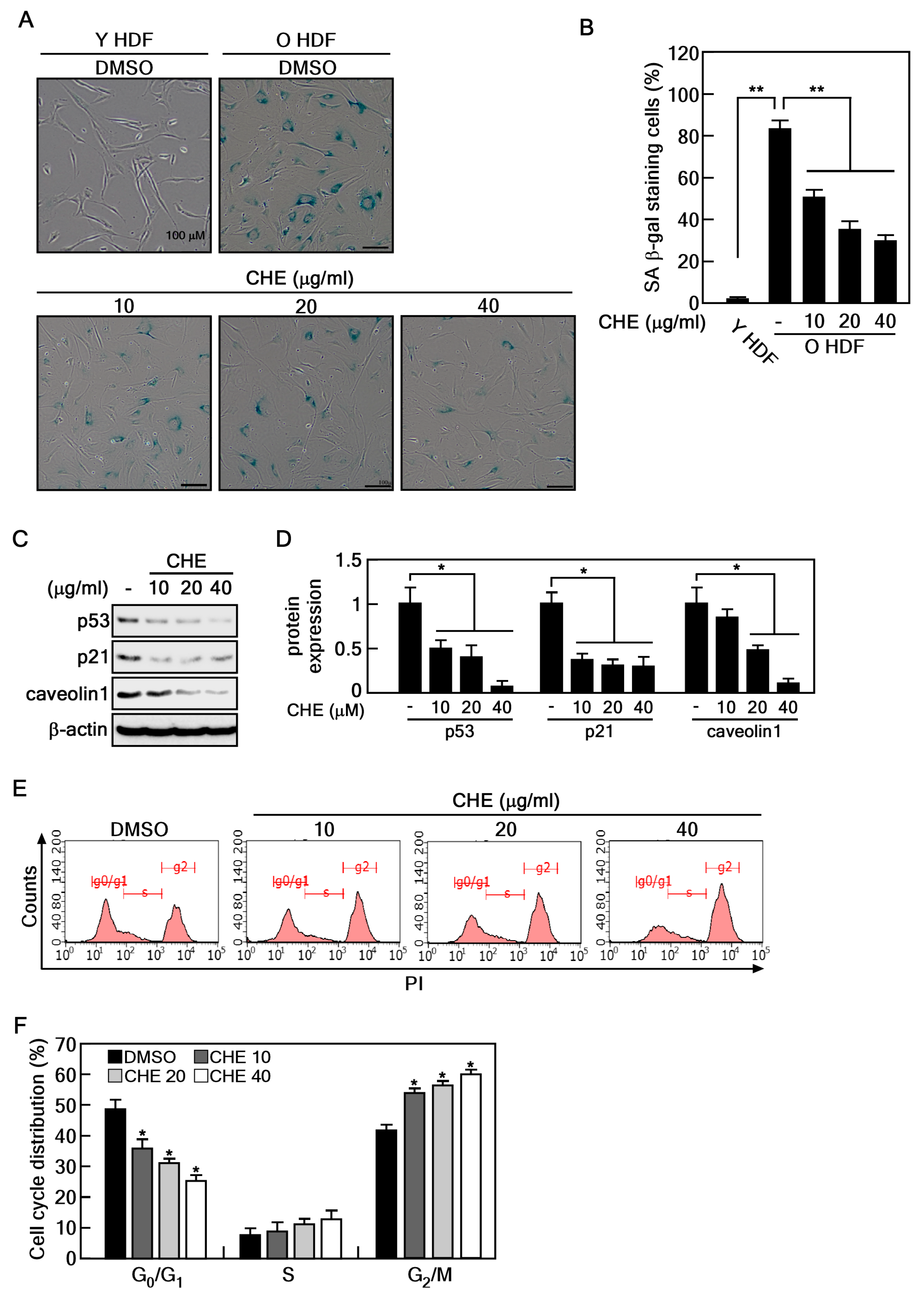
2.1. CHE Reverses the Cellular Senescence in Senescent HDFs
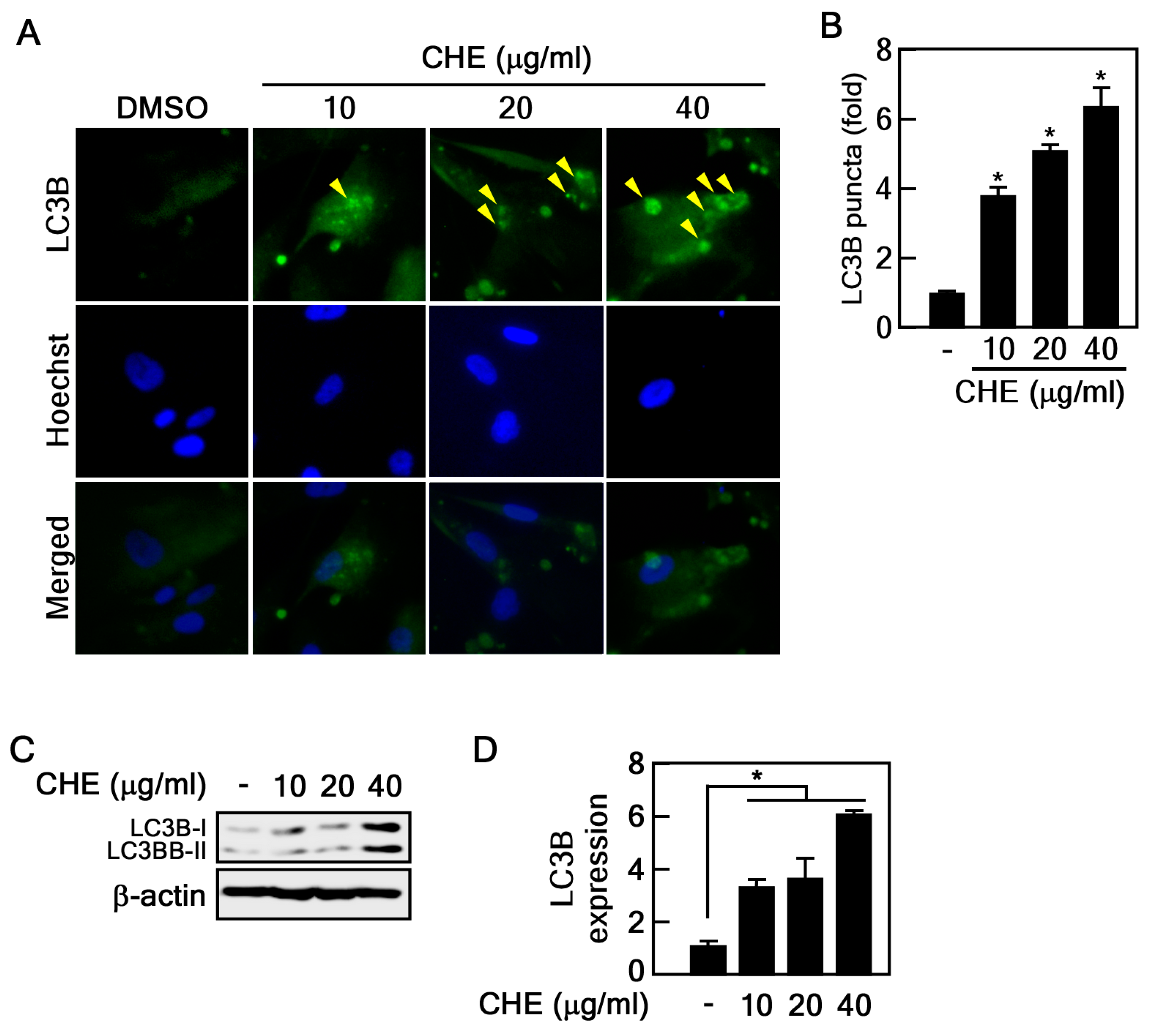
2.2. Regulation of Autophagy by CHE in Senescent HDFs
2.3. CHE Promotes Autophagic Flux
2.4. CHE Affects Leucine-Rich Repeat and Sterile Alpha Motif-Containing 1 (LRSAM1) Expression and Adenosine-Monophosphate Activated-Protein Kinase (AMPK)-Mammalian Target of Rapamycin (mTOR) Pathway
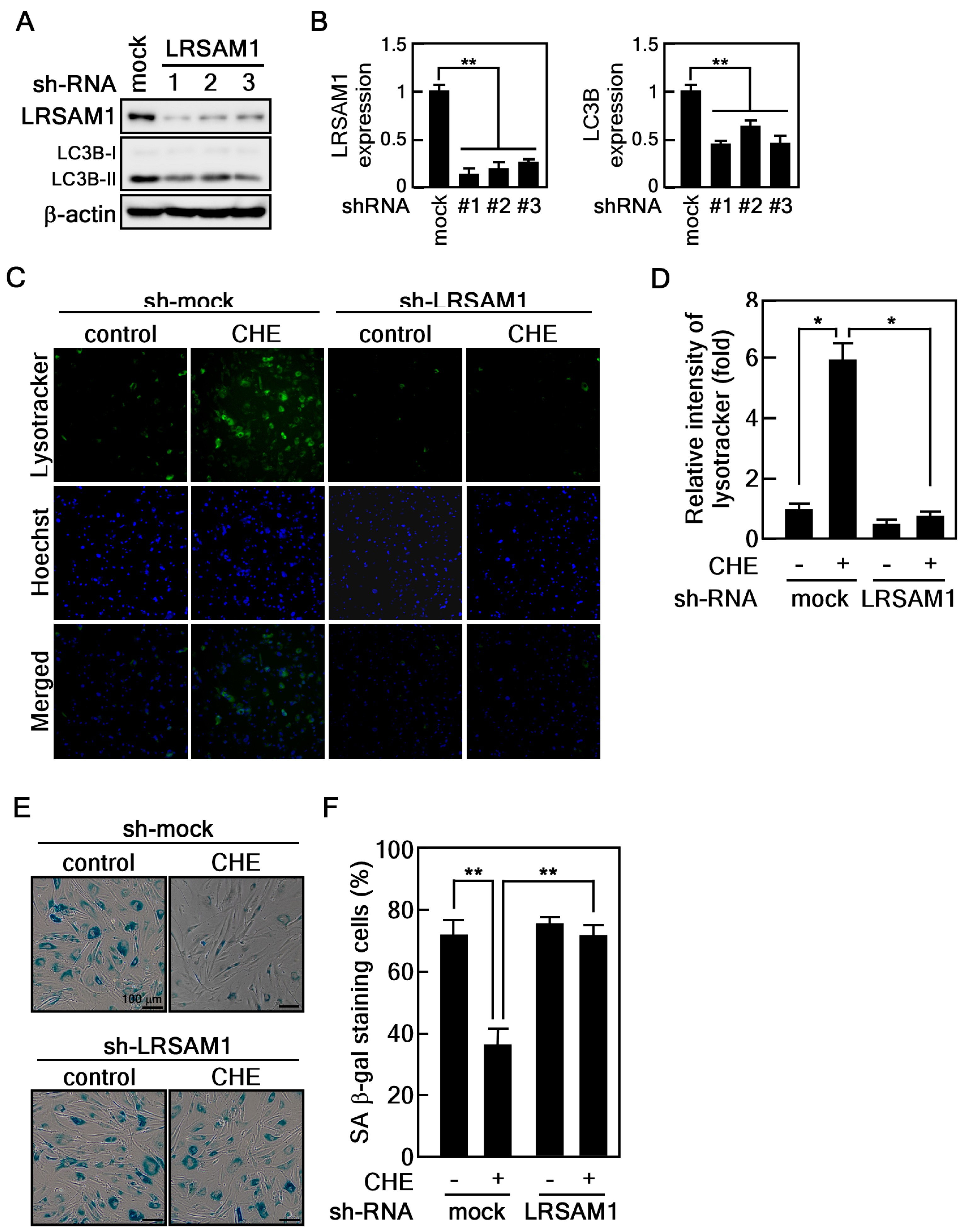
2.5. Depletion of LRSAM1 Suppresses the CHE-Induced Reversal of Cellular Senescence by Inhibiting Autophagy
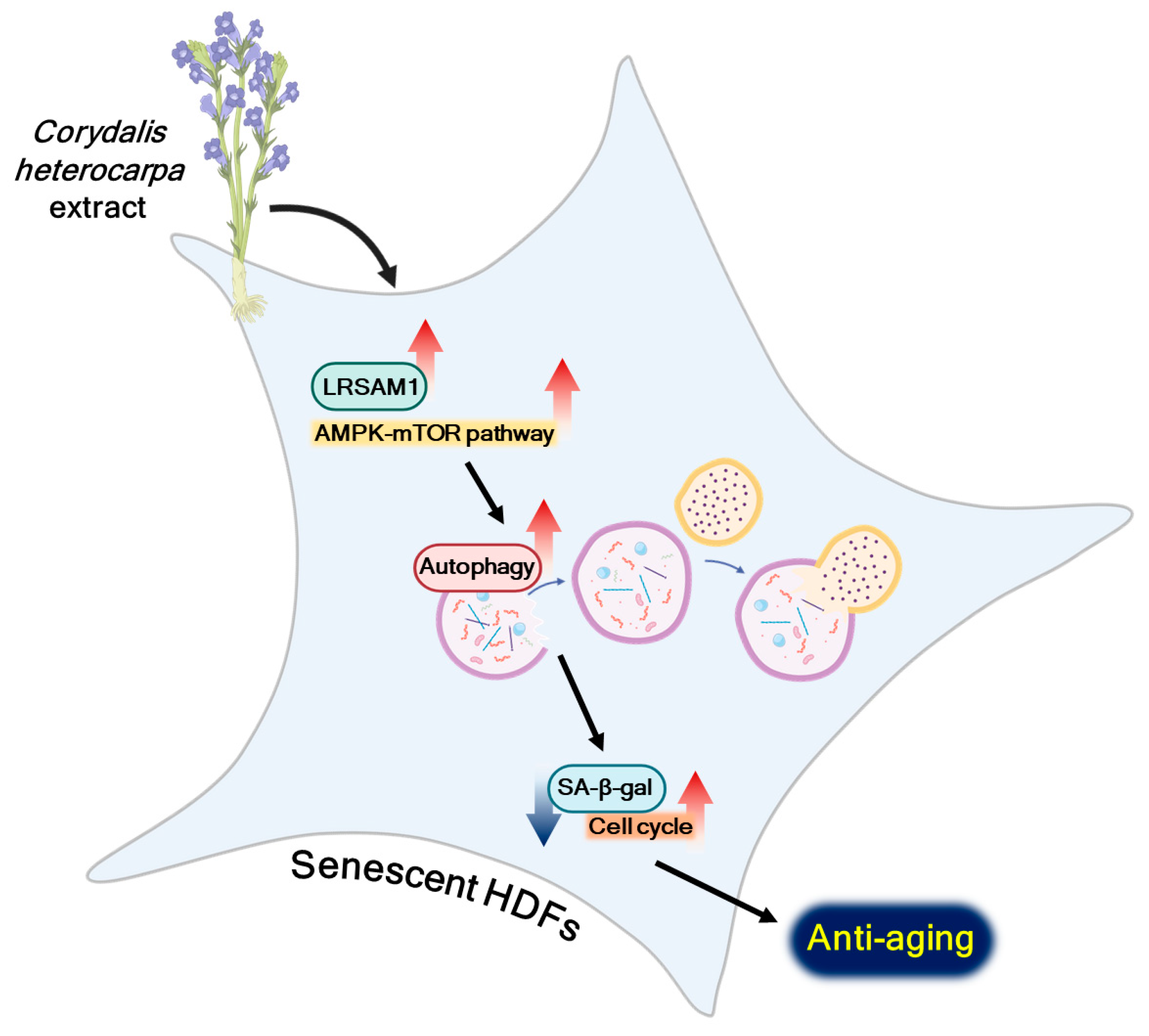
3. Discussion
4. Materials and Methods
4.1. Corydalis Heterocarpa Extract
4.2. Cell Culture and Treatment
4.3. CCK-8 Assay
4.4. Senescence-Associated β-galactosidase Staining Assay
4.5. Western Blotting
4.6. LC3B Puncta Formation
4.7. Measuring Autophagy-Associated Lysosomal Activity
4.8. Identification of Compounds in CHE Using LC/MS
4.9. Cell Cycle Distribution Analysis
4.10. RNA-Sequencing
4.11. Materials
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 1 January 2023).
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Morimoto, R.I.; Pessin, J.E.; et al. Geroscience: Linking aging to chronic disease. Cell 2014, 159, 709–713. [Google Scholar] [CrossRef]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Beauséjour, C.M.; Krtolica, A.; Galimi, F.; Narita, M.; Lowe, S.W.; Yaswen, P.; Campisi, J. Reversal of human cellular senescence: Roles of the p53 and p16 pathways. EMBO J. 2003, 22, 4212–4222. [Google Scholar] [CrossRef]
- Liu, B.; Chen, Y.; St. Clair, D.K. ROS and p53: A versatile partnership. Free Radic. Biol. Med. 2008, 44, 1529–1535. [Google Scholar] [CrossRef]
- Serrano, M.; Lin, A.W.; McCurrach, M.E.; Beach, D.; Lowe, S.W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997, 88, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.E.; Nam, S.-B.; Jang, M.; Park, J.; Lee, G.-E.; Cho, Y.-Y.; Jang, B.-C.; Lee, C.-J.; Choi, J.-S. Ginsenoside Rb2 suppresses cellular senescence of human dermal fibroblasts by inducing autophagy. J. Ginseng Res. 2023, 47, 337–346. [Google Scholar] [CrossRef]
- Cho, K.A.; Ryu, S.J.; Oh, Y.S.; Park, J.H.; Lee, J.W.; Kim, H.P.; Kim, K.T.; Jang, I.S.; Park, S.C. Morphological adjustment of senescent cells by modulating caveolin-1 status. J. Biol. Chem. 2004, 279, 42270–42278. [Google Scholar] [CrossRef]
- Zhang, J.; Lazarenko, O.P.; Blackburn, M.L.; Badger, T.M.; Ronis, M.J.J.; Chen, J.-R. Soy protein isolate down-regulates caveolin-1 expression to suppress osteoblastic cell senescence pathways. FASEB J. 2014, 28, 3134–3145. [Google Scholar] [CrossRef]
- Slobodnyuk, K.; Radic, N.; Ivanova, S.; Llado, A.; Trempolec, N.; Zorzano, A.; Nebreda, A.R. Autophagy-induced senescence is regulated by p38alpha signaling. Cell Death Dis. 2019, 10, 376. [Google Scholar] [CrossRef]
- Garcia-Prat, L.; Martinez-Vicente, M.; Perdiguero, E.; Ortet, L.; Rodriguez-Ubreva, J.; Rebollo, E.; Ruiz-Bonilla, V.; Gutarra, S.; Ballestar, E.; Serrano, A.L.; et al. Autophagy maintains stemness by preventing senescence. Nature 2016, 529, 37–42. [Google Scholar] [CrossRef]
- Chun, Y.; Kim, J. Autophagy: An Essential Degradation Program for Cellular Homeostasis and Life. Cells 2018, 7, 278. [Google Scholar] [CrossRef]
- Matecic, M.; Smith, D.L.; Pan, X.; Maqani, N.; Bekiranov, S.; Boeke, J.D.; Smith, J.S. A microarray-based genetic screen for yeast chronological aging factors. PLoS Genet. 2010, 6, e1000921. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Rubinsztein, D.C.; Walker, D.W. Autophagy as a promoter of longevity: Insights from model organisms. Nat. Rev. Mol. Cell Biol. 2018, 19, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Tóth, M.L.; Sigmond, T.; Borsos, E.; Barna, J.; Erdélyi, P.; Takács-Vellai, K.; Orosz, L.; Kovács, A.L.; Csikós, G.; Sass, M.; et al. Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Autophagy 2008, 4, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Pyo, J.-O.; Yoo, S.-M.; Ahn, H.-H.; Nah, J.; Hong, S.-H.; Kam, T.-I.; Jung, S.; Jung, Y.-K. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat. Commun. 2013, 4, 2300. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.N. Flora of Korea; Kyo-Hak Publishing: Seoul, Republic of Korea, 2002; pp. 218–219. [Google Scholar]
- Kim, Y.A.; Kong, C.-S.; Yea, S.S.; Seo, Y. Constituents of Corydalis heterocarpa and their anti-proliferative effects on human cancer cells. Food Chem. Toxicol. 2010, 48, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.-H.; Kong, C.-S.; Seo, Y.; Kim, M.-M.; Kim, S.-K. Anti-inflammatory effect of coumarins isolated from Corydalis heterocarpa in HT-29 human colon carcinoma cells. Food Chem. Toxicol. 2009, 47, 2129–2134. [Google Scholar] [CrossRef]
- Oh, J.H.; Karadeniz, F.; Seo, Y.; Kong, C.S. Hyunganol II Exerts Antiadipogenic Properties via MAPK-Mediated Suppression of PPARgamma Expression in Human Bone Marrow-Derived Mesenchymal Stromal Cells. Evid.-Based Complement. Altern. Med. 2022, 2022, 4252917. [Google Scholar] [CrossRef]
- Ahn, B.-N.; Kim, J.-A.; Kong, C.-S.; Seo, Y.; Kim, S.-K. Protective effect of (2′S)-columbianetin from Corydalis heterocarpa on UVB-induced keratinocyte damage. J. Photochem. Photobiol. B 2012, 109, 20–27. [Google Scholar] [CrossRef]
- Galbiati, F.; Volonte, D.; Liu, J.; Capozza, F.; Frank, P.G.; Zhu, L.; Pestell, R.G.; Lisanti, M.P. Caveolin-1 expression negatively regulates cell cycle progression by inducing G(0)/G(1) arrest via a p53/p21(WAF1/Cip1)-dependent mechanism. Mol. Biol. Cell 2001, 12, 2229–2244. [Google Scholar] [CrossRef]
- Wang, L.; Chen, M.; Yang, J.; Zhang, Z. LC3 fluorescent puncta in autophagosomes or in protein aggregates can be distinguished by FRAP analysis in living cells. Autophagy 2013, 9, 756–769. [Google Scholar] [CrossRef]
- Yoshii, S.R.; Mizushima, N. Monitoring and Measuring Autophagy. Int. J. Mol. Sci. 2017, 18, 1865. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, J.; Li, G.; Qu, L.; He, Q.; Lou, Y.; Song, Q.; Ma, D.; Chen, Y. PHF23 (plant homeodomain finger protein 23) negatively regulates cell autophagy by promoting ubiquitination and degradation of E3 ligase LRSAM1. Autophagy 2014, 10, 2158–2170. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.N.; Kim, J.A.; Kong, C.S.; Seo, Y.; Kim, S.K. Photoprotective effect of libanoridin isolated from Corydalis heterocarpa on UVB stressed human keratinocyte cells. Exp. Dermatol. 2013, 22, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative stress in the skin: Impact and related protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, S.N.; Kim, K.; Joo, D.H.; Shin, S.; Lee, J.; Lee, H.K.; Kim, J.; Kwon, S.B.; Kim, M.J.; et al. Biological effects of rutin on skin aging. Int. J. Mol. Med. 2016, 38, 357–363. [Google Scholar] [CrossRef]
- Han, T.; Cheng, G.; Liu, Y.; Yang, H.; Hu, Y.-T.; Huang, W. In vitro evaluation of tectoridin, tectorigenin and tectorigenin sodium sulfonate on antioxidant properties. Food Chem. Toxicol. 2012, 50, 409–414. [Google Scholar] [CrossRef]
- Dong, T.; Fan, X.; Zheng, N.; Yan, K.; Hou, T.; Peng, L.; Ci, X. Activation of Nrf2 signalling pathway by tectoridin protects against ferroptosis in particulate matter-induced lung injury. Br. J. Pharmacol. 2023, 180, 2532–2549. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.-L.; Zou, L.-B.; Lin, S.; Shi, J.-G.; Zhu, H.-B. Anti-apoptotic effect of esculin on dopamine-induced cytotoxicity in the human neuroblastoma SH-SY5Y cell line. Neuropharmacology 2007, 53, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Shen, Y.; Du, Y.; Chen, J.; Pei, F.; Fu, W.; Qiao, J. Esculin prevents Lipopolysaccharide/D-Galactosamine-induced acute liver injury in mice. Microb. Pathog. 2018, 125, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Verdú, E.; Ceballos, D.; Vilches, J.J.; Navarro, X. Influence of aging on peripheral nerve function and regeneration. J. Peripher. Nerv. Syst. 2000, 5, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Yang, X.; Wang, L.; Zhang, Q.; Ma, W.; Huang, Z.; Bao, Y.; Zhong, L.; Sun, H.; Ding, F. Isoquercitrin promotes peripheral nerve regeneration through inhibiting oxidative stress following sciatic crush injury in mice. Ann. Transl. Med. 2019, 7, 680. [Google Scholar] [CrossRef] [PubMed]
- Shui, L.; Wang, W.; Xie, M.; Ye, B.; Li, X.; Liu, Y.; Zheng, M. Isoquercitrin induces apoptosis and autophagy in hepatocellular carcinoma cells via AMPK/mTOR/p70S6K signaling pathway. Aging 2020, 12, 24318–24332. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Retzlaff, M.; Roos, T.; Frydman, J. Cellular strategies of protein quality control. Cold Spring Harb. Perspect. Biol. 2011, 3, a004374. [Google Scholar] [CrossRef] [PubMed]
- Cheon, S.Y.; Kim, H.; Rubinsztein, D.C.; Lee, J.E. Autophagy, Cellular Aging and Age-related Human Diseases. Exp. Neurobiol. 2019, 28, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Huett, A.; Heath, R.J.; Begun, J.; Sassi, S.O.; Baxt, L.A.; Vyas, J.M.; Goldberg, M.B.; Xavier, R.J. The LRR and RING domain protein LRSAM1 is an E3 ligase crucial for ubiquitin-dependent autophagy of intracellular Salmonella Typhimurium. Cell Host Microbe 2012, 12, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.R.; Mishra, R.; Sundaria, N.; Jagtap, Y.A.; Kumar, P.; Kinger, S.; Choudhary, A.; Jha, H.C.; Prasad, A.; Gutti, R.K.; et al. Resveratrol Promotes LRSAM1 E3 Ubiquitin Ligase-Dependent Degradation of Misfolded Proteins Linked with Neurodegeneration. Cell Physiol. Biochem. 2022, 56, 530–545. [Google Scholar] [PubMed]
- Alers, S.; Löffler, A.S.; Wesselborg, S.; Stork, B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: Cross talk, shortcuts, and feedbacks. Mol. Cell. Biol. 2012, 32, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Zachari, M.; Ganley, I.G. The mammalian ULK1 complex and autophagy initiation. Essays Biochem. 2017, 61, 585–596. [Google Scholar]
- Koren, I.; Reem, E.; Kimchi, A. DAP1, a novel substrate of mTOR, negatively regulates autophagy. Curr. Biol. 2010, 20, 1093–1098. [Google Scholar] [CrossRef]



| Antibodies | Company | Catalog |
|---|---|---|
| p53 | Santa Cruz Biotechnology | sc-126 |
| p21 | Cell Signaling Technology | 2946 |
| caveolin-1 | Cell Signaling Technology | 3238 |
| β-actin | Santa Cruz Biotechnology | sc-47778 |
| LC3B | Novus Biologicals | NB100-2220 |
| p62 | Novus Biologicals | NBP1-48320 |
| p-AMPK | Cell Signaling Technology | 2531 |
| AMPK | Cell Signaling Technology | 2532 |
| p-mTOR | Cell Signaling Technology | 5536 |
| mTOR | Cell Signaling Technology | 2972 |
| p-ULK1 | Cell Signaling Technology | 6888 |
| ULK1 | Cell Signaling Technology | 4773 |
| LRSAM1 | Cell Signaling Technology | 28405 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.E.; Nam, S.-B.; Lee, G.-E.; Yang, G.; Lee, M.-H.; Bang, G.; Choi, J.H.; Cho, Y.-Y.; Lee, C.-J. Induction of Autophagy by Extract from Corydalis heterocarpa for Skin Anti-Aging. Mar. Drugs 2024, 22, 127. https://doi.org/10.3390/md22030127
Yang KE, Nam S-B, Lee G-E, Yang G, Lee M-H, Bang G, Choi JH, Cho Y-Y, Lee C-J. Induction of Autophagy by Extract from Corydalis heterocarpa for Skin Anti-Aging. Marine Drugs. 2024; 22(3):127. https://doi.org/10.3390/md22030127
Chicago/Turabian StyleYang, Kyeong Eun, Soo-Bin Nam, Ga-Eun Lee, Gabsik Yang, Mee-Hyun Lee, Geul Bang, Jung Hoon Choi, Yong-Yeon Cho, and Cheol-Jung Lee. 2024. "Induction of Autophagy by Extract from Corydalis heterocarpa for Skin Anti-Aging" Marine Drugs 22, no. 3: 127. https://doi.org/10.3390/md22030127
APA StyleYang, K. E., Nam, S.-B., Lee, G.-E., Yang, G., Lee, M.-H., Bang, G., Choi, J. H., Cho, Y.-Y., & Lee, C.-J. (2024). Induction of Autophagy by Extract from Corydalis heterocarpa for Skin Anti-Aging. Marine Drugs, 22(3), 127. https://doi.org/10.3390/md22030127







