Heterocycles and a Sorbicillinoid from the Coral-Derived Fungus Penicillium chrysogenum
Abstract
1. Introduction
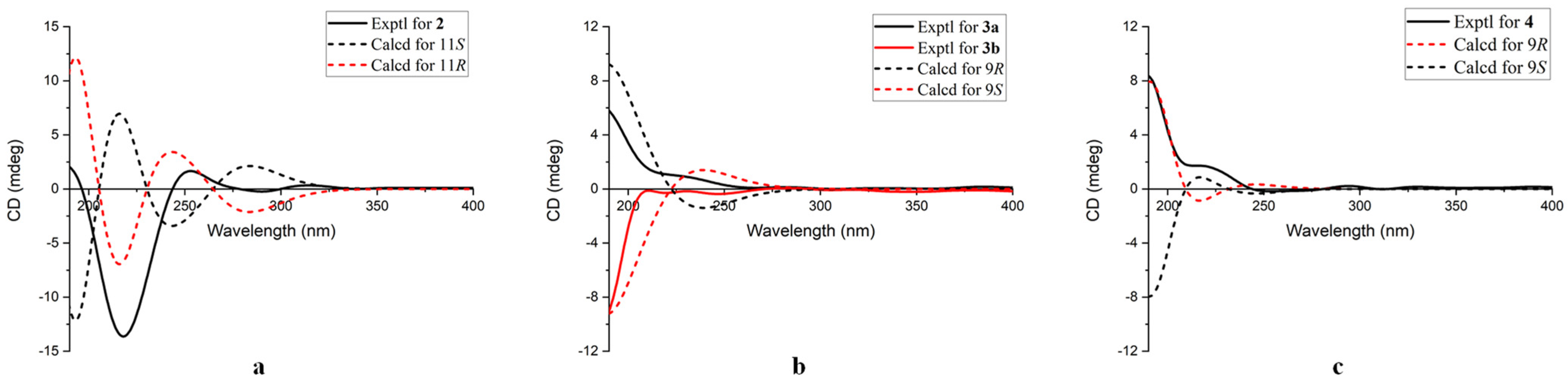
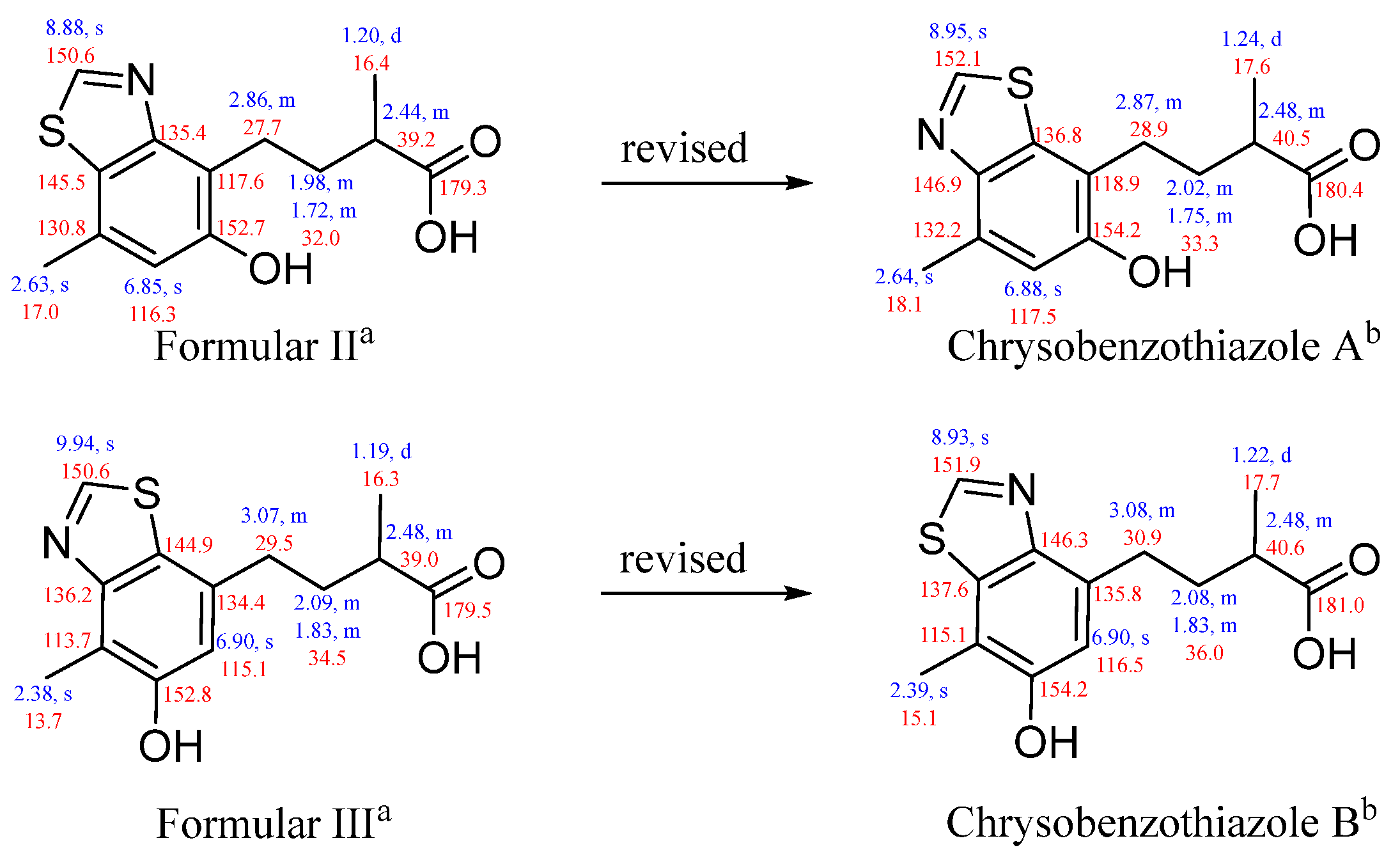
2. Results
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation, Extraction and Isolation
3.4. X-Ray Crystallographic Analysis
3.5. Quantum Chemical Calculations
3.6. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carroll, A.R.; Copp, B.R.; Grkovic, T.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2024, 41, 162–207. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, G.; Lang, G.; Gulder, T.A.M.; Tsuruta, H.; Mühlbacher, J.; Maksimenka, K.; Steffens, S.; Schaumann, K.; Stöhr, R.; Wiese, J.; et al. The first sorbicillinoid alkaloids, the antileukemic sorbicillactones A and B, from a sponge-derived Penicillium chrysogenum strain. Tetrahedron 2005, 61, 7252–7265. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, X.; Du, L.; Wang, W.; Zhu, T.; Cu, Q.; Li, D. Sorbicatechols A and B, Antiviral Sorbicillinoids from the Marine-Derived Fungus Penicillium chrysogenum PJX-17. J. Nat. Prod. 2014, 77, 424–428. [Google Scholar] [CrossRef]
- Cao, M.-J.; Zhu, T.; Liu, J.-T.; Ouyang, L.; Yang, F.; Lin, H.-W. New sorbicillinoid derivatives with GLP-1R and eEF2K affinities from a sponge-derived fungus Penicillium chrysogenum 581F1. Nat. Prod. Res. 2020, 34, 2880–2886. [Google Scholar] [CrossRef]
- Zhen, X.; Gong, T.; Wen, Y.-H.; Yan, D.-J.; Chen, J.-J.; Zhu, P. A Chrysoxanthones A-C, Three New Xanthone-Chromanone Heterdimers from Sponge-Associated Penicillium chrysogenum HLS111 Treated with Histone Deacetylase Inhibitor. Mar. Drugs 2018, 16, 357. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, H.; Li, W.; Zhu, X.; Ding, W.; Li, C. Bioactive Chaetoglobosins from the Mangrove Endophytic Fungus Penicillium chrysogenum. Mar. Drugs 2016, 14, 172. [Google Scholar] [CrossRef]
- Xu, W.-F.; Mao, N.; Xue, X.-J.; Qi, Y.-X.; Wei, M.-Y.; Wang, C.-Y.; Shao, C.-L. Structures and Absolute Configurations of Diketopiperazine Alkaloids Chrysopiperazines A-C from the Gorgonian-Derived Penicillium chrysogenum Fungus. Mar. Drugs 2019, 17, 250. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.-S.; Li, X.-M.; Du, F.-Y.; Li, C.-S.; Proksch, P.; Wang, B.-G. Secondary Metabolites from a Marine-Derived Endophytic Fungus Penicillium chrysogenum QEN-24S. Mar. Drugs 2011, 9, 59–70. [Google Scholar] [CrossRef]
- Guo, W.; Li, D.; Peng, J.; Zhu, T.; Gu, Q.; Li, D. Penicitols A-C and Penixanacid A from the Mangrove-Derived Penicillium chrysogenum HDN11-24. J. Nat. Prod. 2015, 78, 306–310. [Google Scholar] [CrossRef]
- Niu, S.; Xia, M.; Chen, M.; Liu, X.; Li, Z.; Xie, Y.; Shao, Z.; Zhang, G. Cytotoxic Polyketides Isolated from the Deep-Sea-Derived Fungus Penicillium chrysogenum MCCC 3A00292. Mar. Drugs 2019, 17, 686. [Google Scholar] [CrossRef]
- Peng, X.; Wang, Y.; Sun, K.; Liu, P.; Yin, X.; Zhu, W. Cerebrosides and 2-Pyridone Alkaloids from the Halotolerant Fungus Penicillium chrysogenum Grown in a Hypersaline Medium. J. Nat. Prod. 2011, 74, 1298–1302. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.-L.; Yuan, X.-L.; Du, Y.-M.; Zhang, Z.-F.; Zhang, P. Benzophenone Derivatives from an Algal-Endophytic Isolate of Penicillium chrysogenum and Their Cytotoxicity. Molecules 2018, 23, 3378. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, J.; Lin, X.; Zhao, B.; Wei, X.; Li, G.; Kaliaperumal, K.; Liao, S.; Yang, B.; Zhou, X.; et al. Chrysamides A-C, Three Dimeric Nitrophenyl trans-Epoxyamides Produced by the Deep-Sea-Derived Fungus Penicillium chrysogenum SCSIO41001. Org. Lett. 2016, 18, 3650–3653. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.-M.; Wang, C.-Y.; Gu, Y.-C.; Shao, C.-L. Penimethavone A, a flavone from a gorgonian-derived fungus Penicillium chrysogenum. Nat. Prod. Res. 2016, 30, 2274–2277. [Google Scholar] [CrossRef]
- Xu, K.; Wei, X.-L.; Xue, L.; Zhang, Z.-F.; Zhang, P. Antimicrobial Meroterpenoids and Erythritol Derivatives Isolated from the Marine-Algal-Derived Endophytic Fungus Penicillium chrysogenum XNM-12. Mar. Drugs 2020, 18, 578. [Google Scholar] [CrossRef]
- Qiao, H.; Zhang, S.-H.; Dong, Y.; Yang, Y.; Xu, R.; Chen, B.; Wang, Y.; Zhu, T.-J.; Cui, C.-B.; Zhang, G.-G.; et al. Chrysomutanin and related meroterpenoids from a DES mutant of the marine-derived fungus Penicillium chrysogenum S-3-25. Nat. Prod. Res. 2022, 36, 1834–1841. [Google Scholar] [CrossRef]
- Li, H.B.; Fu, G.H.; Zhong, W.H. Natural quinazolinones: From a treasure house to promising anticancer leads. Eur. J. Med. Chem. 2023, 245, 114915. [Google Scholar] [CrossRef]
- Wang, J.J.; Li, K.L.; Luo, X.W.; Wu, Z.Y.; Gu, T.W.; Liao, S.R.; Lin, X.P.; Yang, B.; Liu, Y.H.; Fang, W.; et al. Sorbicillfurans A and B, two novel sorbicillinoid adducts from the fungus Penicillium citrinum SCSIO41402. Org. Biomol. Chem. 2019, 17, 8721–8725. [Google Scholar] [CrossRef]
- Li, D.H.; Chen, L.; Zhu, T.J.; Kurtán, T.; Mándi, A.; Zhao, Z.M.; Li, J.; Gu, Q.Q. Chloctanspirones A and B, novel chlorinated polyketides with an unprecedented skeleton, from marine sediment derived fungus Penicillium terrestre. Tetrahedron 2011, 67, 7913–7918. [Google Scholar] [CrossRef]
- Yu, J.; Han, H.; Zhang, X.; Ma, C.; Sun, C.; Che, Q.; Gu, Q.; Zhu, T.; Zhang, G.; Li, D. Discovery of Two New Sorbicillinoids by Overexpression of the Global Regulator LaeA in a Marine-Derived Fungus Penicillium dipodomyis YJ-11. Mar. Drugs 2019, 17, 446. [Google Scholar] [CrossRef]
- Guo, W.Q.; Peng, J.J.; Zhu, T.Q.; Gu, Q.Q.; Keyzers, R.A.; Li, D.H. Sorbicillamines A-E, Nitrogen-Containing Sorbicillinoids from the Deep-Sea-Derived Fungus Penicillium sp. F23-2. J. Nat. Prod. 2013, 76, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Chen, T.; Sun, B.; Tan, Q.; Ouyang, H.; Wang, B.; Yu, H.J.; She, Z.G. Mono- and Dimeric Sorbicillinoid Inhibitors Targeting IL-6 and IL-1β from the Mangrove-Derived Fungus Trichoderma reesei BGRg-3. Int. J. Mol. Sci. 2023, 24, 16096. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Chen, T.; Yu, J.C.; Jia, H.; Chen, C.; Long, Y.H. New Sorbicillinoids from the Mangrove Endophytic Fungus Trichoderma reesei SCNU-F0042. Mar. Drugs 2023, 21, 442. [Google Scholar] [CrossRef] [PubMed]
- Aayishamma, I.; Matada, G.S.P.; Pal, R.; Ghara, A.; Aishwarya, N.; Kumaraswamy, B.; Hosamani, K.R.; Manjushree, B.V.; Haripriya, E. Benzothiazole a privileged scaffold for Cutting-Edges anticancer agents: Exploring drug design, structure-activity relationship, and docking studies. Eur. J. Med. Chem. 2024, 279, 116831. [Google Scholar]
- Yadav, R.; Meena, D.; Singh, K.; Tyagi, R.; Yadav, Y.; Sagar, R. Recent advances in the synthesis of new benzothiazole based anti-tubercular compounds. Rsc Adv. 2023, 13, 21890–21925. [Google Scholar] [CrossRef]
- Shainyan, B.A.; Zhilitskaya, L.V.; Yarosh, N.O. Synthetic Approaches to Biologically Active C-2-Substituted Benzothiazoles. Molecules 2022, 27, 2598. [Google Scholar] [CrossRef]
- Yang, X.W.; Xie, C.L.; Yan, Q.X.; Zou, Z.B.; He, Z.H. Preparation of Benzothiazole Heteroterpene Derivatives as anticancer Agents. WO2022057223, 24 March 2022. [Google Scholar]
- Tsantrizos, Y.S.; Xu, X.J.; Sauriol, F.; Hynes, R.C. Novel quinazolinones and enniatins from Fusarium lateritium Nees. Can. J. Chem. 1993, 71, 1362–1367. [Google Scholar] [CrossRef]
- Zhang, P.P.; Deng, Y.L.; Lin, X.J.; Chen, B.; Li, J.; Liu, H.J.; Chen, S.H.; Liu, L. Anti-inflammatory Mono- and Dimeric Sorbicillinoids from the Marine-Derived Fungus Trichoderma reesei 4670. J. Nat. Prod. 2019, 82, 947–957. [Google Scholar] [CrossRef]
- Ying, Y.-M.; Zhan, Z.-J.; Ding, Z.-S.; Shan, W.-G. Bioactive metabolites from Penicillium sp. P-1, a fungal endophyte in Huperzia serrata. Chem. Nat. Compd. 2011, 47, 541–544. [Google Scholar] [CrossRef]
- Zong, Y.; Jin, T.Y.; Yang, J.J.; Wang, K.Y.; Shi, X.; Zhang, Y.; Li, P.L. Lemneolemnanes A-D, Four Uncommon Sesquiterpenoids from the Soft Coral Lemnalia sp. Mar. Drugs 2024, 22, 145. [Google Scholar] [CrossRef]
- Wang, C.L.; Zhang, J.R.; Gan, Y.; Wang, M.F.; Li, X.L.; Liu, X.H.; Shi, X.; Mi, Y.; Liu, K.C.; Zhang, Y.; et al. Sarcoelegans A-H, eight undescribed cembranes with anti-inflammatory and anti-thrombotic activities from the South China Sea soft coral Sarcophyton elegans. Phytochemistry 2023, 207, 113578. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]




| Pos. | 1 | ||||
|---|---|---|---|---|---|
| δC a Type | δH b (J in Hz) | 1H–1H COSY | HMBC (H→C) | NOESY | |
| 1 | 66.4, C | ||||
| 2 | 207.2, C | ||||
| 3a | 36.2, CH2 | 2.57, d (19.5) | H-4 | C-2, 4, 5 | H-12 |
| 3b | 2.91 [α] | H-4 | C-2, 4, 8 | H-9b | |
| 4 | 40.2, CH | 2.40, s | H-3a, 3b | C-2, 5, 6, 7 | |
| 5 | 75.9, C | ||||
| 6 | 211.8, C | ||||
| 7 | 43.6, CH | 1.63, m | H-8, 11 | C-1, 2, 6, 8, 9, 11 | H-9a |
| 8 | 38.9, CH | 2.12, m | H-7 | H-11 | |
| 9a | 30.1, CH2 | 2.19, m | H-7 | ||
| 9b | 1.96, m | H-3b | |||
| 10a | 33.8, CH2 | 2.96, m | C-9, 2′ | ||
| 10b | 2.89 [α] | C-2′ | |||
| 11 | 16.2, CH3 | 0.94, d (6.8) | H-7 | C-1, 7, 8 | H-8 |
| 12 | 33.4, CH3 | 1.13, s | C-4, 5, 6 | H-3a | |
| 13 | 9.4, CH3 | 1.07, s | C-1, 2, 6, 7 | ||
| 2′ | 156.0, C | ||||
| 4′ | 164.6, C | ||||
| 5′ | 120.6, C | ||||
| 6′ | 126.3, CH | 8.26, d (7.8) | H-7′ | C-4′, 8′, 10′ | |
| 7′ | 126.9, CH | 7.50, m | H-6′, 8′ | C-5′, 9′ | |
| 8′ | 135.2, CH | 7.79, m | H-7′, 9′ | C-6′, 10′ | |
| 9′ | 127.4, CH | 7.71, d (8.1) | H-8′ | C-5′, 7′ | |
| 10′ | 149.5, C | ||||
| Pos. | 2 | |||
|---|---|---|---|---|
| δC a Type | δH b (J in Hz) | 1H–1H COSY | HMBC (H→C) | |
| 2 | 156.6, C | |||
| 4 | 164.3, C | |||
| 5 | 122.4, C | |||
| 6 | 127.0, CH | 8.20, d (8.0) | H-7 | C-4, 8, 10 |
| 7 | 128.1, CH | 7.53, m | H-6, 8 | C-5, 9 |
| 8 | 135.8, CH | 7.82, m | H-7, 9 | C-6, 10 |
| 9 | 128.6, CH | 7.71, d (8.1) | H-8 | C-5, 7 |
| 10 | 150.1, C | |||
| 11 | 51.2, CH | 5.13, q (7.2) | H-12 | C-2, 12, 2′, 5′ |
| 12 | 15.9, CH3 | 1.63, d (7.2) | H-11 | C-2, 11 |
| 2′ | 178.4, C | |||
| 3′ | 32.1, CH2 | 2.47, t (8.1) | H-4′ | C-2′, 4′, 5′ |
| 4′ | 19.1, CH2 | 2.14, m | H-3′, 5′ | C-2′, 3′, 5′ |
| 5′ | 45.5, CH2 | 3.65, m | H-4′ | C-2′, 3′, 4′ |
| Pos. | 3 | 4 | ||
|---|---|---|---|---|
| δC a Type | δH b (J in Hz) | δC a Type | δH b (J in Hz) | |
| 1 | 118.9, C | 135.8, C | ||
| 2 | 136.8, C | 146.3, C | ||
| 3 | 146.9, C | 137.6, C | ||
| 4 | 132.2, C | 115.1, C | ||
| 5 | 117.5, CH | 6.88, s | 154.2, C | |
| 6 | 154.2, C | 116.5, CH | 6.90, s | |
| 7 | 28.9, CH2 | 2.87, m | 30.9, CH2 | 3.08, m |
| 8a | 33.3, CH2 | 2.02, m | 36.0, CH2 | 2.08, m |
| 8b | 1.75, m | 1.83, m | ||
| 9 | 40.5, CH | 2.48, m | 40.6, CH | 2.48, m |
| 10 | 180.4, C | 181.0, C | ||
| 11 | 17.6, CH3 | 1.24, d (7.0) | 17.7, CH3 | 1.22, d (7.0) |
| 12 | 18.1, CH3 | 2.64, s | 15.1, CH3 | 2.39, s |
| 13 | 152.1, CH | 8.95, s | 151.9, CH | 8.93, s |
| Pos. | 5 | |||
|---|---|---|---|---|
| δC a Type | δH b (J in Hz) | 1H–1H COSY | HMBC (H→C) | |
| 1 | 119.7, C | |||
| 2 | 160.0, C | |||
| 3 | 124.8, C | |||
| 4 | 139.6, C | |||
| 5 | 123.6, C | |||
| 6 | 128.2, CH | 7.51, s | C-2, 4, 7, 13 | |
| 7 | 194.2, C | |||
| 8 | 121.5, CH | 7.00, d (14.9) | H-9 | C-7, 10 |
| 9 | 146.5, CH | 7.53 [α] | H-8, 10 | C-7, 10, 11 |
| 10 | 130.6, CH | 6.38 [α] | H-9 | C-9, 12 |
| 11 | 143.0, CH | 6.37 [α] | H-12 | C-9, 12 |
| 12 | 19.2, CH3 | 1.94, d (5.3) | H-11 | C-10, 11 |
| 13 | 19.3, CH3 | 2.37, s | C-4, 5, 6 | |
| 14 | 171.8, C | |||
| 15 | 12.8, CH3 | 2.31, s | C-2, 3, 4 | |
| -OH | 13.09, s | C-1, 2, 3 | ||
| Compound | IC50 (μM) | |||||
|---|---|---|---|---|---|---|
| K562 | L-02 | ASPC-1 | MDA-MB-231 | NCI-H446 | NCI-H446/EP | |
| 1 | >30 | >30 | >30 | >30 | >30 | >30 |
| 2 | >30 | >30 | >30 | >30 | >30 | >30 |
| 3a | >30 | >30 | >30 | >30 | >30 | >30 |
| 3b | >30 | >30 | >30 | >30 | >30 | >30 |
| 4 | >30 | >30 | >30 | >30 | >30 | >30 |
| 5 | 15.00 | >30 | >30 | >30 | 16.87 | >30 |
| Doxorubicin a | <1 | <1 | >1 | <1 | <1 | >1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Zong, Y.; Wang, C.; Li, K.; Zhang, Y.; Li, P. Heterocycles and a Sorbicillinoid from the Coral-Derived Fungus Penicillium chrysogenum. Mar. Drugs 2024, 22, 517. https://doi.org/10.3390/md22110517
Yang J, Zong Y, Wang C, Li K, Zhang Y, Li P. Heterocycles and a Sorbicillinoid from the Coral-Derived Fungus Penicillium chrysogenum. Marine Drugs. 2024; 22(11):517. https://doi.org/10.3390/md22110517
Chicago/Turabian StyleYang, Junjie, Yuan Zong, Cili Wang, Kai Li, Yue Zhang, and Pinglin Li. 2024. "Heterocycles and a Sorbicillinoid from the Coral-Derived Fungus Penicillium chrysogenum" Marine Drugs 22, no. 11: 517. https://doi.org/10.3390/md22110517
APA StyleYang, J., Zong, Y., Wang, C., Li, K., Zhang, Y., & Li, P. (2024). Heterocycles and a Sorbicillinoid from the Coral-Derived Fungus Penicillium chrysogenum. Marine Drugs, 22(11), 517. https://doi.org/10.3390/md22110517







