Bio-Calcium from Skipjack Tuna Frame Attenuates Bone Loss in Ovariectomy-Induced Osteoporosis Rats
Abstract
1. Introduction
2. Results
2.1. Bioavailability and Chemical Blood Test
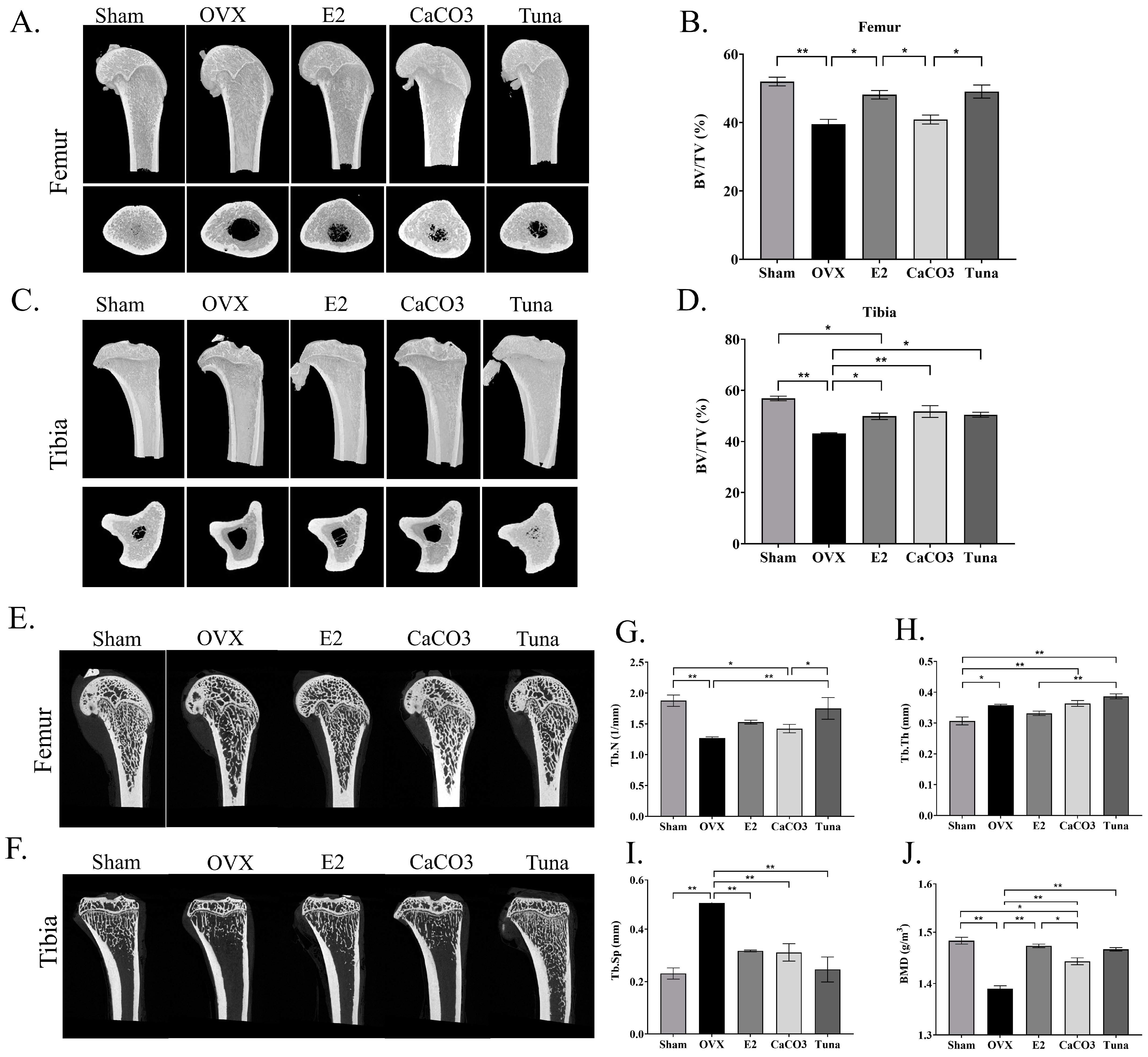
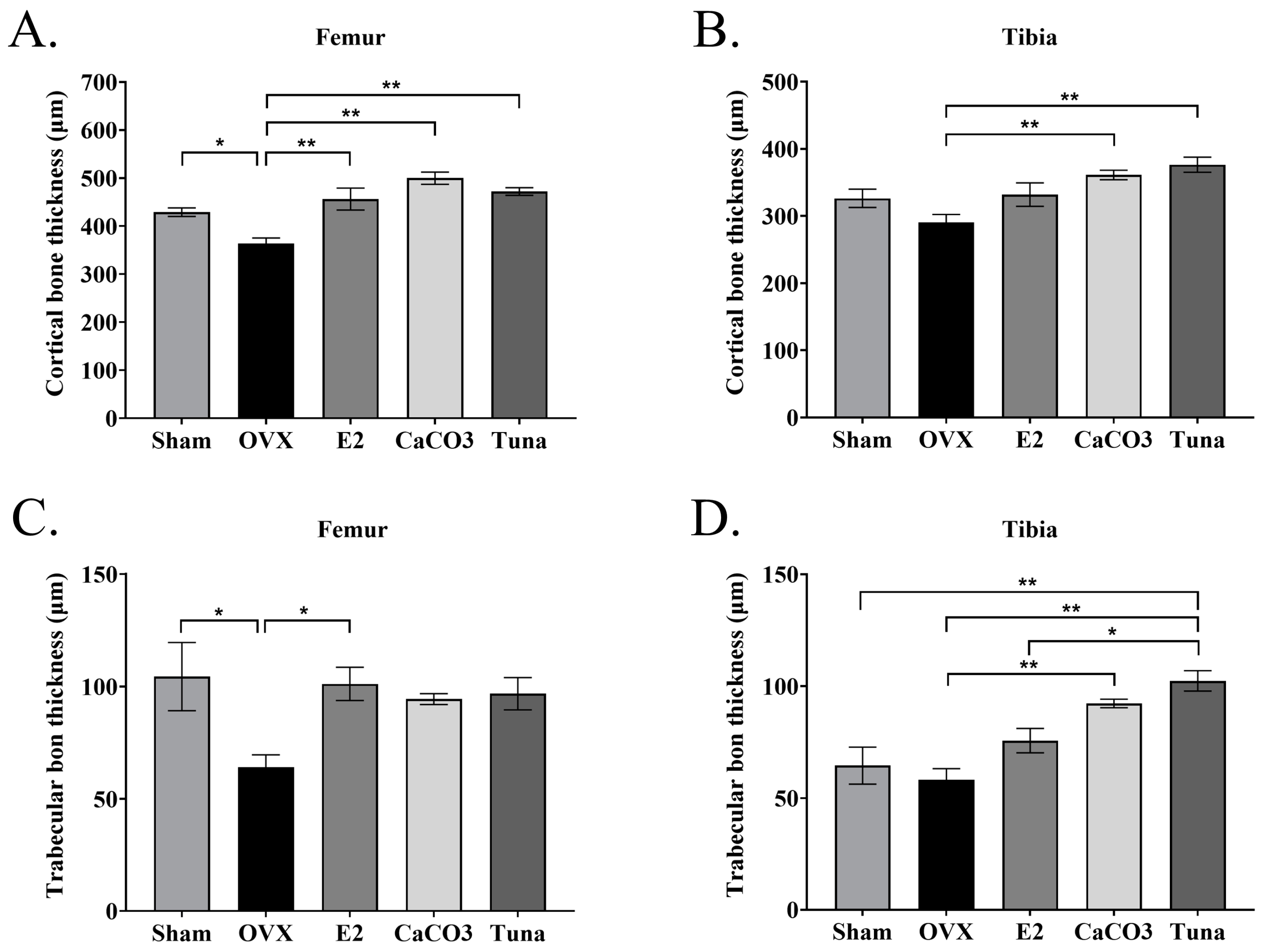
2.2. microCT Analysis of the Bones
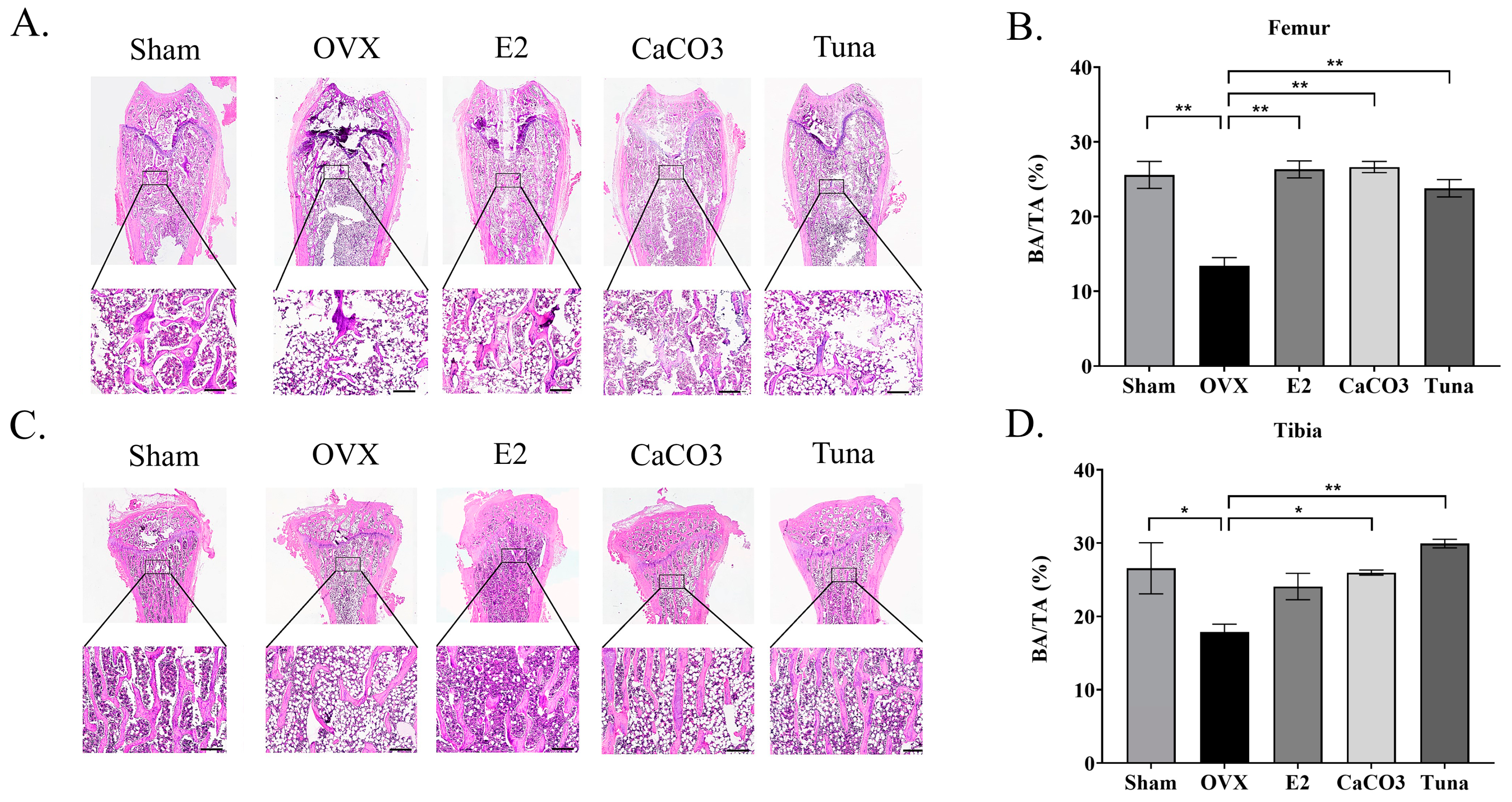
2.3. Bone Histomorphometry
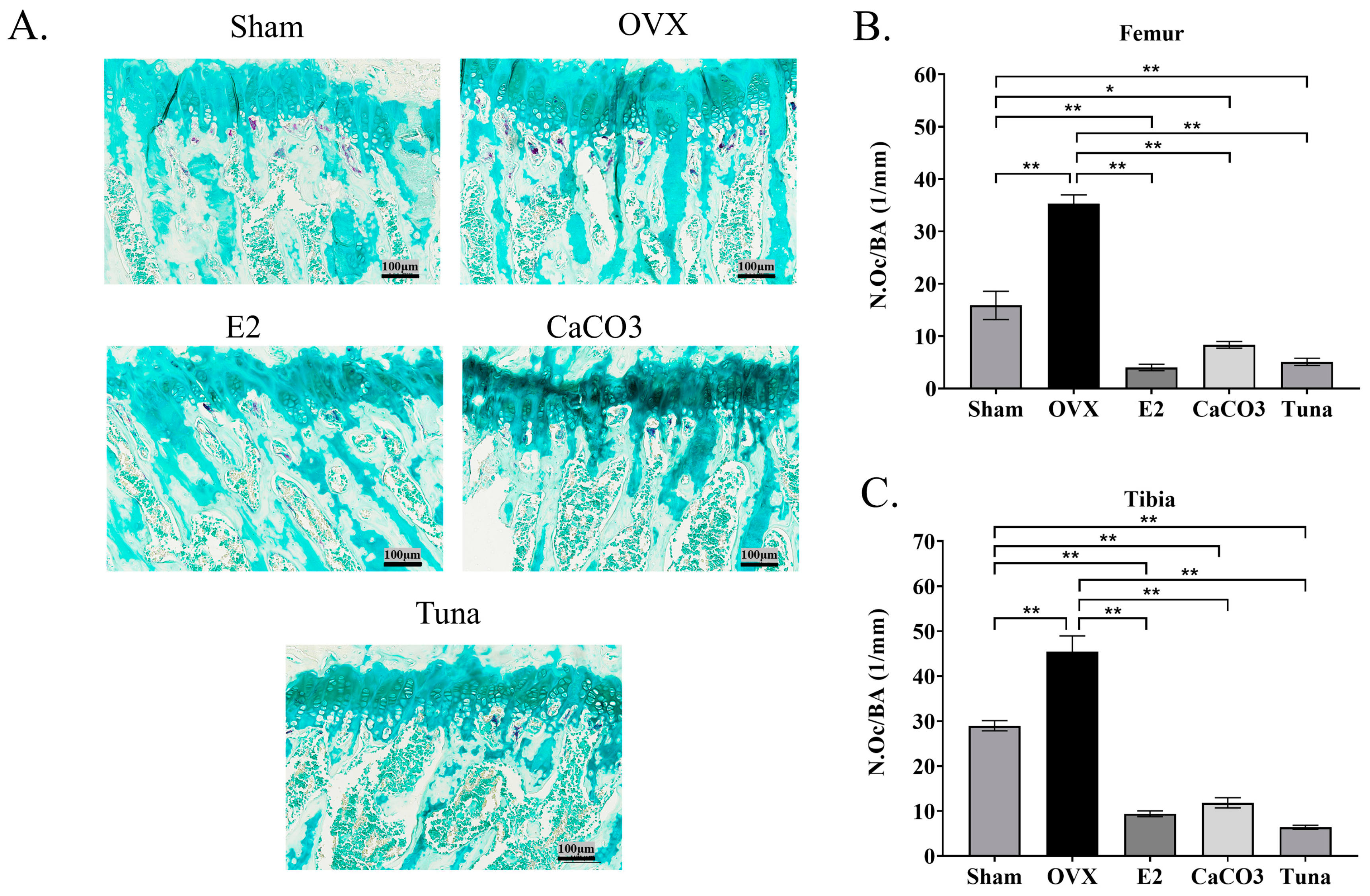
2.4. Osteoclast Analysis
3. Discussion
4. Materials and Methods
4.1. Skipjack Tuna Bio-Calcium Preparation
4.2. Preparation of Digested Bio-Calcium
4.3. Bioavailability Evaluation of Skipjack Tuna Derived Bio-Calcium
4.4. Animal Model
4.5. Biochemical Analysis
4.6. Quantitative 3D Analyses of Bone
4.7. Histomorphological Study
4.8. Osteoclast Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeLucia, M.C.; Mitnick, M.E.; Carpenter, T.O. Nutritional Rickets with Normal Circulating 25-Hydroxyvitamin D: A Call for Reexamining the Role of Dietary Calcium Intake in North American Infants. J. Clin. Endocrinol. Metab. 2003, 88, 3539–3545. [Google Scholar] [CrossRef]
- Reeve, J.; Meunier, P.J.; Parsons, J.A.; Bernat, M.; Bijvoet, O.L.; Courpron, P.; Edouard, C.; Klenerman, L.; Neer, R.M.; Renier, J.C.; et al. Anabolic Effect of Human Parathyroid Hormone Fragment on Trabecular Bone in Involutional Osteoporosis: A Multicentre Trial. Br. Med. J. 1980, 280, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Lanham-New, S.A. Importance of Calcium, Vitamin D and Vitamin K for Osteoporosis Prevention and Treatment: Symposium on ‘Diet and Bone Health’. Proc. Nutr. Soc. 2008, 67, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.-X.; Yu, Q. Primary Osteoporosis in Postmenopausal Women. Chronic Dis. Transl. Med. 2015, 1, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Lerner, U.H. Bone Remodeling in Post-Menopausal Osteoporosis. J. Dent. Res. 2006, 85, 584–595. [Google Scholar] [CrossRef]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the Skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef]
- Reid, I.R. Should We Prescribe Calcium Supplements for Osteoporosis Prevention? J. Bone Metab. 2014, 21, 21–28. [Google Scholar] [CrossRef]
- Wijayanti, I.; Singh, A.; Benjakul, S.; Sookchoo, P. Textural, Sensory, and Chemical Characteristic of Threadfin Bream (Nemipterus sp.) Surimi Gel Fortified with Bio-Calcium from Bone of Asian Sea Bass (Lates calcarifer). Foods 2021, 10, 976. [Google Scholar] [CrossRef]
- Wijayanti, I.; Sookchoo, P.; Prodpran, T.; Mohan, C.O.; Aluko, R.E.; Benjakul, S. Physical and Chemical Characteristics of Asian Sea Bass Bio-Calcium Powders as Affected by Ultrasonication Treatment and Drying Method. J. Food Biochem. 2021, 45, e13652. [Google Scholar] [CrossRef]
- Benjakul, S.; Mad-Ali, S.; Senphan, T.; Sookchoo, P. Biocalcium Powder from Precooked Skipjack Tuna Bone: Production and Its Characteristics. J. Food Biochem. 2017, 41, e12412. [Google Scholar] [CrossRef]
- Idowu, A.T.; Benjakul, S.; Sinthusamran, S.; Sae-leaw, T.; Suzuki, N.; Kitani, Y.; Sookchoo, P. Effect of Alkaline Treatment on Characteristics of Bio-Calcium and Hydroxyapatite Powders Derived from Salmon Bone. Appl. Sci. 2020, 10, 4141. [Google Scholar] [CrossRef]
- Huo, J.; Deng, S.; Xie, C.; Tong, G. Preparation and Biological Efficacy of Haddock Bone Calcium Tablets. Chin. J. Oceanol. Limnol. 2010, 28, 371–378. [Google Scholar] [CrossRef]
- Li, K.; Wang, X.-F.; Li, D.-Y.; Chen, Y.-C.; Zhao, L.-J.; Liu, X.-G.; Guo, Y.-F.; Shen, J.; Lin, X.; Deng, J.; et al. The Good, the Bad, and the Ugly of Calcium Supplementation: A Review of Calcium Intake on Human Health. Clin. Interv. Aging 2018, 13, 2443–2452. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.-Q.; Luo, Q.-B.; Suo, S.-K.; Zhao, Y.-Q.; Chi, C.-F.; Wang, B. Preparation, Characterization, and Cytoprotective Effects on HUVECs of Fourteen Novel Angiotensin-I-Converting Enzyme Inhibitory Peptides From Protein Hydrolysate of Tuna Processing By-Products. Front. Nutr. 2022, 9, 868681. [Google Scholar] [CrossRef]
- Kling, J.M.; Clarke, B.L.; Sandhu, N.P. Osteoporosis Prevention, Screening, and Treatment: A Review. J. Womens Health 2014, 23, 563–572. [Google Scholar] [CrossRef]
- Drake, M.T.; Clarke, B.L.; Lewiecki, E.M. The Pathophysiology and Treatment of Osteoporosis. Clin. Ther. 2015, 37, 1837–1850. [Google Scholar] [CrossRef]
- Flammini, L.; Martuzzi, F.; Vivo, V.; Ghirri, A.; Salomi, E.; Bignetti, E.; Barocelli, E. Hake Fish Bone as a Calcium Source for Efficient Bone Mineralization. Int. J. Food Sci. Nutr. 2016, 67, 265–273. [Google Scholar] [CrossRef]
- Lecerf, J.-M.; Lamotte, C.; Boukandoura, B.; Cayzeele, A.; Libersa, C.; Delannoy, C.; Borgiès, B. Effects of Two Marine Dietary Supplements with High Calcium Content on Calcium Metabolism and Biochemical Marker of Bone Resorption. Eur. J. Clin. Nutr. 2008, 62, 879–884. [Google Scholar] [CrossRef][Green Version]
- Hu, G.; Li, X.; Su, R.; Corazzin, M.; Dou, L.; Sun, L.; Hou, P.; Su, L.; Jin, Y.; Zhao, L. Effects of Sheep Bone Peptide-Chelated Calcium on Calcium Absorption and Bone Deposition in Rats Fed a Low-Calcium Diet. J. Food Biochem. 2024, 2024, 8434888. [Google Scholar] [CrossRef]
- Zhang, K.; Li, B.; Chen, Q.; Zhang, Z.; Zhao, X.; Hou, H. Functional Calcium Binding Peptides from Pacific Cod (Gadus macrocephalus) Bone: Calcium Bioavailability Enhancing Activity and Anti-Osteoporosis Effects in the Ovariectomy-Induced Osteoporosis Rat Model. Nutrients 2018, 10, 1325. [Google Scholar] [CrossRef]
- Torres, B.; Pérez, A.; García, P.; Jiménez, P.; Abrigo, K.; Valencia, P.; Ramírez, C.; Pinto, M.; Almonacid, S.; Ruz, M. Fish Bones as Calcium Source: Bioavailability of Micro and Nano Particles. Foods 2024, 13, 1840. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Chen, T.; Liao, D.; Wang, Y.; Su, Y.; Liu, S.; Chen, X.; Ruifang, Q.; Jiang, L.; Liu, Z. Novel Peptides Extracted from Muraenesox cinereus Bone Promote Calcium Transport, Osteoblast Differentiation, and Calcium Absorption. J. Funct. Foods 2022, 95, 105157. [Google Scholar] [CrossRef]
- Heo, S.-Y.; Ko, S.-C.; Nam, S.Y.; Oh, J.; Kim, Y.-M.; Kim, J.-I.; Kim, N.; Yi, M.; Jung, W.-K. Fish Bone Peptide Promotes Osteogenic Differentiation of MC3T3-E1 Pre-Osteoblasts through Upregulation of MAPKs and Smad Pathways Activated BMP-2 Receptor. Cell Biochem. Funct. 2018, 36, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Cianferotti, L.; Gomes, A.R.; Fabbri, S.; Tanini, A.; Brandi, M.L. The Calcium-Sensing Receptor in Bone Metabolism: From Bench to Bedside and Back. Osteoporos. Int. 2015, 26, 2055–2071. [Google Scholar] [CrossRef]
- Goltzman, D.; Hendy, G.N. The Calcium-Sensing Receptor in Bone—Mechanistic and Therapeutic Insights. Nat. Rev. Endocrinol. 2015, 11, 298–307. [Google Scholar] [CrossRef]
- Piri, F.; Khosravi, A.; Moayeri, A.; Moradipour, A.; Derakhshan, S. The Effects of Dietary Supplements of Calcium, Vitamin D and Estrogen Hormone on Serum Levels of OPG and RANKL Cytokines and Their Relationship with Increased Bone Density in Rats. J. Clin. Diagn. Res. 2016, 10, AF01–AF04. [Google Scholar] [CrossRef]
- Li, H.; Xiao, Z.; Quarles, L.D.; Li, W. Osteoporosis: Mechanism, Molecular Target, and Current Status on Drug Development. Curr. Med. Chem. 2021, 28, 1489–1507. [Google Scholar] [CrossRef]
- Straub, D.A. Calcium Supplementation in Clinical Practice: A Review of Forms, Doses, and Indications. Nutr. Clin. Pract. 2007, 22, 286–296. [Google Scholar] [CrossRef]
- Savi, F.M.; Brierly, G.I.; Baldwin, J.; Theodoropoulos, C.; Woodruff, M.A. Comparison of Different Decalcification Methods Using Rat Mandibles as a Model. J. Histochem. Cytochem. 2017, 65, 705–722. [Google Scholar] [CrossRef]
- Doube, M.; Kłosowski, M.M.; Arganda-Carreras, I.; Cordelières, F.P.; Dougherty, R.P.; Jackson, J.S.; Schmid, B.; Hutchinson, J.R.; Shefelbine, S.J. BoneJ: Free and Extensible Bone Image Analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saetang, J.; Issuriya, A.; Suyapoh, W.; Sornying, P.; Nilsuwan, K.; Benjakul, S. Bio-Calcium from Skipjack Tuna Frame Attenuates Bone Loss in Ovariectomy-Induced Osteoporosis Rats. Mar. Drugs 2024, 22, 472. https://doi.org/10.3390/md22100472
Saetang J, Issuriya A, Suyapoh W, Sornying P, Nilsuwan K, Benjakul S. Bio-Calcium from Skipjack Tuna Frame Attenuates Bone Loss in Ovariectomy-Induced Osteoporosis Rats. Marine Drugs. 2024; 22(10):472. https://doi.org/10.3390/md22100472
Chicago/Turabian StyleSaetang, Jirakrit, Acharaporn Issuriya, Watcharapol Suyapoh, Peerapon Sornying, Krisana Nilsuwan, and Soottawat Benjakul. 2024. "Bio-Calcium from Skipjack Tuna Frame Attenuates Bone Loss in Ovariectomy-Induced Osteoporosis Rats" Marine Drugs 22, no. 10: 472. https://doi.org/10.3390/md22100472
APA StyleSaetang, J., Issuriya, A., Suyapoh, W., Sornying, P., Nilsuwan, K., & Benjakul, S. (2024). Bio-Calcium from Skipjack Tuna Frame Attenuates Bone Loss in Ovariectomy-Induced Osteoporosis Rats. Marine Drugs, 22(10), 472. https://doi.org/10.3390/md22100472







