Indole Diketopiperazine Alkaloids from the Marine Sediment-Derived Fungus Aspergillus chevalieri against Pancreatic Ductal Adenocarcinoma
Abstract
:1. Introduction
2. Results and Discussion
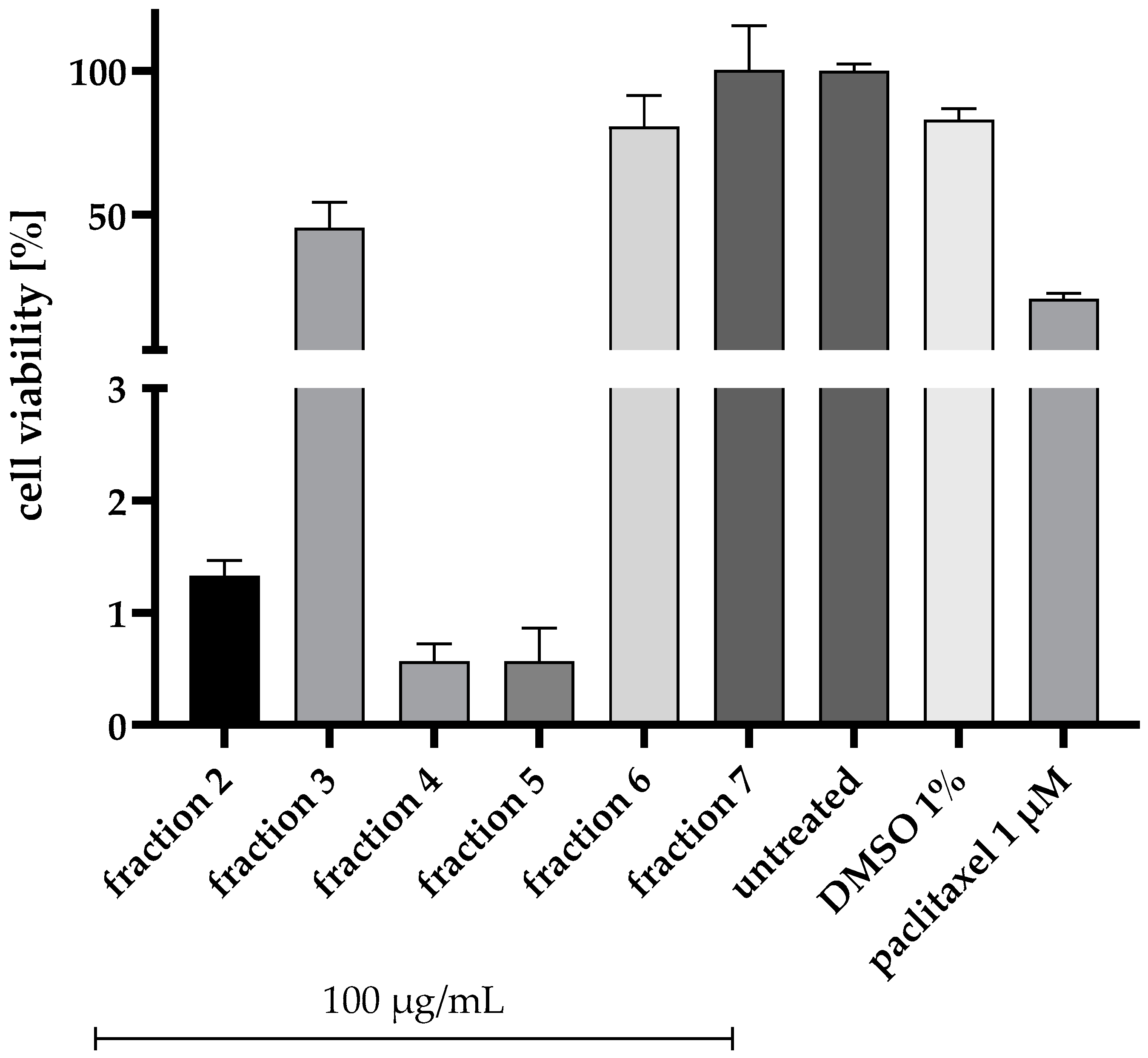
2.1. Bioactivity-Guided Fractionation of the Ethyl Acetate Extract of Aspergillus chevalieri
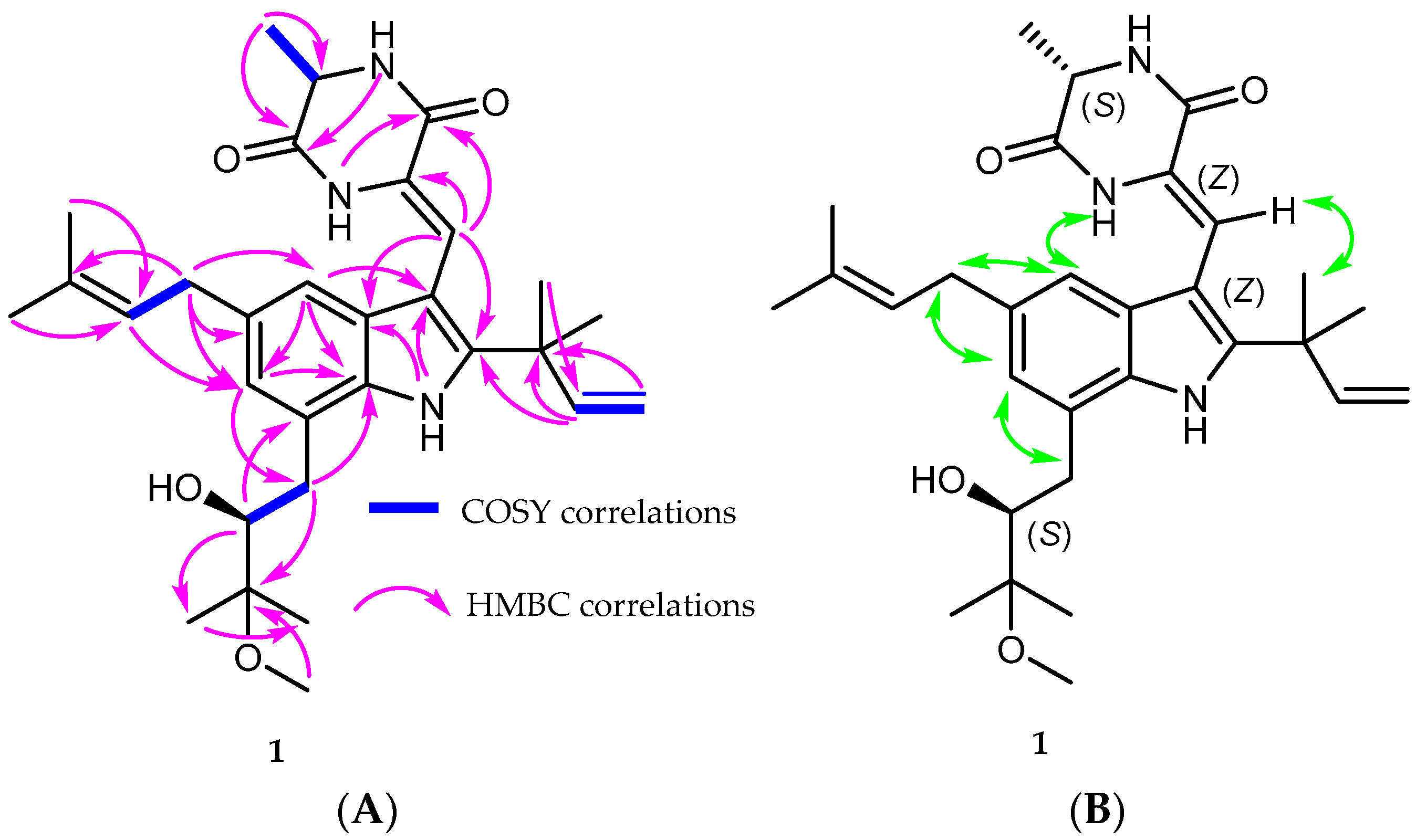
2.2. Structure Elucidation
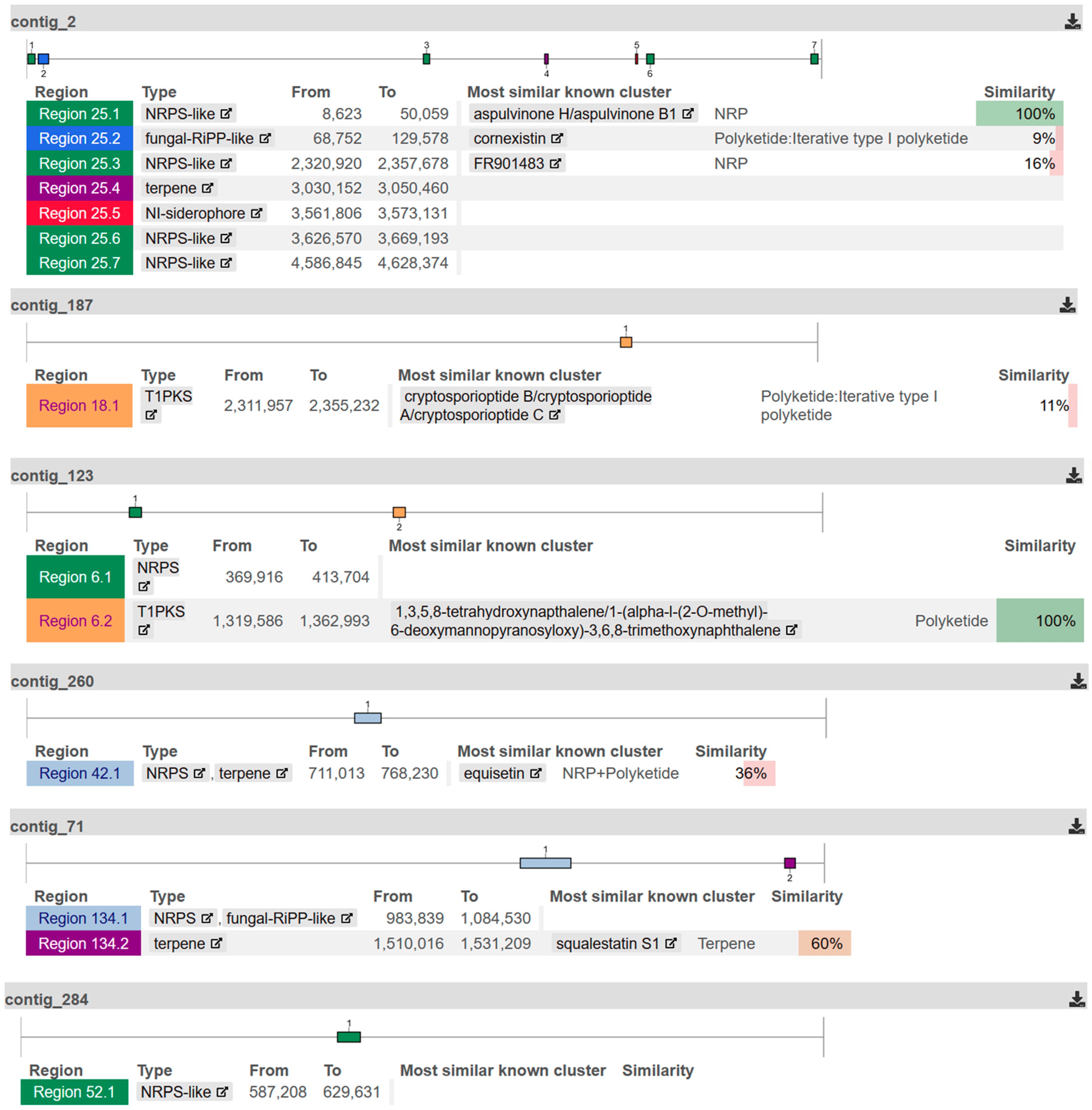
2.3. Genome Sequencing
2.4. Cytotoxicity and Cell Cycle Analyses in the Pancreatic Ductal Adenocarcinoma Cell Line PANC-1
3. Materials and Methods
3.1. General Compound Spectroscopic Analysis and Purification Procedures
3.2. Fungal Material
3.3. Fermentation, Extraction, and Isolation
3.4. Crystallographic Analysis of Compound 2
3.5. DNA Isolation and Genome Sequencing
3.6. Cell Culture and Cytotoxic Activity
3.7. Cell Cycle Analysis
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Słodkowski, M.; Wroński, M.; Karkocha, D.; Kraj, L.; Śmigielska, K.; Jachnis, A. Current Approaches for the Curative-Intent Surgical Treatment of Pancreatic Ductal Adenocarcinoma. Cancers 2023, 15, 2584. [Google Scholar] [CrossRef] [PubMed]
- Adamska, A.; Domenichini, A.; Falasca, M. Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies. Int. J. Mol. Sci. 2017, 18, 1338. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, N.; Zhang, Y.; Huang, X.; Wang, Y. The therapeutic potential of natural products for treating pancreatic cancer. Front. Pharmacol. 2022, 13, 1051952. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Liu, B.; Liu, Y.; Shi, X.; Li, W.; Xin, H.; Xin, J.; Hao, C. Anticancer effects of herbal medicines in pancreatic ductal adenocarcinoma through modulation of steroid hormone response proteins. Sci. Rep. 2022, 12, 9910. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Ha, J.; Kim, J.; Cho, Y.; Ahn, J.; Cheon, C.; Kim, S.-H.; Ko, S.-G.; Kim, B. Natural Products for Pancreatic Cancer Treatment: From Traditional Medicine to Modern Drug Discovery. Nutrients 2021, 13, 3801. [Google Scholar] [CrossRef]
- Xie, B.; Hänsel, J.; Mundorf, V.; Betz, J.; Reimche, I.; Erkan, M.; Büdeyri, I.; Gesell, A.; Kerr, R.G.; Ariantari, N.P.; et al. Pseudopterosin and O-Methyltylophorinidine Suppress Cell Growth in a 3D Spheroid Co-Culture Model of Pancreatic Ductal Adenocarcinoma. Bioengineering 2020, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, V.; Kindinger, F.; Li, S.M. Convenient synthetic approach for tri- and tetraprenylated cyclodipeptides by consecutive enzymatic prenylations. Appl. Microbiol. Biotechnol. 2018, 102, 2671–2681. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, V.; Kindinger, F.; Xie, X.; Wang, B.G.; Li, S.M. Two Prenyltransferases Govern a Consecutive Prenylation Cascade in the Biosynthesis of Echinulin and Neoechinulin. Org. Lett. 2017, 19, 5928–5931. [Google Scholar] [CrossRef]
- Ma, Y.-M.; Liang, X.-A.; Kong, Y.; Jia, B. Structural Diversity and Biological Activities of Indole Diketopiperazine Alkaloids from Fungi. J. Agric. Food Chem. 2016, 64, 6659–6671. [Google Scholar] [CrossRef]
- Xu, W.; Gavia, D.J.; Tang, Y. Biosynthesis of fungal indole alkaloids. Nat. Prod. Rep. 2014, 31, 1474–1487. [Google Scholar] [CrossRef]
- Giessen, T.W.; Marahiel, M.A. Rational and combinatorial tailoring of bioactive cyclic dipeptides. Front. Microbiol. 2015, 6, 785. [Google Scholar] [CrossRef] [PubMed]
- Winkelblech, J.; Fan, A.; Li, S.-M. Prenyltransferases as key enzymes in primary and secondary metabolism. Appl. Microbiol. Biotechnol. 2015, 99, 7379–7397. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-M. Applications of dimethylallyltryptophan synthases and other indole prenyltransferases for structural modification of natural products. Appl. Microbiol. Biotechnol. 2009, 84, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Bahukhandi, A.; Dhyani, P.; Sati, P.; Capanoglu, E.; Docea, A.O.; Al-Harrasi, A.; Dey, A.; Calina, D. Therapeutic Potential of Neoechinulins and Their Derivatives: An Overview of the Molecular Mechanisms Behind Pharmacological Activities. Front. Nutr. 2021, 8, 664197. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhu, T.; Li, D.; Gu, Q.; Liu, W. Prenylated indole diketopiperazine alkaloids from a mangrove rhizosphere soil derived fungus Aspergillus effuses H1-1. Arch. Pharmacal Res. 2013, 36, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Sun, S.; Peng, J.; Kong, X.; Zhou, H.; Zhu, T.; Gu, Q.; Li, D. Okaramines S–U, three new indole diketopiperazine alkaloids from Aspergillus taichungensis ZHN-7-07. Tetrahedron 2015, 71, 3715–3719. [Google Scholar] [CrossRef]
- Akashi, S.; Kimura, T.; Takeuchi, T.; Kuramochi, K.; Kobayashi, S.; Sugawara, F.; Watanabe, N.; Arai, T. Neoechinulin a impedes the progression of rotenone-induced cytotoxicity in PC12 cells. Biol. Pharm. Bull. 2011, 34, 243–248. [Google Scholar] [CrossRef]
- Wijesekara, I.; Li, Y.-X.; Vo, T.-S.; Van Ta, Q.; Ngo, D.-H.; Kim, S.-K. Induction of apoptosis in human cervical carcinoma HeLa cells by neoechinulin A from marine-derived fungus Microsporum sp. Process Biochem. 2013, 48, 68–72. [Google Scholar] [CrossRef]
- Terao, J.; Mukai, R. Prenylation modulates the bioavailability and bioaccumulation of dietary flavonoids. Arch. Biochem. Biophys. 2014, 559, 12–16. [Google Scholar] [CrossRef]
- Botta, B.; Vitali, A.; Menendez, P.; Misiti, D.; Delle Monache, G. Prenylated flavonoids: Pharmacology and biotechnology. Curr. Med. Chem. 2005, 12, 717–739. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, H.; Wu, W.; Li, H.; An, Z.; Zhou, F. C7-Prenylation of Tryptophan-Containing Cyclic Dipeptides by 7-Dimethylallyl Tryptophan Synthase Significantly Increases the Anticancer and Antimicrobial Activities. Molecules 2020, 25, 3676. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-M. Prenylated indole derivatives from fungi: Structure diversity, biological activities, biosynthesis and chemoenzymatic synthesis. Nat. Prod. Rep. 2010, 27, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Si, L.; Liu, D.; Proksch, P.; Zhang, L.; Zhou, D.; Lin, W. Neoechinulin B and its analogues as potential entry inhibitors of influenza viruses, targeting viral hemagglutinin. Eur. J. Med. Chem. 2015, 93, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Alhadrami, H.A.; Burgio, G.; Thissera, B.; Orfali, R.; Jiffri, S.E.; Yaseen, M.; Sayed, A.M.; Rateb, M.E. Neoechinulin A as a Promising SARS-CoV-2 Mpro Inhibitor: In Vitro and In Silico Study Showing the Ability of Simulations in Discerning Active from Inactive Enzyme Inhibitors. Mar. Drugs 2022, 20, 163. [Google Scholar] [CrossRef] [PubMed]
- Dossena, A.; Marchelli, R.; Pochini, A. Neoechinulin D, a new isoprenylated dehydrotryptophyl metabolite from Aspergillus amstelodami. Experientia 1975, 31, 1249. [Google Scholar] [CrossRef]
- Wang, W.-L.; Lu, Z.-Y.; Tao, H.-W.; Zhu, T.-J.; Fang, Y.-C.; Gu, Q.-Q.; Zhu, W.-M. Isoechinulin-type Alkaloids, Variecolorins A–L, from Halotolerant Aspergillus variecolor. J. Nat. Prod. 2007, 70, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-L.; Li, X.-M.; Li, T.-G.; Dang, H.-Y.; Wang, B.-G. Dioxopiperazine Alkaloids Produced by the Marine Mangrove Derived Endophytic Fungus Eurotium rubrum. Helv. Chim. Acta 2008, 91, 1888–1893. [Google Scholar] [CrossRef]
- Marchelli, R.; Dossena, A.; Pochini, A.; Dradi, E. The structures of five new didehydropeptides related to neoechinulin, isolated from Aspergillus amstelodami. J. Chem. Soc. Perkin Trans. 1977, 1, 713–717. [Google Scholar] [CrossRef]
- Meng, L.-H.; Du, F.-Y.; Li, X.-M.; Pedpradab, P.; Xu, G.-M.; Wang, B.-G. Rubrumazines A–C, Indolediketopiperazines of the Isoechinulin Class from Eurotium rubrum MA-150, a Fungus Obtained from Marine Mangrove-Derived Rhizospheric Soil. J. Nat. Prod. 2015, 78, 909–913. [Google Scholar] [CrossRef]
- Genome Assembly AchevalieriM1_assembly01. Available online: www.ncbi.nlm.nih.gov/datasets/genome/GCF_016861735.1 (accessed on 28 November 2023).
- Li, W.; Xie, X.; Liu, J.; Yu, H.; Li, S.M. Prenylation of dimeric cyclo-L-Trp-L-Trp by the promiscuous cyclo-L-Trp-L-Ala prenyltransferase EchPT1. Appl. Microbiol. Biotechnol. 2023, 107, 6887–6895. [Google Scholar] [CrossRef]
- Yu, X.; Li, S.-M. Chapter Thirteen—Prenyltransferases of the Dimethylallyltryptophan Synthase Superfamily. In Methods in Enzymology; Hopwood, D.A., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 516, pp. 259–278. [Google Scholar]
- Brumskill, S.; Barrera, L.N.; Calcraft, P.; Phillips, C.; Costello, E. Inclusion of cancer-associated fibroblasts in drug screening assays to evaluate pancreatic cancer resistance to therapeutic drugs. J. Physiol. Biochem. 2023, 79, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Kjer, J.; Debbab, A.; Aly, A.H.; Proksch, P. Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Nat. Protoc. 2010, 5, 479–490. [Google Scholar] [CrossRef] [PubMed]
- CrysAlisPRO, v171.42; Oxford Diffraction/Agilent Technologies UK Ltd.: Yarnton, UK, 2022.
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Nagamachi, T.; Yoshikawa, A.; Yamada, T.; Sugita, T. A joint PCR-based gene-targeting method using electroporation in the pathogenic fungus Trichosporon asahii. AMB Express 2022, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Kolmogorov, M.; Bickhart, D.M.; Behsaz, B.; Gurevich, A.; Rayko, M.; Shin, S.B.; Kuhn, K.; Yuan, J.; Polevikov, E.; Smith, T.P.L.; et al. metaFlye: Scalable long-read metagenome assembly using repeat graphs. Nat. Methods 2020, 17, 1103–1110. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; van Wezel, G.P.; Medema, M.H.; Weber, T. antiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef]
- Paysan-Lafosse, T.; Blum, M.; Chuguransky, S.; Grego, T.; Pinto, B.L.; Salazar, G.A.; Bileschi, M.L.; Bork, P.; Bridge, A.; Colwell, L.; et al. InterPro in 2022. Nucleic Acids Res. 2023, 51, D418–D427. [Google Scholar] [CrossRef]



| No. | δC, Type | δH (Mult, J in Hz) | HMBC (from H to C) |
|---|---|---|---|
| 2 | 144.7, C | ||
| 3 | 102.8, C | ||
| 3a | 126.6, C | ||
| 4 | 116.6, CH | 6.93, d (1.5) | 3, 6, 7a, 21 |
| 5 | 134.5, C | ||
| 6 | 123.8, CH | 6.74, d (1.5) | 4, 7a, 26 |
| 7 | 123.5, C | ||
| 7a | 132.9, C | ||
| 8 | 113.2, CH | 7.23, s | 2, 3a, 10 |
| 9 | 124.0, C | ||
| 10 | 160.4, C | ||
| 12 | 51.8, CH | 4.27, q (6.9) | 10, 13, 20 |
| 13 | 165.5, C | ||
| 15 | 39.4, C | ||
| 16 | 144.6, CH | 6.05, dd (17.4, 10.5) | 2, 15, 18, 19 |
| 17 | 112.7, CH2 | 5.14, d (10.5) | 15, 16 |
| 5.12, d (17.4) | |||
| 18 | 27.6, CH3 | 1.51, s | 2, 15, 16, 19 |
| 19 | 27.5, CH3 | 1.50, s | 2, 15, 16, 18 |
| 20 | 21.1, CH3 | 1.60, d (6.9) | 12, 13 |
| 21 | 36.3, CH2 | 2.85, d (15.0) | 7, 7a, 28 |
| 2.94, dd (15.0, 8.7) | |||
| 22 | 78.2, CH | 3.80, d (8.7) | 7, 26, 28, 29, 30 |
| 23 | 77.8, C | ||
| 24 | 20.5, CH3 | 1.28, s | 27, 28, 30 |
| 25 | 18.5, CH3 | 1.23, s | 27, 28, 29 |
| 26 | 34.6, CH2 | 3.38, m | 4, 5, 6, 22, 23 |
| 27 | 124.3, CH | 5.35, m | 24, 25 |
| 28 | 132.0, C | ||
| 29 | 25.9, CH3 | 1.72, s | 22, 23, 25 |
| 30 | 18.0, CH3 | 1.71, s | 22, 23, 24 |
| 1-NH | 10.21, s | 2, 3, 3a, 7a | |
| 11-NH | 6.34, br s | 10, 13 | |
| 14-NH | 7.47, br s | 10, 12, 13 | |
| 23-OCH3 | 49.4, OCH3 | 3.28, s | 28 |
| Contig No. | Length (bp) | Coverage (x-Fold) | Secondary Metabolite Regions |
|---|---|---|---|
| 2 | 4,649,651 | 4036 | NRPS-like; fungal-RiPP-like; terpene; NI-siderophore |
| 187 | 3,079,001 | 4045 | T1PKS |
| 123 | 2,864,395 | 4042 | NRPS; T1PKS |
| 260 | 1,732,854 | 4039 | NRPS |
| 71 | 1,589,188 | 4047 | NRPS; fungal-RiPP-like; terpene |
| 284 | 1,488,407 | 4039 | NRPS-like |
| 328 | 1,357,148 | 4052 | no secondary metabolite region found |
| 315 | 1,177,976 | 4057 | no secondary metabolite region found |
| 149 | 21,917 | 3994 | Terpenoid cyclases/Protein prenyltransferases |
| Compound | IC50 (±SD) [µM] |
|---|---|
| PANC-1 | |
| 1 | 25.8 ± 1.3 |
| 4 | 23.4 ± 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Kashef, D.H.; Obidake, D.D.; Schiedlauske, K.; Deipenbrock, A.; Scharf, S.; Wang, H.; Naumann, D.; Friedrich, D.; Miljanovic, S.; Haj Hassani Sohi, T.; et al. Indole Diketopiperazine Alkaloids from the Marine Sediment-Derived Fungus Aspergillus chevalieri against Pancreatic Ductal Adenocarcinoma. Mar. Drugs 2024, 22, 5. https://doi.org/10.3390/md22010005
El-Kashef DH, Obidake DD, Schiedlauske K, Deipenbrock A, Scharf S, Wang H, Naumann D, Friedrich D, Miljanovic S, Haj Hassani Sohi T, et al. Indole Diketopiperazine Alkaloids from the Marine Sediment-Derived Fungus Aspergillus chevalieri against Pancreatic Ductal Adenocarcinoma. Marine Drugs. 2024; 22(1):5. https://doi.org/10.3390/md22010005
Chicago/Turabian StyleEl-Kashef, Dina H., Deborah D. Obidake, Katja Schiedlauske, Alina Deipenbrock, Sebastian Scharf, Hao Wang, Daniela Naumann, Daniel Friedrich, Simone Miljanovic, Takin Haj Hassani Sohi, and et al. 2024. "Indole Diketopiperazine Alkaloids from the Marine Sediment-Derived Fungus Aspergillus chevalieri against Pancreatic Ductal Adenocarcinoma" Marine Drugs 22, no. 1: 5. https://doi.org/10.3390/md22010005
APA StyleEl-Kashef, D. H., Obidake, D. D., Schiedlauske, K., Deipenbrock, A., Scharf, S., Wang, H., Naumann, D., Friedrich, D., Miljanovic, S., Haj Hassani Sohi, T., Janiak, C., Pfeffer, K., & Teusch, N. (2024). Indole Diketopiperazine Alkaloids from the Marine Sediment-Derived Fungus Aspergillus chevalieri against Pancreatic Ductal Adenocarcinoma. Marine Drugs, 22(1), 5. https://doi.org/10.3390/md22010005









