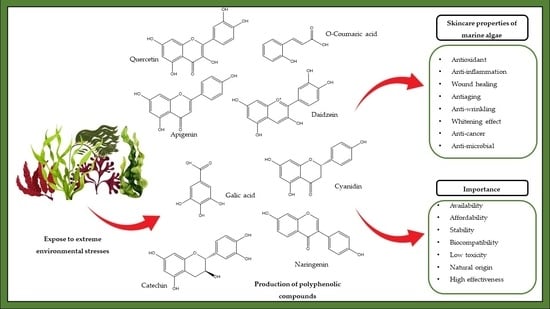
Marine Algal Polyphenols as Skin Protective Agents: Current Status and Future Prospectives
Abstract
1. Introduction
1.1. Cosmetic Industrial Background
1.2. Introduction to Marine Algal Polyphenols
| Constituents | Chlorophyta | Rhodophyta | Phaeophyceae | References |
|---|---|---|---|---|
| Polysaccharide | Ulvans | Carrageenans | Alginate and Fucan | [37,38] |
| Lipid and fatty acids | Hexadecatetraenoic, oleic, Palmitic acids, and PUFAs (linoleic acid and α-linolenic acid) | Eicosapentaenoic acid (EPA) and Arachidonic acid (AA) | EPA and AA | [38,39] |
| Sterol | Ucocholesterol, Cholesterol, and ß-sitosterol | Desmosterol, Cholesterol, Sitosterol, Fucosterol, and Chalinasterol | Fucosterol, Cholesterol, and Brassicasterol | [40,41] |
| Pigment | Chlorophyll | Phycobilin | Fucoxanthin | [12,13] |
| Phenolic compounds | phlorotannins (phloroglucinol, eckol, 7-phloroeckol, 6,6-bieckol, phlorofucofuroeckol A, fucodiphloroethol) | [42,43] | ||
| hydroxybenzoic acid derivatives (gallic, p-hydroxybenzoic, vanillic, and syringic acids), hydroxycinnamic acids (caffeic, ferulic, sinapic, and p-coumaric acids), flavonoids (epicatechin, epigallocatechin, rutin, quercitrin, hesperidin, myricetin, and kaempferol), and bromophenols | ||||
| Seaweeds | Activity Potential | Active Component | References |
|---|---|---|---|
| Brown algae | |||
| Gongolaria nodicaulis (formerly Cystoseira nodicaulis), Ericaria selaginoides (formerly Cystoseira tamariscifolia), Gongolaria usneoides (formerly Cystoseira usneoides), Fucus spiralis | Anti-aging, antioxidant, anti-wrinkling (inhibition of hyaluronidase), anti-inflammation | Plorotannins-Fucophloroethol, fucodiphloroethol, fucotriphloroethol, 7-phloroeckol, phlorofucofuroeckol and bieckol/diecko | [44] |
| Ecklonia cava | Anti-allergic | Plorotannins-dieckol and 6,6-bieckol | [45] |
| Ecklonia cava | Inhibition of tyrosinase and melanin synthesis | Phlorotannins (ethanolic extract and ethyl acetate soluble fraction) | [46] |
| Ecklonia cava | Inhibition of tyrosinase and melanin synthesis | Phlorotaninns-phloroglucinol, eckol, and dieckol | [47] |
| Ecklonia cava | Anti-inflammation, inhibited MMP expression | Phloroglucinol derivatives | [48] |
| Ecklonia cava | Antioxidant, anti-photoaging | 100% methanol extract–phlorotannins (dieckol) | [49] |
| Ecklonia cava | Antioxidant, anti-inflammation, anti-photoaging | Dieckol | [50] |
| Eisenia arborea Sargassum thunbergii | Anti-allergic | Polyphenolic compounds (Methanol extract) | [51] |
| Eisenia arborea | Anti-allergic | phlorofucofuroeckol-B | [52] |
| Eisenia bicyclis Ecklonia cava Ecklonia cava subsp. stolonifera (formerly Ecklonia stolonifera) | Anti-photoaging | Phlorotannins- eckol and dieckol | [53] |
| Eisenia bicyclis Ecklonia cava subsp. kurome (formerly Ecklonia kurome) | Anti-wrinkling (inhibition of hyaluronidase) | Phlorotannins- phloroglucinol, eckol, phlorofucofuroeckol A, dieckol, and 8,8- bieckol | [54] |
| Ecklonia cava subsp. stolonifera (formerly Ecklonia stolonifera) | Inhibition of tyrosinase | phloroglucinol, eckstolonol, eckol, phlorofucofuroeckol A, and dieckol | [55] |
| Ishige okamurae | Inhibition of tyrosinase, antioxidant, whitening | Phenolic compounds (methanol extract) | [56] |
| Sargassum longifolium | Antioxidant, anti-bacterial | Phenolic compounds (ethanol extract) | [57] |
| Eisenia bicyclis Ecklonia cava Ecklonia cava subsp. kurome (formerly Ecklonia kurome) | Antioxidant | Phloroglucinol, Eckol, Phlorofucofuroeckol A, Dieckol and 8,8′-bieckol | [58] |
| Sargassum carpophyllum | Anti-allergic | Phlorotannins –
| [59] |
| Colpmenia sinuosa | Anti-inflammation, antimicrobial | Ethanolic and dichloromethane extract respectively | [60] |
| Green algae | |||
| Dunaliella tertiolecta (green microalga) Tetraselmis (green microalga) Nannochloropsis sp. (Eustigmatophyceae) | Anti-aging | Phenolic compounds (Ethanolic extract) | [61] |
| Turbinaria conoides | Antioxidant | Phenolic compounds (ethanolic extract and ethyl acetate) | [62] |
| Ulva lactuca | Antioxidant | 80% methanolic extract | [63] |
| Ulva linza | Anti-inflammation, antimicrobial | Ethanolic extract | [60] |
| Caulerpa racemosa | Antioxidant, anticancer | clionasterol-rich hexane fraction | [64] |
| Ulva compressa (formerly Enteromorpha compressa) Capsosiphon fulvescens Chaetomorpha moniligera Ulva australis (formerly Ulva pertusa) | Antioxidant | Ethanolic extract | [65] |
| Red algae | |||
| Acanthophora spicifera | Antioxidant | Flavonoids-acanthophorin A, acanthophorin B, tilimside, (-)-catechin, quercetin | [66] |
| Vertebrata fucoides (formerly Polysiphonia fucoides) | Antioxidant | Phenolic compounds (ethanolic extract) | [67] |
| Corallina pilulifera | Antioxidant, anti-photoaging | Phenolic compounds (methanol extract) | [68] |
| Gracilaria foliifera | Antioxidant, anti-bacterial | Phenolic compounds (ethanol extract) | [57] |
| Kappaphycopsis cottonii (formerly Eucheuma cottonii) | Antioxidant | Phenolic compounds (ethanolic extract and ethyl acetate) | [62] |
| Hypnea musciformis Hypnea valentiae Jania rubens | Antioxidant | Methanol extract and its solvent fractions (n-hexane, dichloromethane, and ethyl acetate) | [69] |
1.3. Classification of Marine Algal Polyphenols
| Isolated Compound | Seaweed | Reference | |
|---|---|---|---|
| Flavonols | Quercetin | Acanthophora spicifera Caulerpa racemosa, Caulerpa racemosa, Caulerpa racemosa, Caulerpa scalpelliformis, Codium dwarkense, Ulva fasciata, and Ulva lactuca (formerly Ulva fasciat) (Chlorophyta) | [66,79] |
| Flavones | Apigenin | Acanthophora spicifera Caulerpa racemosa, Caulerpa racemosa, Caulerpa racemosa, Caulerpa scalpelliformis, Codium dwarkense, Ulva fasciata, and Ulva lactuca | [79,80] |
| Isoflavones | Daidzein | Sargassum muticum, Sargassum vulgare (Phaeophyceae), Hypnea spinella, Porphyra sp. (Rhodophyta), Undaria pinnatifida (Phaeophyceae), Chondrus crispus and Halopytis incurvus (Rhodophyta) | [81] |
| Flavanones | Naringenin | Undaria pinnatifida | [82] |
| Anthocyanidins | Cyanidin | Caulerpa racemosa, Caulerpa racemosa, Caulerpa racemosa, Caulerpa scalpelliformis, Codium dwarkense, Ulva fasciata, and Ulva lactuca | [79] |
| Flavanols | Catechin | Acanthophora spicifera | [66] |
| Hydrobenzoic acid | Gallic acid | Laminaria digitata, Dictyota dichotoma, Fucus vesiculosus, Fucus serratus, Fucus distichus, Fucus spiralis, Mastocarpus stellatus, Vertebrata fucoides (formerly Polysiphonia fucoides) (Rhodophyta), Saccharina latissima, Gracilaria vermiculophyllum (Rhodophyta), Palmaria palmata, Porphyra purpurea, Chondrus crispus, Ulva intestinalis (formerly Enteromorpha intestinalis) (Chlorophyta), Ulva lactuca, Sargassum muticum | [67] |
| Hydrocinnamic acid | Coumaric acid | Kelp Jania rubens | [83,84] |
| Tannins | Eckol | Eisenia bicyclis, Ecklonia cava subsp. kurome (formerly Ecklonia kurome) (Phaeophyceae) Eisenia bicyclis, Ecklonia cava, Ecklonia cava subsp. Kurome (formerly Ecklonia kurome) (Phaeophyceae) | [54] |
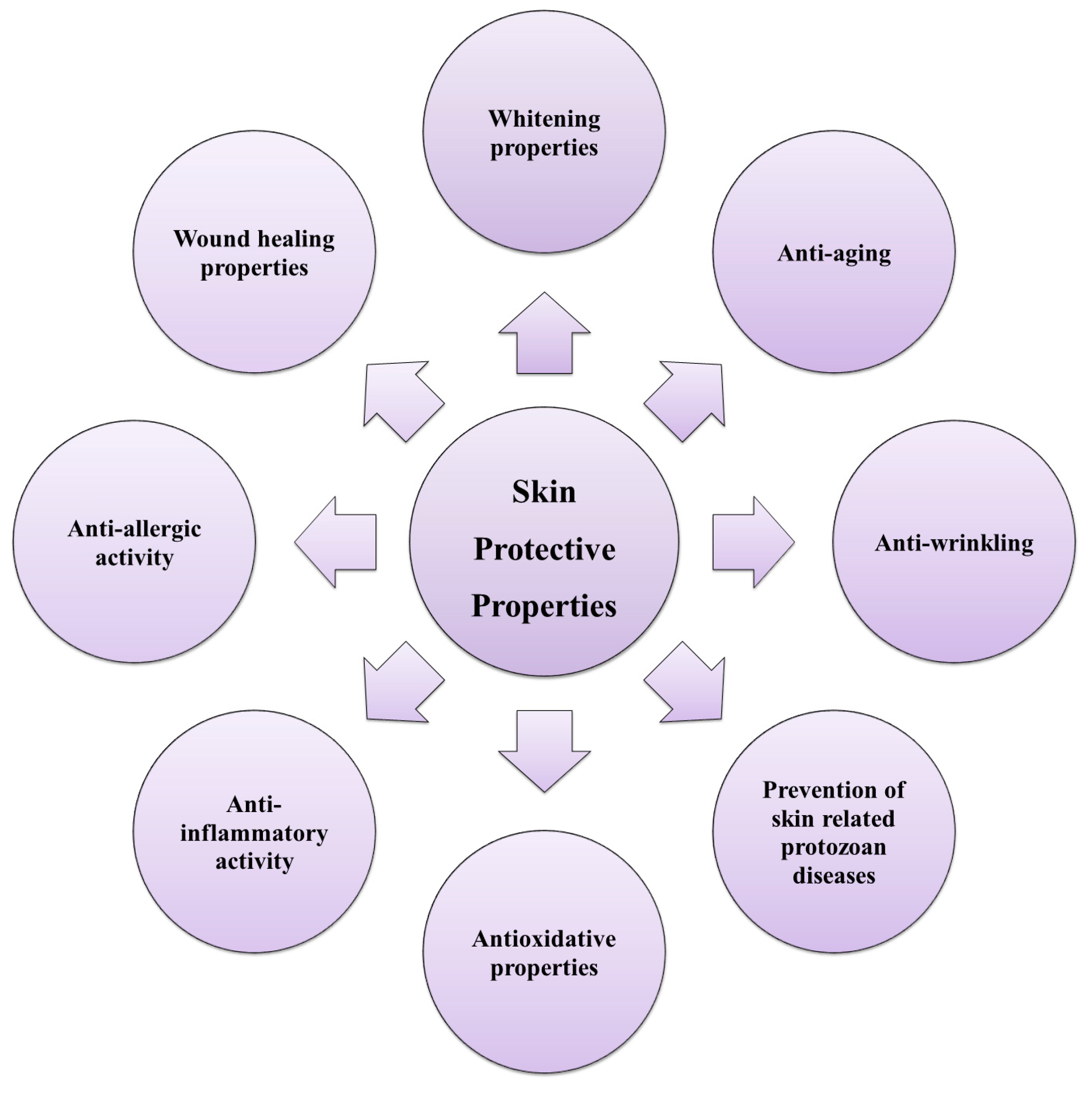
1.4. Skin Protective Properties
2. Marine Algal Polyphenols as Skin Protective Agents
2.1. Antioxidative Activity (ROS Scavenging Activity)
2.2. Anti-Inflammatory and Anti-Allergic Activity
2.3. Wound Healing of the Skin
2.4. Anti-Aging Activity and Anti-Wrinkling Activity
2.5. Whitening Properties
2.6. Anticancer Activity
2.7. Anti-Microbial and Antiprotozoal Activity
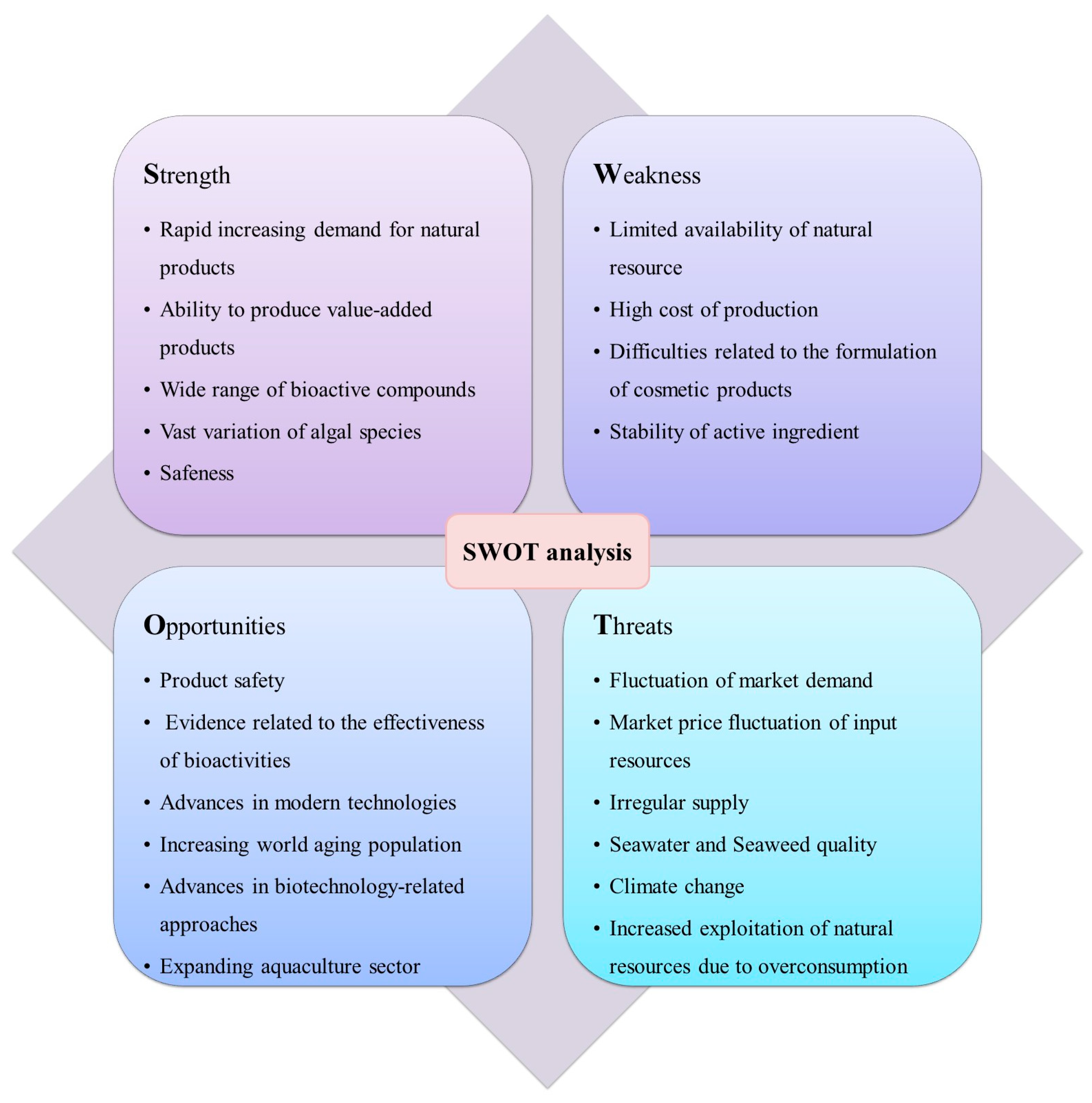
3. Conclusions and Future Prospectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, S. Exploratory analysis of global cosmetic industry: Major players, technology and market trends. Technovation 2005, 25, 1263–1272. [Google Scholar] [CrossRef]
- Dureja, H.; Kaushik, D.; A Gupta, M.; Kumar, V.; Lather, V. Cosmeceuticals: An emerging concept. Indian J. Pharmacol. 2005, 37, 155. [Google Scholar] [CrossRef]
- Alam, M.A.; Xu, J.-L.; Wang, Z. Microalgae Biotechnology for Food, Health and High Value Products; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Kalasariya, H.S.; Pereira, L. Dermo-Cosmetic Benefits of Marine Macroalgae-Derived Phenolic Compounds. Appl. Sci. 2022, 12, 11954. [Google Scholar] [CrossRef]
- Balboa, E.M.; Conde, E.; Soto, M.L.; Pérez-Armada, L.; Domínguez, H. Cosmetics from marine sources. In Springer Handbook of Marine Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1015–1042. [Google Scholar]
- Guillerme, J.-B.; Couteau, C.; Coiffard, L. Applications for Marine Resources in Cosmetics. Cosmetics 2017, 4, 35. [Google Scholar] [CrossRef]
- Kim, S.-K.; Ravichandran, Y.D.; Khan, S.B.; Kim, Y.T. Prospective of the cosmeceuticals derived from marine organisms. Biotechnol. Bioprocess Eng. 2008, 13, 511–523. [Google Scholar] [CrossRef]
- Bhadury, P.; Wright, P.C. Exploitation of marine algae: Biogenic compounds for potential antifouling applications. Planta 2004, 219, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Christie, H.; Norderhaug, K.M.; Fredriksen, S. Macrophytes as habitat for fauna. Mar. Ecol. Prog. Ser. 2009, 396, 221–233. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.L.; Pinto, D.C.G.A.; Michalak, I.; Trincone, A.; Mishra, A.P.; Nigam, M.; Zam, W.; Martins, N. Current Trends on Seaweeds: Looking at Chemical Composition, Phytopharmacology, and Cosmetic Applications. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef] [PubMed]
- Anantharaman, P. Manual on identification of seaweed. All India coordinate project on survey and Inventorization of coastal and marine biodiversity. J. Mar. Biol. Assoc. India 2002, 29, 1–9. [Google Scholar]
- Dawczynski, C.; Schäfer, U.; Leiterer, M.; Jahreis, G. Nutritional and Toxicological Importance of Macro, Trace, and Ultra-Trace Elements in Algae Food Products. J. Agric. Food Chem. 2007, 55, 10470–10475. [Google Scholar] [CrossRef]
- Gómez-Guzmán, M.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J. Potential Role of Seaweed Polyphenols in Cardiovascular-Associated Disorders. Mar. Drugs 2018, 16, 250. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L. Seaweeds as source of bioactive substances and skin care therapy—Cosmeceuticals, algotheraphy, and thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef]
- Dillehay, T.D.; Ramírez, C.; Pino, M.; Collins, M.B.; Rossen, J.; Pino-Navarro, J.D. Monte Verde: Seaweed, food, medicine, and the peopling of South America. Science 2008, 320, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.A.J.P.; Jeon, Y.-J. Biological activities and potential cosmeceutical applications of bioactive components from brown seaweeds: A review. Phytochem. Rev. 2011, 10, 431–443. [Google Scholar] [CrossRef]
- Roohinejad, S.; Koubaa, M.; Barba, F.J.; Saljoughian, S.; Amid, M.; Greiner, R. Application of seaweeds to develop new food products with enhanced shelf-life, quality and health-related beneficial properties. Food Res. Int. 2017, 99, 1066–1083. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Kamthania, M.C.; Kumar, A. Bioactive Compounds and Properties of Seaweeds—A Review. Oalib 2014, 1, e752. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algal extracts: Technology and advances. Eng. Life Sci. 2014, 14, 581–591. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Herrero, M. Bioactive Compounds from Marine Foods: Plant and Animal Sources; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Wijesekara, I.; Senevirathne, M.; Li, Y.; Kim, S. Functional Ingredients from Marine Algae as Potential Antioxidants in the Food Industry. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; Wiley: New York, NY, USA, 2012; pp. 398–402. [Google Scholar] [CrossRef]
- Vo, T.-S.; Ngo, D.-H.; Kim, S.-K. Potential targets for anti-inflammatory and anti-allergic activities of marine algae: An overview. Inflamm. Allergy-Drug Targets (Former. Curr. Drug Targets-Inflamm. Allergy) (Discontin.) 2012, 11, 90–101. [Google Scholar] [CrossRef]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.-E.F.; Moustafa, M.S.; El-Wahed, A.A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef]
- Lee, S.-H.; Jeon, Y.-J. Anti-diabetic effects of brown algae derived phlorotannins, marine polyphenols through diverse mechanisms. Fitoterapia 2013, 86, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Ianora, A. Marine Organisms with Anti-Diabetes Properties. Mar. Drugs 2016, 14, 220. [Google Scholar] [CrossRef]
- Gunathilaka, T.L.; Samarakoon, K.; Ranasinghe, P.; Peiris, L.D.C. Antidiabetic Potential of Marine Brown Algae—A Mini Review. J. Diabetes Res. 2020, 2020, 1230218. [Google Scholar] [CrossRef] [PubMed]
- Nagahawatta, D.; Liyanage, N.; Je, J.-G.; Jayawardhana, H.; Jayawardena, T.U.; Jeong, S.-H.; Kwon, H.-J.; Choi, C.S.; Jeon, Y.-J. Polyphenolic Compounds Isolated from Marine Algae Attenuate the Replication of SARS-CoV-2 in the Host Cell through a Multi-Target Approach of 3CLpro and PLpro. Mar. Drugs 2022, 20, 786. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.-S.; Ngo, D.-H.; Kim, S.-K. Marine algae as a potential pharmaceutical source for anti-allergic therapeutics. Process Biochem. 2012, 47, 386–394. [Google Scholar] [CrossRef]
- Ding, Y.; Kim, S.H.; Lee, J.J.; Hong, J.T.; Kim, E.-A.; Kang, D.-H.; Heo, S.-J.; Lee, S.-H. Anti-melanogenesis activity of Ecklonia cava extract cultured in tanks with magma seawater of Jeju Island. Algae 2019, 34, 177–185. [Google Scholar] [CrossRef]
- Manandhar, B.; Wagle, A.; Seong, S.H.; Paudel, P.; Kim, H.-R.; Jung, H.A.; Choi, J.S. Phlorotannins with Potential Anti-Tyrosinase and Antioxidant Activity Isolated from the Marine Seaweed Ecklonia stolonifera. Antioxidants 2019, 8, 240. [Google Scholar] [CrossRef]
- Namjooyan, F.; Farasat, M.; Alishahi, M.; Jahangiri, A.; Mousavi, H. The Anti-melanogenesis Activities of Some Selected Brown Macroalgae from Northern Coasts of the Persian Gulf. Braz. Arch. Biol. Technol. 2019, 62, e19180198. [Google Scholar] [CrossRef]
- Riani, M.K.L.; Anwar, E.; Nurhayati, T. Antioxidant and Anti-Collagenase Activity of Sargassum Plagyophyllum Extract as an Anti-Wrinkle Cosmetic Ingredient. Pharmacogn. J. 2018, 10, 932–936. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Kim, E.-A.; Son, K.-T.; Jeon, Y.-J. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: A review. J. Photochem. Photobiol. B Biol. 2016, 162, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.M.; Patel, I.C. A review on phytoconstituents of marine brown algae. Future J. Pharm. Sci. 2020, 6, 129. [Google Scholar] [CrossRef]
- Biris-Dorhoi, E.-S.; Michiu, D.; Pop, C.R.; Rotar, A.M.; Tofana, M.; Pop, O.L.; Socaci, S.A.; Farcas, A.C. Macroalgae—A Sustainable Source of Chemical Compounds with Biological Activities. Nutrients 2020, 12, 3085. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Troy, D.J. Chapter 1-Seaweed sustainability–food and nonfood applications. In Seaweed Sustainability; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 1–6. [Google Scholar]
- Sánchez-Machado, D.; López-Hernández, J.; Paseiro-Losada, P.; López-Cervantes, J. An HPLC method for the quantification of sterols in edible seaweeds. Biomed. Chromatogr. 2004, 18, 183–190. [Google Scholar] [CrossRef]
- Whittaker, M.H.; Frankos, V.H.; Wolterbeek, A.; Waalkens-Berendsen, D. Effects of dietary phytosterols on cholesterol metabolism and atherosclerosis: Clinical and experimental evidence. Am. J. Med. 2000, 109, 600–601. [Google Scholar] [CrossRef]
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweed’s Bioactive Candidate Compounds to Food Industry and Global Food Security. Life 2020, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.A.O.; Félix, R.; Pais, A.C.S.; Rocha, S.M.; Silvestre, A.J.D. The Quest for Phenolic Compounds from Macroalgae: A Review of Extraction and Identification Methodologies. Biomolecules 2019, 9, 847. [Google Scholar] [CrossRef]
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin extracts from fucales characterized by HPLC-DAD-ESI-MS n: Approaches to hyaluronidase inhibitory capacity and antioxidant properties. Mar. Drugs 2012, 10, 2766–2781. [Google Scholar] [CrossRef]
- Le, Q.-T.; Li, Y.; Qian, Z.-J.; Kim, M.-M.; Kim, S.-K. Inhibitory effects of polyphenols isolated from marine alga Ecklonia cava on histamine release. Process Biochem. 2009, 44, 168–176. [Google Scholar] [CrossRef]
- Yoon, N.Y.; Eom, T.-K.; Kim, M.-M.; Kim, S.-K. Inhibitory Effect of Phlorotannins Isolated from Ecklonia cava on Mushroom Tyrosinase Activity and Melanin Formation in Mouse B16F10 Melanoma Cells. J. Agric. Food Chem. 2009, 57, 4124–4129. [Google Scholar] [CrossRef]
- Heo, S.-J.; Ko, S.-C.; Cha, S.-H.; Kang, D.-H.; Park, H.-S.; Choi, Y.-U.; Kim, D.; Jung, W.-K.; Jeon, Y.-J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. Vitr. 2009, 23, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.-S.; Kim, J.-A.; Ahn, B.-N.; Kim, S.-K. Potential effect of phloroglucinol derivatives from Ecklonia cava on matrix metalloproteinase expression and the inflammatory profile in lipopolysaccharide-stimulated human THP-1 macrophages. Fish. Sci. 2011, 77, 867–873. [Google Scholar] [CrossRef]
- Ko, S.-C.; Cha, S.-H.; Heo, S.-J.; Lee, S.-H.; Kang, S.-M.; Jeon, Y.-J. Protective effect of Ecklonia cava on UVB-induced oxidative stress: In vitro and in vivo zebrafish model. J. Appl. Phycol. 2011, 23, 697–708. [Google Scholar] [CrossRef]
- Wang, L.; Je, J.-G.; Yang, H.-W.; Jeon, Y.-J.; Lee, S. Dieckol, an Algae-Derived Phenolic Compound, Suppresses UVB-Induced Skin Damage in Human Dermal Fibroblasts and Its Underlying Mechanisms. Antioxidants 2021, 10, 352. [Google Scholar] [CrossRef]
- Sugiura, Y.; Takeuchi, Y.; Kakinuma, M.; Amano, H. Inhibitory effects of seaweeds on histamine release from rat basophile leukemia cells (RBL-2H3). Fish. Sci. 2006, 72, 1286–1291. [Google Scholar] [CrossRef]
- Sugiura, Y.; Matsuda, K.; Yamada, Y.; Nishikawa, M.; Shioya, K.; Katsuzaki, H.; Imai, K.; Amano, H. Isolation of a New Anti-Allergic Phlorotannin, Phlorofucofuroeckol-B, from an Edible Brown Alga, Eisenia arborea. Biosci. Biotechnol. Biochem. 2006, 70, 2807–2811. [Google Scholar] [CrossRef]
- Joe, M.-J.; Kim, S.-N.; Choi, H.-Y.; Shin, W.-S.; Park, G.-M.; Kang, D.-W.; Kim, Y.K. The Inhibitory Effects of Eckol and Dieckol from Ecklonia stolonifera on the Expression of Matrix Metalloproteinase-1 in Human Dermal Fibroblasts. Biol. Pharm. Bull. 2006, 29, 1735–1739. [Google Scholar] [CrossRef]
- Shibata, T.; Fujimoto, K.; Nagayama, K.; Yamaguchi, K.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against hyaluronidase. Int. J. Food Sci. Technol. 2002, 37, 703–709. [Google Scholar] [CrossRef]
- Kang, H.S.; Kim, H.R.; Byun, D.S.; Son, B.W.; Nam, T.J.; Choi, J.S. Tyrosinase inhibitors isolated from the edible brown algaEcklonia stolonifera. Arch. Pharmacal Res. 2004, 27, 1226–1232. [Google Scholar] [CrossRef]
- Heo, S.-J.; Ko, S.-C.; Kang, S.-M.; Cha, S.-H.; Lee, S.-H.; Kang, D.-H.; Jung, W.-K.; Affan, A.; Oh, C.; Jeon, Y.-J. Inhibitory effect of diphlorethohydroxycarmalol on melanogenesis and its protective effect against UV-B radiation-induced cell damage. Food Chem. Toxicol. 2010, 48, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Thanigaivel, S.; Hindu, S.V.; Vijayakumar, S.; Mukherjee, A.; Chandrasekaran, N.; Thomas, J. Differential solvent extraction of two seaweeds and their efficacy in controlling Aeromonas salmonicida infection in Oreochromis mossambicus: A novel therapeutic approach. Aquaculture 2015, 443, 56–64. [Google Scholar] [CrossRef]
- Shibata, T.; Ishimaru, K.; Kawaguchi, S.; Yoshikawa, H.; Hama, Y. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. In Nineteenth International Seaweed Symposium; Springer: Berlin/Heidelberg, Germany, 2007; pp. 255–261. [Google Scholar] [CrossRef]
- Matsui, T.; Ito, C.; Itoigawa, M.; Shibata, T. Three phlorotannins from Sargassum carpophyllum are effective against the secretion of allergic mediators from antigen-stimulated rat basophilic leukemia cells. Food Chem. 2022, 377, 131992. [Google Scholar] [CrossRef]
- Shobier, A.H.; Ismail, M.M.; Hassan, S.W.M. Variation in Anti-inflammatory, Anti-arthritic, and Antimicrobial Activities of Different Extracts of Common Egyptian Seaweeds with an Emphasis on Their Phytochemical and Heavy Metal Contents. Biol. Trace Element Res. 2022, 201, 2071–2087. [Google Scholar] [CrossRef] [PubMed]
- Norzagaray-Valenzuela, C.D.; Valdez-Ortiz, A.; Shelton, L.M.; Jiménez-Edeza, M.; Rivera-López, J.; Valdez-Flores, M.A.; Germán-Báez, L.J. Residual biomasses and protein hydrolysates of three green microalgae species exhibit antioxidant and anti-aging activity. J. Appl. Phycol. 2017, 29, 189–198. [Google Scholar] [CrossRef]
- Yanuarti, R.; Nurjanah, N.; Anwar, E.; Hidayat, T. Profile of Phenolic and Antioxidants Activity from Seaweed Extract Turbinaria conoides and Eucheuma cottonii. J. Pengolah. Has. Perikan. Indones. 2017, 20, 230–237. [Google Scholar] [CrossRef]
- Abkener, A.M. Seasonal Variation of Antioxidant Activity and Properties in Ulva Lactuca Linnaeus, 1753: A compression Study in Pre and Post Monsoon to the Northern Coasts of the Oman Sea. 2022. Available online: https://assets.researchsquare.com/files/rs-1657482/v1/2b501e14-ec7c-4cb7-9972-de8189eb1104.pdf?c=1653660031 (accessed on 16 January 2023).
- Liyanage, N.M.; Nagahawatta, D.P.; Jayawardena, T.U.; Jayawardhana, H.H.A.C.K.; Lee, H.-G.; Kim, Y.-S.; Jeon, Y.-J. Clionasterol-Rich Fraction of Caulerpa racemosa against Particulate Matter-Induced Skin Damage via Inhibition of Oxidative Stress and Apoptosis-Related Signaling Pathway. Antioxidants 2022, 11, 1941. [Google Scholar] [CrossRef]
- Cho, M.; Kang, I.-J.; Won, M.-H.; Lee, H.-S.; You, S. The Antioxidant Properties of Ethanol Extracts and Their Solvent-Partitioned Fractions from Various Green Seaweeds. J. Med. Food 2010, 13, 1232–1239. [Google Scholar] [CrossRef]
- Zeng, L.-M.; Wang, C.-J.; Su, J.-Y.; Li, D.; Owen, N.L.; Lu, Y.; Lu, N.; Zheng, Q.-T. Flavonoids from the red alga Acanthophora spicifera. Chin. J. Chem. 2011, 19, 1097–1100. [Google Scholar] [CrossRef]
- Farvin, K.S.; Jacobsen, C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef]
- Ryu, B.; Qian, Z.-J.; Kim, M.-M.; Nam, K.W.; Kim, S.-K. Anti-photoaging activity and inhibition of matrix metalloproteinase (MMP) by marine red alga, Corallina pilulifera methanol extract. Radiat. Phys. Chem. 2009, 78, 98–105. [Google Scholar] [CrossRef]
- Chakraborty, K.; Joseph, D.; Praveen, N.K. Antioxidant activities and phenolic contents of three red seaweeds (Division: Rhodophyta) harvested from the Gulf of Mannar of Peninsular India. J. Food Sci. Technol. 2015, 52, 1924–1935. [Google Scholar] [CrossRef]
- Tutino, V.; Gigante, I.; Milella, R.A.; De Nunzio, V.; Flamini, R.; De Rosso, M.; Scavo, M.P.; Depalo, N.; Fanizza, E.; Caruso, M.G.; et al. Flavonoid and Non-Flavonoid Compounds of Autumn Royal and Egnatia Grape Skin Extracts Affect Membrane PUFA’s Profile and Cell Morphology in Human Colon Cancer Cell Lines. Molecules 2020, 25, 3352. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From Chemistry to Biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Ferrazzano, G.F.; Amato, I.; Ingenito, A.; Zarrelli, A.; Pinto, G.; Pollio, A. Plant Polyphenols and Their Anti-Cariogenic Properties: A Review. Molecules 2011, 16, 1486–1507. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Ito, H. Tannins of Constant Structure in Medicinal and Food Plants—Hydrolyzable Tannins and Polyphenols Related to Tannins. Molecules 2011, 16, 2191–2217. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Tsopmo, A.; Awah, F.M.; Kuete, V. Lignans and Stilbenes from African Medicinal Plants. In Medicinal Plant Research in Africa; Kuete, V., Ed.; Elsevier: Oxford, UK, 2013; pp. 435–478. [Google Scholar] [CrossRef]
- Mateos, R.; Pérez-Correa, J.R.; Domínguez, H. Bioactive Properties of Marine Phenolics. Mar. Drugs 2020, 18, 501. [Google Scholar] [CrossRef]
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kryzhanovsky, S.P.; Kuznetsova, T.A.; Fedyanina, L.N.; Makarenkova, I.D.; Zvyagintseva, T.N. Algae Polyphenolic Compounds and Modern Antibacterial Strategies: Current Achievements and Immediate Prospects. Biomedicines 2020, 8, 342. [Google Scholar] [CrossRef]
- Tanna, B.; Choudhary, B.; Mishra, A. Metabolite profiling, antioxidant, scavenging and anti-proliferative activities of selected tropical green seaweeds reveal the nutraceutical potential of Caulerpa spp. Algal Res. 2018, 36, 96–105. [Google Scholar] [CrossRef]
- El Shoubaky, G.A.; Abdel-Daim, M.M.; Mansour, M.H.; Salem, E.A. Isolation and Identification of a Flavone Apigenin from Marine Red Alga Acanthophora spicifera with Antinociceptive and Anti-Inflammatory Activities. J. Exp. Neurosci. 2016, 10, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Klejdus, B.; Lojková, L.; Plaza, M.; Šnóblová, M.; Štěrbová, D. Hyphenated technique for the extraction and determination of isoflavones in algae: Ultrasound-assisted supercritical fluid extraction followed by fast chromatography with tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 7956–7965. [Google Scholar] [CrossRef]
- Lee, H.-H.; Kim, J.-S.; Jeong, J.-H.; Park, S.M.; Sathasivam, R.; Lee, S.Y.; Kim, C.S. Effect of Different Solvents on the Extraction of Compounds from Different Parts of Undaria pinnatifida (Harvey) Suringar. J. Mar. Sci. Eng. 2022, 10, 1193. [Google Scholar] [CrossRef]
- Arin, A.; Rahaman, S.; Farwa, U.; Lee, B. Faster and Protective Wound Healing Mechanistic of Para-Coumaric Acid Loaded Liver ECM Scaffold Cross-linked with Acellular Marine Kelp. Adv. Funct. Mater. 2023, 33, 2212325. [Google Scholar] [CrossRef]
- Dixit, D.; Reddy, C.R.K. Non-Targeted Secondary Metabolite Profile Study for Deciphering the Cosmeceutical Potential of Red Marine Macro Alga Jania rubens—An LCMS-Based Approach. Cosmetics 2017, 4, 45. [Google Scholar] [CrossRef]
- Pallela, R.; Na-Young, Y.; Kim, S.-K. Anti-photoaging and Photoprotective Compounds Derived from Marine Organisms. Mar. Drugs 2010, 8, 1189–1202. [Google Scholar] [CrossRef]
- Kendall, A.C.; Nicolaou, A. Bioactive lipid mediators in skin inflammation and immunity. Prog. Lipid Res. 2013, 52, 141–164. [Google Scholar] [CrossRef]
- Susano, P.; Silva, J.; Alves, C.; Martins, A.; Pinteus, S.; Gaspar, H.; Goettert, M.I.; Pedrosa, R. Mitigating the negative impacts of marine invasive species–Sargassum muticum-a key seaweed for skincare products development. Algal Res. 2022, 62, 102634. [Google Scholar] [CrossRef]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of Photoaging and Chronological Skin Aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef]
- Jenkins, G. Molecular mechanisms of skin ageing. Mech. Ageing Dev. 2002, 123, 801–810. [Google Scholar] [CrossRef]
- Gao, X.-H.; Zhang, L.; Wei, H.; Chen, H.-D. Efficacy and safety of innovative cosmeceuticals. Clin. Dermatol. 2008, 26, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Ferdouse, F.; Holdt, S.L.; Smith, R.; Murúa, P.; Yang, Z. The global status of seaweed production, trade and utilization. Globefish Res. Programme 2018, 124, I. [Google Scholar]
- Fernando, S.; Kim, M.; Son, K.-T.; Jeong, Y.; Jeon, Y.-J. Antioxidant Activity of Marine Algal Polyphenolic Compounds: A Mechanistic Approach. J. Med. Food 2016, 19, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Jesumani, V.; Du, H.; Pei, P.; Aslam, M.; Huang, N. Comparative study on skin protection activity of polyphenol-rich extract and polysaccharide-rich extract from Sargassum vachellianum. PLoS ONE 2020, 15, e0227308. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Liu, Y.; Xiao, J.; Wang, Y. Protective Effects of Tea Polysaccharides and Polyphenols on Skin. J. Agric. Food Chem. 2009, 57, 7757–7762. [Google Scholar] [CrossRef]
- Wang, H.-M.D.; Chen, C.-C.; Huynh, P.; Chang, J.-S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef]
- Urquiaga, I.; Leighton, F. Plant Polyphenol Antioxidants and Oxidative Stress. Biol. Res. 2000, 33, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Suganthy, N.; Kesika, P.; Pandian, S.K. Bioprotective properties of seaweeds: In vitro evaluation of antioxidant activity and antimicrobial activity against food borne bacteria in relation to polyphenolic content. BMC Complement. Altern. Med. 2008, 8, 38. [Google Scholar] [CrossRef]
- Heo, S.-J.; Jeon, Y.-J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B Biol. 2009, 95, 101–107. [Google Scholar] [CrossRef]
- Gautam, R.; Jachak, S.M. Recent developments in anti-inflammatory natural products. Med. Res. Rev. 2009, 29, 767–820. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Talero, E.; García-Mauriño, S.; Ávila-Román, J.; Rodríguez-Luna, A.; Alcaide, A.; Motilva, V. Bioactive Compounds Isolated from Microalgae in Chronic Inflammation and Cancer. Mar. Drugs 2015, 13, 6152–6209. [Google Scholar] [CrossRef]
- Xu, H.; Zheng, Y.-W.; Liu, Q.; Liu, L.-P.; Luo, F.-L.; Zhou, H.-C.; Isoda, H.; Ohkohchi, N.; Li, Y.-M. Reactive oxygen species in skin repair, regeneration, aging, and inflammation. React. Oxyg. Species (ROS) Living Cells 2018, 8, 69–88. [Google Scholar]
- Fernando, I.P.S.; Kim, H.-S.; Sanjeewa, K.K.A.; Oh, J.-Y.; Jeon, Y.-J.; Lee, W.W. Inhibition of inflammatory responses elicited by urban fine dust particles in keratinocytes and macrophages by diphlorethohydroxycarmalol isolated from a brown alga Ishige okamurae. Algae 2017, 32, 261–273. [Google Scholar] [CrossRef]
- Barker, J.; Griffiths, C.; Nickoloff, B.; Mitra, R.; Dixit, V. Keratinocytes as initiators of inflammation. Lancet 1991, 337, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lin, H.; Li, Z.; Mou, Q. The anti-allergic activity of polyphenol extracted from five marine algae. J. Ocean Univ. China 2015, 14, 681–684. [Google Scholar] [CrossRef]
- Novak, N.; Bieber, T. Allergic and nonallergic forms of atopic diseases. J. Allergy Clin. Immunol. 2003, 112, 252–262. [Google Scholar] [CrossRef]
- Mihindukulasooriya, S.P.; Kim, H.J.; Cho, J.; Herath, K.H.I.N.M.; Yang, J.; Dinh, D.T.T.; Ko, M.-O.; Jeon, Y.-J.; Ahn, G.; Jee, Y. Polyphenol-rich Sargassum horneri alleviates atopic dermatitis-like skin lesions in NC/Nga mice by suppressing Th2-mediated cytokine IL-13. Algae 2022, 37, 331–347. [Google Scholar] [CrossRef]
- Sugiura, Y.; Katsuzaki, H.; Imai, K.; Amano, H. The Anti-Allergic and Anti-Inflammatory Effects of Phlorotannins from the Edible Brown Algae, Ecklonia sp. and Eisenia sp. Nat. Prod. Commun. 2021, 16, 1934578X211060924. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Syarina, P.N.A.; Karthivashan, G.; Abas, F.; Arulselvan, P.; Fakurazi, S. Wound healing potential of Spirulina platensis extracts on human dermal fibroblast cells. EXCLI J. 2015, 14, 385. [Google Scholar] [CrossRef] [PubMed]
- Dorai, A.A. Wound care with traditional, complementary and alternative medicine. Indian J. Plast. Surg. 2012, 45, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Bigas, M.; Cruz, N.I.; Suarez, A. Comparative Evaluation of Aloe Vera in the Management of Burn Wounds in Guinea Pigs. Plast. Reconstr. Surg. 1988, 81, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Samaneh, G.F.; Fatemeh, T.S.; Mozhdeh, E.; Meng, G.; Kharidah, M.; Suhaila, M. Ethanolic extract of Eucheuma cottonii promotes in vivo hair growth and wound healing. J. Anim. Vet. Adv. 2011, 10, 601–605. [Google Scholar]
- Dreifke, M.B.; Jayasuriya, A.A.; Jayasuriya, A.C. Current wound healing procedures and potential care. Mater. Sci. Eng. C 2015, 48, 651–662. [Google Scholar] [CrossRef]
- Gokarneshan, N. Role of Chitosan in wound healing—A review of the recent advances. Glob. J. Addict. Rehabil. Med. 2017, 4, 61–64. [Google Scholar]
- Korzeniowska, K.; Górka, B.; Lipok, J.; Wieczorek, P.P. Algae and their extracts in medical treatment. In Algae Biomass: Characteristics and Applications; Springer: Berlin/Heidelberg, Germany, 2018; pp. 73–87. [Google Scholar]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; Gomez d’Ayala, G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef]
- Kim, M.-S.; Oh, G.-W.; Jang, Y.-M.; Ko, S.-C.; Park, W.-S.; Choi, I.-W.; Kim, Y.-M.; Jung, W.-K. Antimicrobial hydrogels based on PVA and diphlorethohydroxycarmalol (DPHC) derived from brown alga Ishige okamurae: An in vitro and in vivo study for wound dressing application. Mater. Sci. Eng. C 2020, 107, 110352. [Google Scholar] [CrossRef]
- McCullough, J.L.; Kelly, K.M. Prevention and treatment of skin aging. Ann. N. Y. Acad. Sci. 2006, 1067, 323–331. [Google Scholar] [CrossRef]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; De Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Heo, S.-J.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.-J.; Kim, M.-J.; Sanjeewa, K.K.A.; Lee, K.; Ahn, G. (−)-Loliolide Isolated from Sargassum horneri Abate UVB-Induced Oxidative Damage in Human Dermal Fibroblasts and Subside ECM Degradation. Mar. Drugs 2021, 19, 435. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-M.; Van Ta, Q.; Mendis, E.; Rajapakse, N.; Jung, W.-K.; Byun, H.-G.; Jeon, Y.-J.; Kim, S.-K. Phlorotannins in Ecklonia cava extract inhibit matrix metalloproteinase activity. Life Sci. 2006, 79, 1436–1443. [Google Scholar] [CrossRef]
- Phang, S.J.; Teh, H.X.; Looi, M.L.; Arumugam, B.; Fauzi, M.B.; Kuppusamy, U.R. Phlorotannins from brown algae: A review on their antioxidant mechanisms and applications in oxidative stress-mediated diseases. J. Appl. Phycol. 2023, 35, 867–892. [Google Scholar] [CrossRef]
- Hempel, M.d.S.S.; Colepicolo, P.; Zambotti-Villela, L. Macroalgae Biorefinery for the Cosmetic Industry: Basic Concept, Green Technology, and Safety Guidelines. Phycology 2023, 3, 211–241. [Google Scholar] [CrossRef]
- Berson, D.S.; Cohen, J.L.; Rendon, M.I.; Roberts, W.E.; Starker, I.; Wang, B. Clinical role and application of superficial chemical peels in today’s practice. J. Drugs Dermatol. 2009, 8, 803–811. [Google Scholar]
- Ebanks, J.P.; Wickett, R.R.; Boissy, R.E. Mechanisms Regulating Skin Pigmentation: The Rise and Fall of Complexion Coloration. Int. J. Mol. Sci. 2009, 10, 4066–4087. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, M.E.; Kijjoa, A.; Pinto, M. Marine-Derived Compounds with Potential Use as Cosmeceuticals and Nutricosmetics. Molecules 2020, 25, 2536. [Google Scholar] [CrossRef]
- Parvez, S.; Kang, M.; Chung, H.S.; Cho, C.; Hong, M.C.; Shin, M.K.; Bae, H. Survey and mechanism of skin depigmenting and lightening agents. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006, 20, 921–934. [Google Scholar] [CrossRef]
- Roy, A.; Sahu, R.K.; Matlam, M.; Deshmukh, V.K.; Dwivedi, J.; Jha, A.K. In vitro techniques to assess the proficiency of skin care cosmetic formulations. Pharmacogn. Rev. 2013, 7, 97. [Google Scholar] [CrossRef]
- Berthon, J.-Y.; Kappes, R.N.; Bey, M.; Cadoret, J.-P.; Renimel, I.; Filaire, E. Marine algae as attractive source to skin care. Free. Radic. Res. 2017, 51, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.-W.; Su, C.-C.; Peng, H.-Y.; Chou, S.-T. Melaleuca quinquenervia essential oil inhibits α-melanocyte-stimulating hormone-induced melanin production and oxidative stress in B16 melanoma cells. Phytomedicine 2017, 34, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-Y.; Perez, J.M.; Laursen, R.; Hara, M.; Gilchrest, B.A. Protein Kinase C-β Activates Tyrosinase by Phosphorylating Serine Residues in Its Cytoplasmic Domain. J. Biol. Chem. 1999, 274, 16470–16478. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. p-Coumaric Acid as An Active Ingredient in Cosmetics: A Review Focusing on its Antimelanogenic Effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef]
- Masum, M.N.; Yamauchi, K.; Mitsunaga, T. Tyrosinase Inhibitors from Natural and Synthetic Sources as Skin-lightening Agents. Rev. Agric. Sci. 2019, 7, 41–58. [Google Scholar] [CrossRef]
- Azam, M.S.; Choi, J.; Lee, M.-S.; Kim, H.-R. Hypopigmenting Effects of Brown Algae-Derived Phytochemicals: A Review on Molecular Mechanisms. Mar. Drugs 2017, 15, 297. [Google Scholar] [CrossRef]
- Paudel, P.; Wagle, A.; Seong, S.H.; Park, H.J.; Jung, H.A.; Choi, J.S. A New Tyrosinase Inhibitor from the Red Alga Symphyocladia latiuscula (Harvey) Yamada (Rhodomelaceae). Mar. Drugs 2019, 17, 295. [Google Scholar] [CrossRef]
- Chan, Y.Y.; Kim, K.H.; Cheah, S.H. Inhibitory effects of Sargassum polycystum on tyrosinase activity and melanin formation in B16F10 murine melanoma cells. J. Ethnopharmacol. 2011, 137, 1183–1188. [Google Scholar] [CrossRef]
- Shimoda, H.; Tanaka, J.; Shan, S.-J.; Maoka, T. Anti-pigmentary activity of fucoxanthin and its influence on skin mRNA expression of melanogenic molecules. J. Pharm. Pharmacol. 2010, 62, 1137–1145. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kang, S.-M.; Sok, C.H.; Hong, J.T.; Oh, J.-Y.; Jeon, Y.-J. Cellular activities and docking studies of eckol isolated from Ecklonia cava (Laminariales, Phaeophyceae) as potential tyrosinase inhibitor. Algae 2015, 30, 163–170. [Google Scholar] [CrossRef]
- Kim, K.-N.; Yang, H.-M.; Kang, S.-M.; Kim, D.; Ahn, G.; Jeon, Y.-J. Octaphlorethol A isolated from Ishige foliacea inhibits α-MSH-stimulated induced melanogenesis via ERK pathway in B16F10 melanoma cells. Food Chem. Toxicol. 2013, 59, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.-S.; Park, H.-Y.; Nam, K.-H. Whitening effects of 4-hydroxyphenethyl alcohol isolated from water boiled with Hizikia fusiformis. Food Sci. Biotechnol. 2014, 23, 555–560. [Google Scholar] [CrossRef]
- Alam, S.; Pal, A.; Singh, D.; Ansari, K.M. Topical application of Nexrutine inhibits ultraviolet B-induced cutaneous inflammatory responses in SKH-1 hairless mouse. Photodermatol. Photoimmunol. Photomed. 2018, 34, 82–90. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Pal, H.C.; Prasad, R. Dietary proanthocyanidins prevent ultraviolet radiation-induced non-melanoma skin cancer through enhanced repair of damaged DNA-dependent activation of immune sensitivity. Semin. Cancer Biol. 2017, 46, 138–145. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Bahramsoltani, R.; Iranpanah, A.; Patra, J.K.; Das, G.; Gouda, S.; Rahimi, R.; Rezaeiamiri, E.; Cao, H.; Giampieri, F.; et al. Advances on Natural Polyphenols as Anticancer Agents for Skin Cancer. Pharmacol. Res. 2020, 151, 104584. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.-J.; Ahn, G. Fucoidan refined by Sargassum confusum indicate protective effects suppressing photo-oxidative stress and skin barrier perturbation in UVB-induced human keratinocytes. Int. J. Biol. Macromol. 2020, 164, 149–161. [Google Scholar] [CrossRef]
- Simões, M.C.F.; Sousa, J.J.S.; Pais, A.A.C.C. Skin cancer and new treatment perspectives: A review. Cancer Lett. 2015, 357, 8–42. [Google Scholar] [CrossRef]
- Hwang, H.; Chen, T.; Nines, R.G.; Shin, H.-C.; Stoner, G.D. Photochemoprevention of UVB-induced skin carcinogenesis in SKH-1 mice by brown algae polyphenols. Int. J. Cancer 2006, 119, 2742–2749. [Google Scholar] [CrossRef]
- Namvar, F.; Mohamed, S.; Fard, S.G.; Behravan, J.; Mustapha, N.M.; Alitheen, N.B.M.; Othman, F. Polyphenol-rich seaweed (Eucheuma cottonii) extract suppresses breast tumour via hormone modulation and apoptosis induction. Food Chem. 2012, 130, 376–382. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.-K. Beneficial Effects of Marine Algal Compounds in Cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Naughton, L.M.; Montánchez, I.; Dobson, A.D.W.; Rai, D.K. Current Status and Future Prospects of Marine Natural Products (MNPs) as Antimicrobials. Mar. Drugs 2017, 15, 272. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial Action of Compounds from Marine Seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Sameeh, M.; Mohamed, A.; Elazzazy, A. Polyphenolic contents and antimicrobial activity of different extracts of Padina boryana Thivy and Enteromorpha sp marine algae. J. Appl. Pharm. Sci. 2016, 6, 087–092. [Google Scholar] [CrossRef]
- TÜney, İ.; Cadirci, B.H.; Ünal, D.; Sukatar, A. Antimicrobial activities of the extracts of marine algae from the coast of Urla (Izmir, Turkey). Turk. J. Biol. 2006, 30, 171–175. [Google Scholar]
- Fouladvand, M.; Barazesh, A.; Farokhzad, F.; Malekizadeh, H.; Sartavi, K. Evaluation of in vitro anti-Leishmanial activity of some brown, green and red algae from the Persian Gulf. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 597–600. [Google Scholar]
- Torres, F.A.; Passalacqua, T.G.; Velásquez, A.M.; de Souza, R.A.; Colepicolo, P.; Graminha, M.A. New drugs with antiprotozoal activity from marine algae: A review. Rev. Bras. De Farm. 2014, 24, 265–276. [Google Scholar] [CrossRef]
- Singh, B.; Mal, G.; Sharma, D.; Gautam, S.K.; Kumar, M.; Solimene, U.; Metalla, M.; Marotta, F. Chapter 30-Plant Polyphenols: The Futuristic Bioactive Therapeutics for Skin Care. In Polyphenols: Prevention and Treatment of Human Disease, 2nd ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 385–394. [Google Scholar]
- Dos Santos, A.O.; Britta, E.A.; Bianco, E.M.; Ueda-Nakamura, T.; Filho, B.P.D.; Pereira, R.C.; Nakamura, C.V. 4-Acetoxydolastane Diterpene from the Brazilian Brown Alga Canistrocarpus cervicornis as Antileishmanial Agent. Mar. Drugs 2011, 9, 2369–2383. [Google Scholar] [CrossRef]
- Dos Santos, A.O.; Veiga-Santos, P.; Ueda-Nakamura, T.; Filho, B.P.D.; Sudatti, D.B.; Bianco, M.; Pereira, R.C.; Nakamura, C.V. Effect of Elatol, Isolated from Red Seaweed Laurencia dendroidea, on Leishmania amazonensis. Mar. Drugs 2010, 8, 2733–2743. [Google Scholar] [CrossRef]
- Soares, D.C.; Calegari-Silva, T.C.; Lopes, U.G.; Teixeira, V.L.; Paixão, I.C.N.D.P.; Cirne-Santos, C.; Bou-Habib, D.C.; Saraiva, E.M. Dolabelladienetriol, a Compound from Dictyota pfaffii Algae, Inhibits the Infection by Leishmania amazonensis. PLOS Neglected Trop. Dis. 2012, 6, e1787. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J. Metabolites from Macroalgae and Its Applications in the Cosmetic Industry: A Circular Economy Approach. Resources 2020, 9, 101. [Google Scholar] [CrossRef]
- Gupta, V.; Mohapatra, S.; Mishra, H.; Farooq, U.; Kumar, K.; Ansari, M.J.; Aldawsari, M.F.; Alalaiwe, A.S.; Mirza, M.A.; Iqbal, Z. Nanotechnology in Cosmetics and Cosmeceuticals—A Review of Latest Advancements. Gels 2022, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarasaiyar, K.; Goh, B.-H.; Jeon, Y.-J.; Yow, Y.-Y. Algae Metabolites in Cosmeceutical: An Overview of Current Applications and Challenges. Mar. Drugs 2020, 18, 323. [Google Scholar] [CrossRef] [PubMed]
- Puchkova, T.V.; Khapchaeva, S.A.; Zotov, V.S.; Lukyanov, A.A.; Solovchenko, A.E. Marine and freshwater microalgae as a sustainable source of cosmeceuticals. Mar. Biol. J. 2021, 6, 67–81. [Google Scholar] [CrossRef]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal Carotenoids: A Review of Production, Current Markets, Regulations, and Future Direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayawardhana, H.H.A.C.K.; Jayawardena, T.U.; Sanjeewa, K.K.A.; Liyanage, N.M.; Nagahawatta, D.P.; Lee, H.-G.; Kim, J.-I.; Jeon, Y.-J. Marine Algal Polyphenols as Skin Protective Agents: Current Status and Future Prospectives. Mar. Drugs 2023, 21, 285. https://doi.org/10.3390/md21050285
Jayawardhana HHACK, Jayawardena TU, Sanjeewa KKA, Liyanage NM, Nagahawatta DP, Lee H-G, Kim J-I, Jeon Y-J. Marine Algal Polyphenols as Skin Protective Agents: Current Status and Future Prospectives. Marine Drugs. 2023; 21(5):285. https://doi.org/10.3390/md21050285
Chicago/Turabian StyleJayawardhana, H.H.A.C.K., Thilina U. Jayawardena, K.K.A. Sanjeewa, N.M. Liyanage, D.P. Nagahawatta, Hyo-Geun Lee, Jae-Il Kim, and You-Jin Jeon. 2023. "Marine Algal Polyphenols as Skin Protective Agents: Current Status and Future Prospectives" Marine Drugs 21, no. 5: 285. https://doi.org/10.3390/md21050285
APA StyleJayawardhana, H. H. A. C. K., Jayawardena, T. U., Sanjeewa, K. K. A., Liyanage, N. M., Nagahawatta, D. P., Lee, H.-G., Kim, J.-I., & Jeon, Y.-J. (2023). Marine Algal Polyphenols as Skin Protective Agents: Current Status and Future Prospectives. Marine Drugs, 21(5), 285. https://doi.org/10.3390/md21050285