Extraction, Structural Characterization, and In Vivo Anti-Inflammatory Effect of Alginate from Cystoseira crinita (Desf.) Borry Harvested in the Bulgarian Black Sea
Abstract
1. Introduction
2. Results
2.1. Extraction Yield and Chemical Composition of C. crinita Alginate
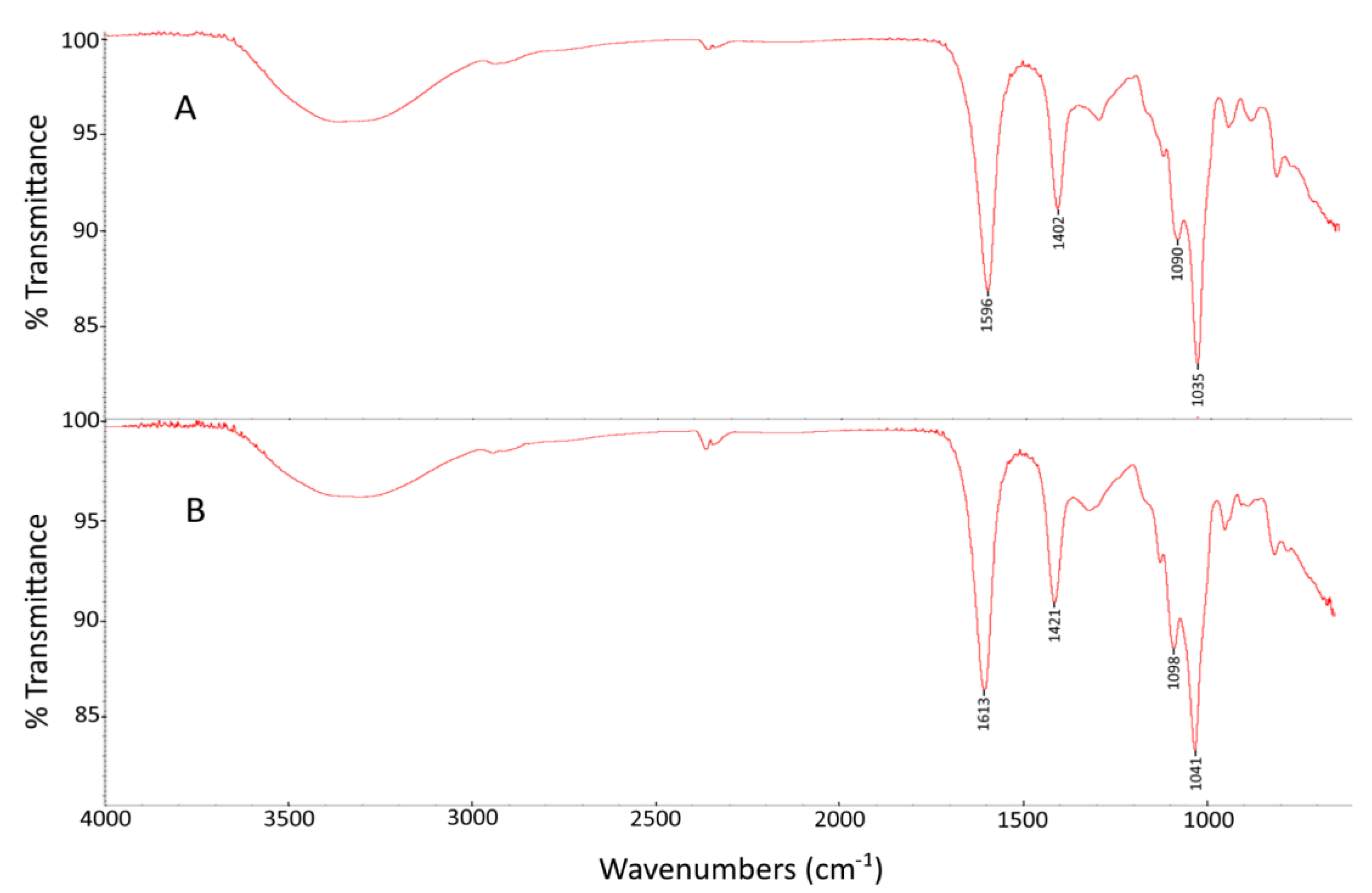
2.2. FTIR Spectroscopy Analysis
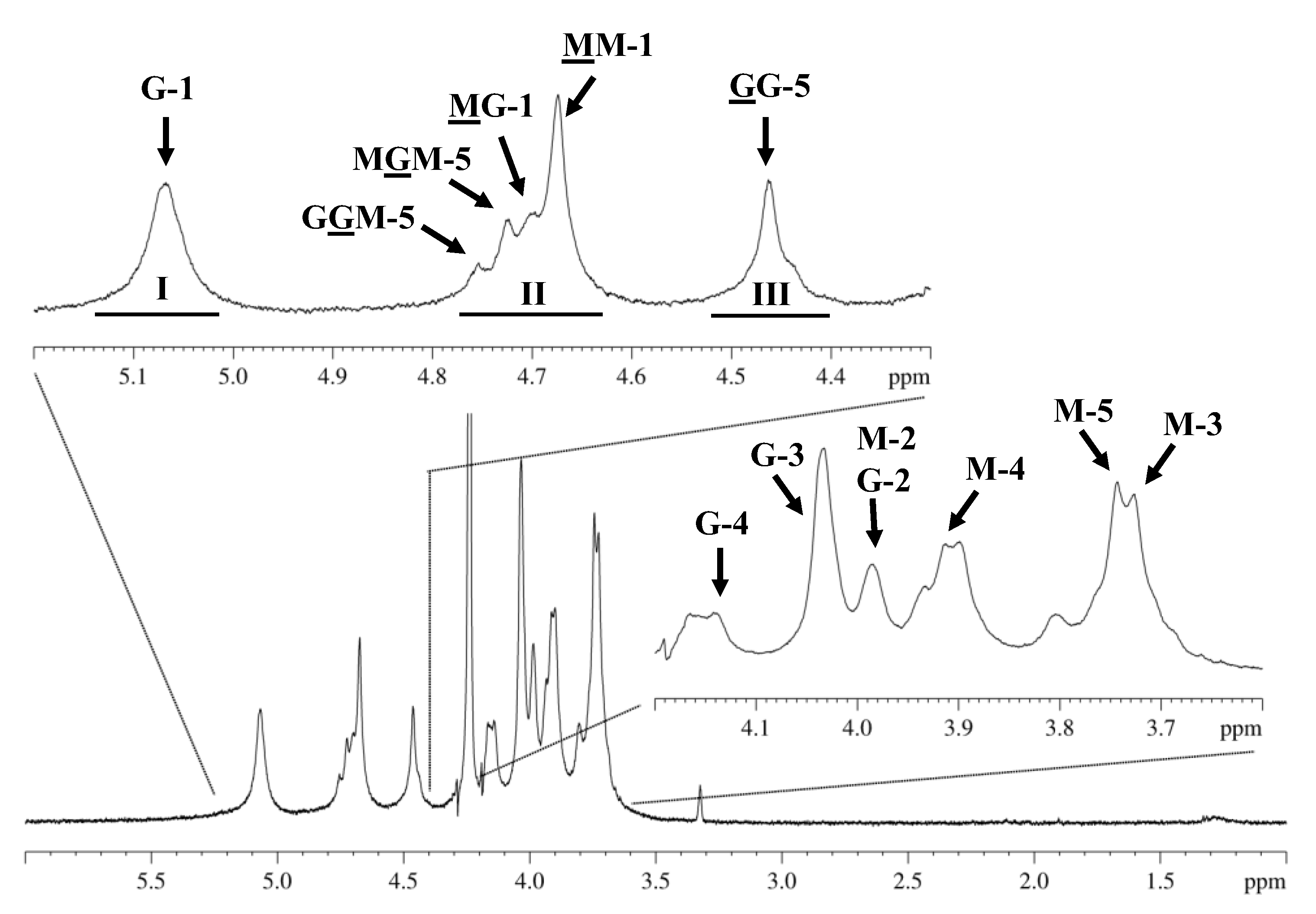
2.3. Proton Nuclear Magnetic Resonance (1H NMR) Spectroscopy
2.4. SEC-MALS Analysis
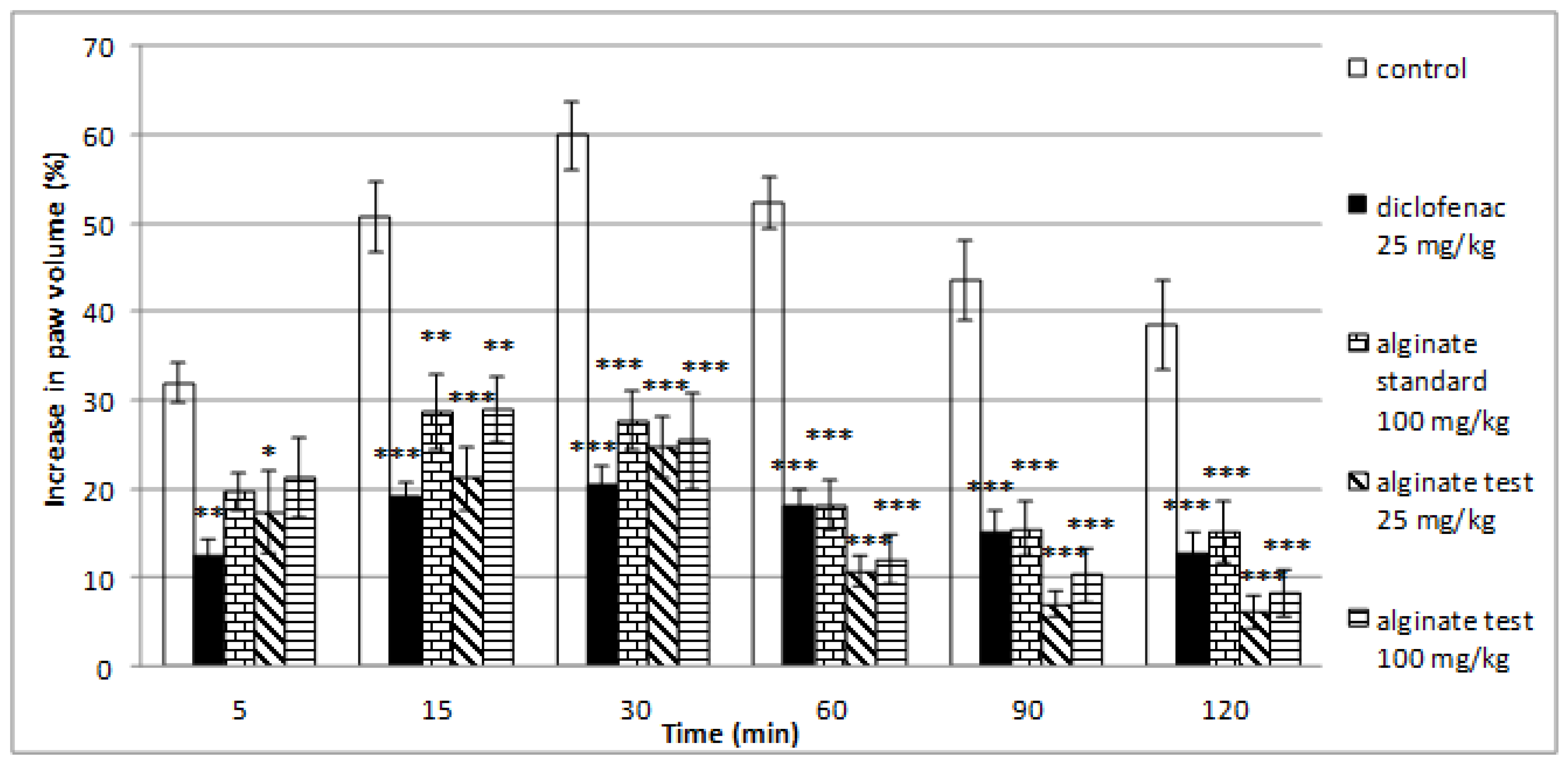
2.5. Effect of Alginate on Histamine-Induced Paw Edema
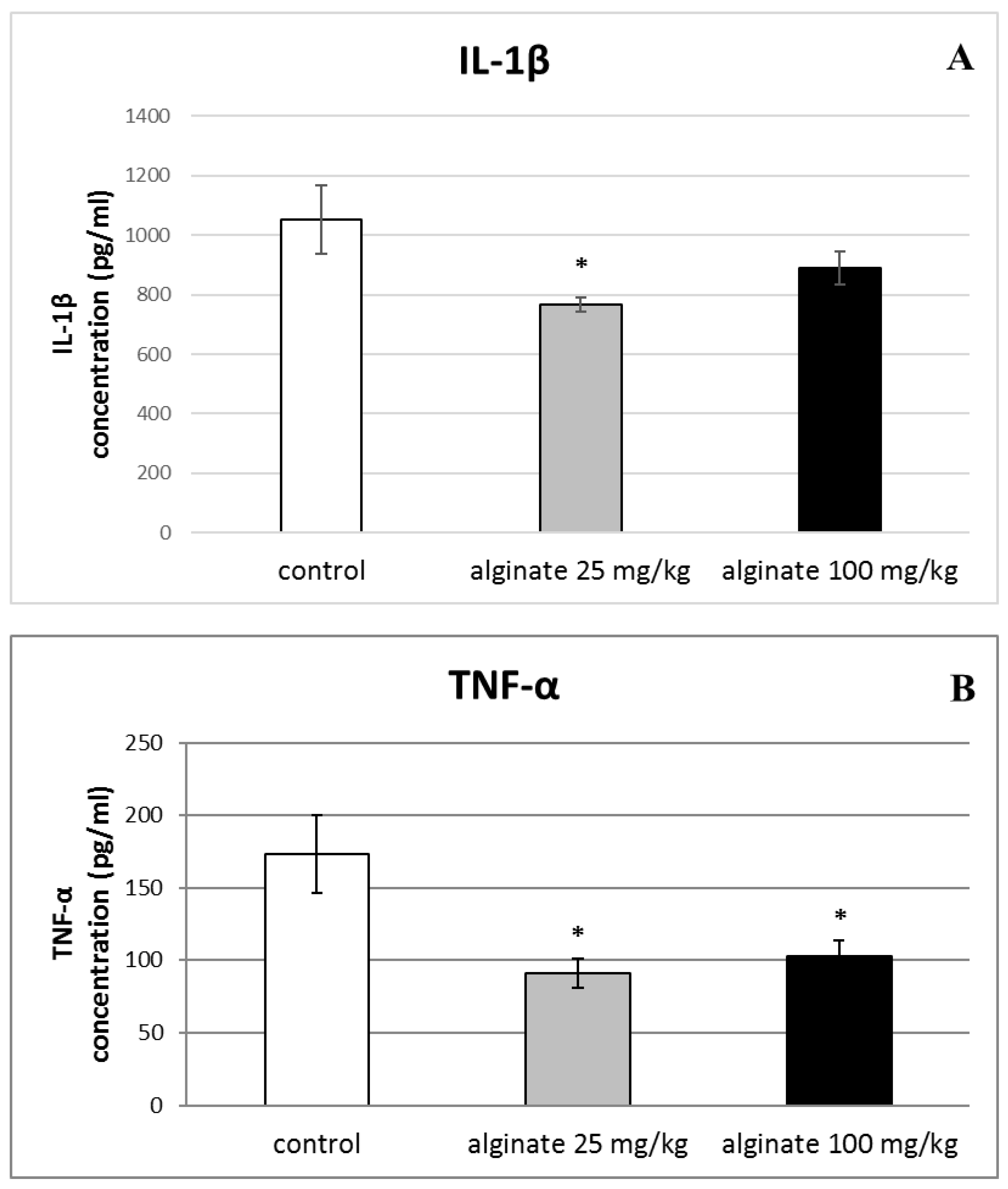
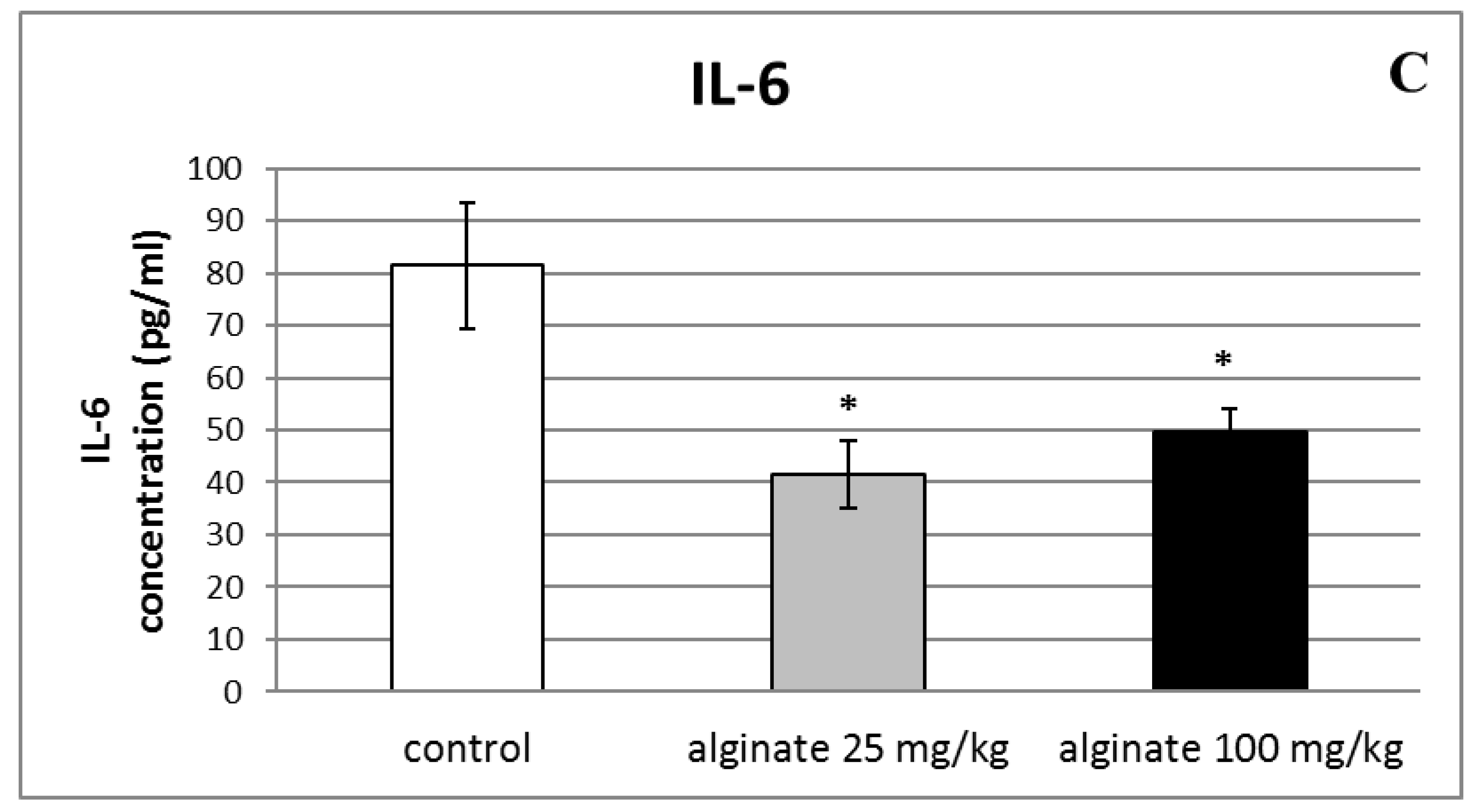
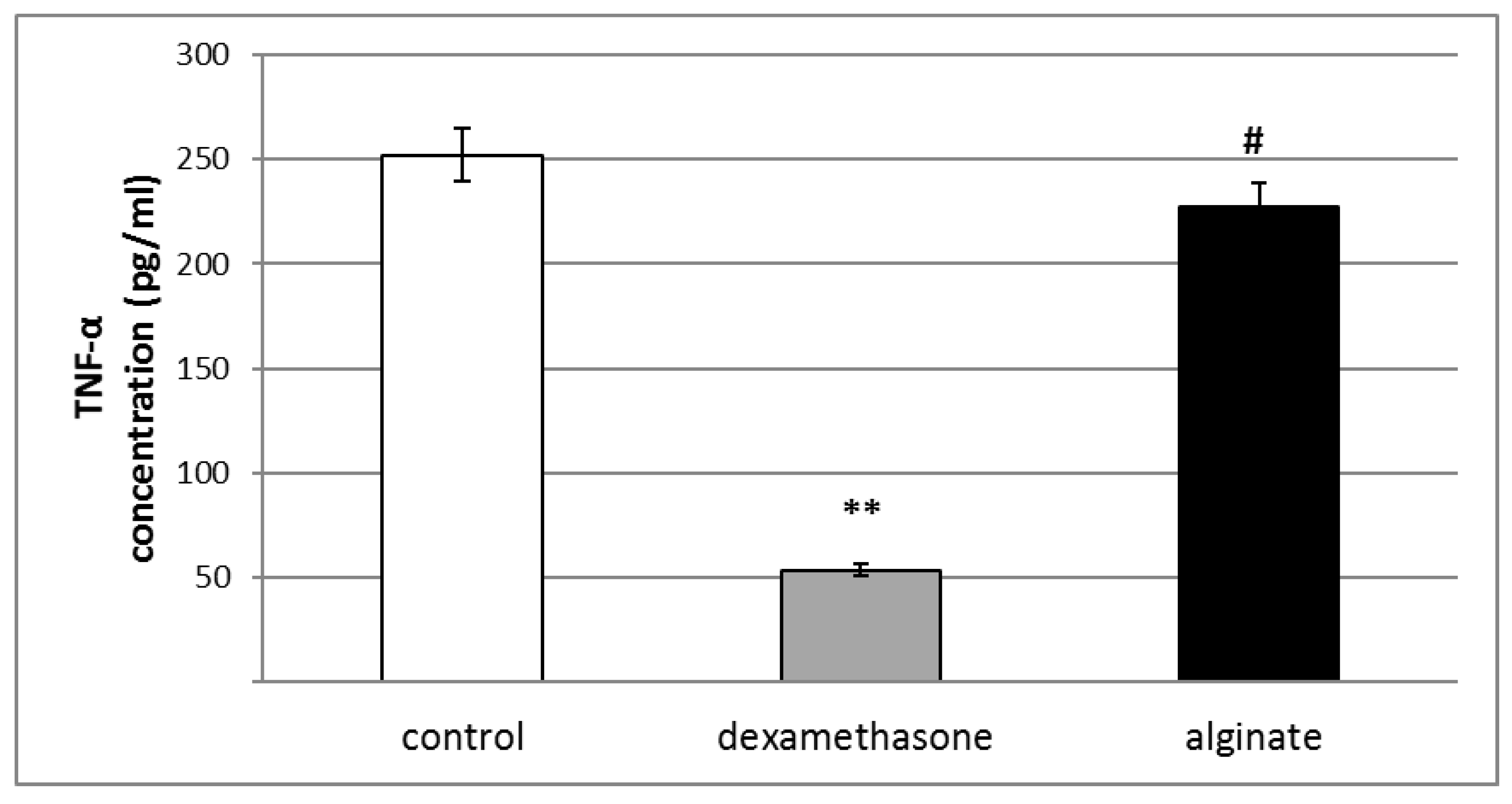
2.6. Effect of Alginate on the Levels of Pro-Inflammatory Cytokines (IL-1β, TNF-α, and IL-6) in Serum and Peritoneal Fluid
2.7. Effect of Alginate on the Levels of Anti-Inflammatory Cytokine (IL-10) in Blood Serum
3. Discussion
4. Materials and Methods
4.1. Algae Material and Chemicals
4.2. Animals
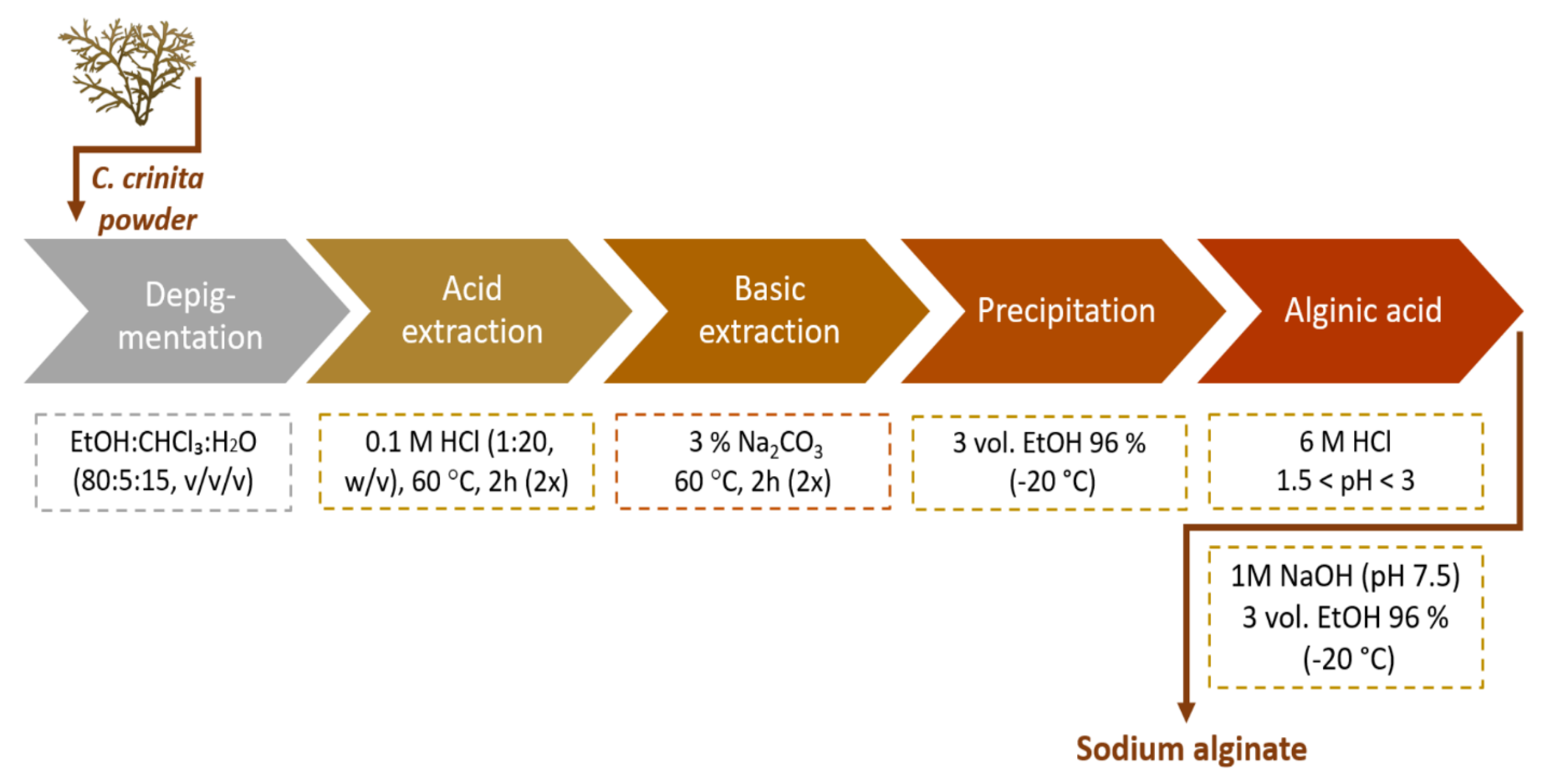
4.3. Extraction of Alginate
4.4. Chemical Analyses of C. crinita Alginate
4.5. FTIR Spectroscopy
4.6. SEC-MALS
4.7. 1H NMR Analysis
4.8. Histamine-Induced Paw Edema
4.9. Detection of Pro-Inflammatory and Anti-Inflammatory Cytokines
4.10. Carrageenan-Induced Peritonitis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mišurcová, L.; Škrovánková, S.; Samek, D.; Ambrožová, J.; Machů, L. Health benefits of algal polysaccharides in human nutrition. Adv. Food Nutr. Res. 2012, 66, 75–145. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Ye, H.; Wang, K.; Zhou, C.; Liu, J.; Zeng, X. Purification, antitumor and antioxidant activities in vitro of polysaccharides from the brown seaweed Sargassum pallidum. Food Chem. 2008, 111, 428–432. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; De Morais, A.M.B.; De Morais, R.M.S.C. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef] [PubMed]
- Okolie, C.L.; CK Rajendran, S.R.; Udenigwe, C.C.; Aryee, A.N.; Mason, B. Prospects of brown seaweed polysaccharides (BSP) as prebiotics and potential immunomodulators. J. Food Biochem. 2017, 41, 12392. [Google Scholar] [CrossRef]
- Lim, S.J.; Aida, W.M.W. Extraction of Sulfated Polysaccharides (Fucoidan) From Brown Seaweed. In Seaweed Polysaccharides; Venkatesan, J., Anil, S., Kim, S.K., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 27–46. [Google Scholar] [CrossRef]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Delattre, C.; Molinié, R.; Petit, E.; Elboutachfaiti, R.; Nikolova, M.; Iliev, I.; Murdjeva, M.; et al. Structural characterization and in vivo anti-inflammatory activity of fucoidan from Cystoseira crinita (Desf.) Borry. Mar. Drugs 2022, 20, 714. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’donnell, C.P. Extraction, structure and biofunctional activities of laminarin from brown algae. Int. J. Food Sci. Technol. 2015, 50, 24–31. [Google Scholar] [CrossRef]
- Déléris, P.; Nazih, H.; Bard, J.M. Seaweeds in human health. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 319–367. [Google Scholar] [CrossRef]
- Priyan Shanura Fernando, I.; Kim, K.N.; Kim, D.; Jeon, Y.J. Algal polysaccharides: Potential bioactive substances for cosmeceutical applications. Crit. Rev. Biotechnol. 2019, 39, 99–113. [Google Scholar] [CrossRef]
- Rioux, L.-E.; Turgeon, S.L.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Sun, J.C.; Tan, H.P. Alginate-based biomaterials for regenerative medicine applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Cao, Q.; Wang, Y.; Xiao, H.; Zhao, J.; Zhang, Q.; Ji, A.; Song, S. Advances in research on the bioactivity of alginate oligosaccharides. Mar. Drugs. 2020, 18, 144. [Google Scholar] [CrossRef]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current use and future perspectives in pharmaceutical and biomedical applications. Int. J. Polym. Sci. 2016, 2016, 7697031. [Google Scholar] [CrossRef]
- Milkova, T.; Talev, G.; Christov, R.; Dimitrova-Konaklieva, S.; Popov, S. Sterols and volatiles in Cystoseira barbata and Cystoseira crinita from the black sea. Phytochemistry 1997, 45, 93–95. [Google Scholar] [CrossRef]
- Sellimi, S.; Younes, I.; Ayed, H.B.; Maalej, H.; Montero, V.; Rinaudo, M.; Dahia, M.; Mechichi, T.; Hajji, M.; Nasri, M. Structural, physicochemical and antioxidant properties of sodium alginate isolated from a Tunisian brown seaweed. Int. J. Biol. Macromol. 2015, 72, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Hentati, F.; Delattre, C.; Ursu, A.V.; Desbrières, J.; Le Cerf, D.; Gardarin, C.; Abdelkafi, S.; Michaud, P.; Pierre, G. Structural characterization and antioxidant activity of water-soluble polysaccharides from the Tunisian brown seaweed Cystoseira compressa. Carbohydr. Polym. 2018, 198, 589–600. [Google Scholar] [CrossRef]
- Benslima, A.; Sellimi, S.; Hamdi, M.; Nasri, R.; Jridi, M.; Cot, D.; Li, S.; Nasri, M.; Zouari, N. The brown seaweed Cystoseira schiffneri as a source of sodium alginate: Chemical and structural characterization, and antioxidant activities. Food Biosci. 2021, 40, 100873. [Google Scholar] [CrossRef]
- Pereira, L.; Sousa, A.; Coelho, H.; Amado, A.M.; Ribeiro-Claro, P.J. Use of FTIR, FT-Raman and 13C-NMR spectroscopy for identification of some seaweed phycocolloids. Biomed. Eng. 2003, 20, 223–228. [Google Scholar] [CrossRef]
- Kaidi, S.; Bentiss, F.; Jama, C.; Khaya, K.; Belattmania, Z.; Reani, A.; Sabour, B. Isolation and structural characterization of alginates from the Kelp species Laminaria ochroleuca and Saccorhiza polyschides from the Atlantic Coast of Morocco. Colloids Interfaces 2022, 6, 51. [Google Scholar] [CrossRef]
- Aarstad, O.A.; Tøndervik, A.; Sletta, H.; Skjåk-Bræk, G. Alginate sequencing: An analysis of block distribution in alginates using specific alginate degrading enzymes. Biomacromolecules 2012, 13, 106–116. [Google Scholar] [CrossRef]
- Fenoradosoa, T.A.; Ali, G.; Delattre, C.; Laroche, C.; Petit, E.; Wadouachi, A.; Michaud, P. Extraction and characterization of an alginate from the brown seaweed Sargassum turbinarioides Grunow. J. Appl. Phycol. 2010, 22, 131–137. [Google Scholar] [CrossRef]
- Trica, B.; Delattre, C.; Gros, F.; Ursu, A.V.; Dobre, T.; Djelveh, G.; Michaud, P.; Oancea, F. Extraction and characterization of alginate from an edible brown seaweed (Cystoseira barbata) harvested in the Romanian Black Sea. Mar. Drugs 2019, 17, 405. [Google Scholar] [CrossRef] [PubMed]
- Grasdalen, H.; Larsen, B.; Smidsrød, O. A pmr study of the composition and sequence of uronate residues in alginates. Carbohydr. Res. 1979, 68, 23–31. [Google Scholar] [CrossRef]
- Heyraud, A.; Gey, C.; Leonard, C.; Rochas, C.; Girond, S.; Kloareg, B. NMR spectroscopy analysis of oligoguluronates and oligomannuronates prepared by acid or enzymatic hydrolysis of homopolymeric blocks of alginic acid. Application to the determination of the substrate specificity of Haliotis tuberculata alginate lyase. Carbohydr. Res. 1996, 289, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Ramirez, M.; Mucci, A.; Larsen, B. Extraction, isolation and cadmium binding of alginate from Sargassum spp. J. Appl. Psychol. 2004, 16, 275–284. [Google Scholar] [CrossRef]
- Rhein-Knudsen, N.; Ale, M.T.; Ajalloueian, F.; Meyer, A.S. Characterization of alginates from Ghanaian brown seaweeds: Sargassum spp. and Padina spp. Food Hydrocoll. 2017, 71, 236–244. [Google Scholar] [CrossRef]
- Rashedy, S.H.; Abd El Hafez, M.S.; Dar, M.A.; Cotas, J.; Pereira, L. Evaluation and characterization of alginate extracted from brown seaweed collected in the Red Sea. Appl. Sci. 2021, 11, 6290. [Google Scholar] [CrossRef]
- Permatasari, A.A.A.P.; Rosiana, I.W.; Wiradana, P.A.; Lestari, M.D.; Widiastuti, N.K.; Kurniawan, S.B.; Widhiantara, I.G. Extraction and characterization of sodium alginate from three brown algae collected from Sanur Coastal Waters, Bali as biopolymer agent. Biodiversitas J. Biol. Divers. 2022, 23, 1655–1663. [Google Scholar] [CrossRef]
- Kelly, B.J.; Brown, M.T. Variations in the alginate content and composition of Durvillaea antarctica and D. willana from southern New Zealand. J. Appl. Phycol. 2000, 12, 317–324. [Google Scholar] [CrossRef]
- Bouissil, S.; El Alaoui-Talibi, Z.; Pierre, G.; Michaud, P.; El Modafar, C.; Delattre, C. Use of alginate extracted from Moroccan brown algae to stimulate natural defense in date palm roots. Molecules 2020, 25, 720. [Google Scholar] [CrossRef]
- Saji, S.; Hebden, A.; Goswami, P.; Du, C. A brief review on the development of alginate extraction process and its sustainability. Sustainability 2022, 14, 5181. [Google Scholar] [CrossRef]
- Aitouguinane, M.; Alaoui-Talibi, Z.E.; Rchid, H.; Fendri, I.; Abdelkafi, S.; El-Hadj, M.D.O.; Boual, Z.; Dubessay, P.; Michaud, P.; Traïkia, M.; et al. Polysaccharides from Moroccan green and brown seaweed and their derivatives stimulate natural defenses in olive tree leaves. Appl. Sci. 2022, 12, 8842. [Google Scholar] [CrossRef]
- Hachemi-Benmalek, N.; Nouani, A.; Benchabane, A. Valorization of brown algae (Cystoseira caespitosa) from local region in Algeria for sodium alginate extraction and their application in the immobilization of microbial pectinases. ALJEST 2019, 5, 1155–1162. [Google Scholar]
- Day, D.F. Alginates. In Biopolymers from Renewable Resources; Kaplan, D.L., Ed.; Springer Berlin: Heidelberg, Germany, 1998; pp. 119–143. [Google Scholar]
- Gacesa, P. Alginates. Carbohydr. Polym. 1988, 8, 161–182. [Google Scholar] [CrossRef]
- Abka-Khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, properties and applications of alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Hadj Ammar, H.; Lajili, S.; Ben Said, R.; Le Cerf, D.; Bouraoui, A.; Majdoub, H. Physico-chemical characterization and pharmacological evaluation of sulfated polysaccharides from three species of Mediterranean brown algae of the genus Cystoseira. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2015, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Siebers, U.; Horcher, A.; Bretzel, R.G.; Federlin, K.; Zekorn, T. Alginate-based microcapsules for immunoprotected islet transplantation. Ann. N. Y. Acad. Sci. 1997, 831, 304–312. [Google Scholar] [CrossRef]
- Kammerlander, G.; Eberlein, T. An assessment of the wound healing properties of Algisite M dressings. Nurs. Times 2003, 99, 54–56. [Google Scholar]
- Ohsumi, H.; Hirata, H.; Nagakura, T.; Tsujii, M.; Sugimoto, T.; Miyamoto, K.; Horiuchi, T.; Nagao, M.; Nakashima, T.; Uchida, A. Enhancement of perineurial repair and inhibition of nerve adhesion by viscous injectable pure alginate sol. Plast. Reconstr. Surg. 2005, 116, 823–830. [Google Scholar] [CrossRef]
- Hori, Y.; Winans, A.M.; Huang, C.C.; Horrigan, E.M.; Irvine, D.J. Injectable dendritic cell-carrying alginate gels for immunization and immunotherapy. Biomaterials 2008, 29, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Abramowitz, L.; Weyandt, G.H.; Havlickova, B.; Matsuda, Y.; Didelot, J.M.; Rothhaar, A.; Sobrado, C.; Szabadi, A.; Vitalyos, T.; Wiesel, P. The diagnosis and management of haemorrhoidal disease from a global perspective. Aliment. Pharmacol. Ttherap. 2010, 31, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Slezka, I.E.; Miroshichenko, V.A.; Vostrikova, O.G.; Ziganshina, O.A. Applications of bioactive compounds from marine organisms in atherosclerosis prophylaxis in children, new biomedical technologies using bioactive additives. In Proceedings of the All Russian Conference; IMKVL Siberian Branch, Ross Akad Med Nauk: Vladivostok, Russia, 1998; pp. 90–94. [Google Scholar]
- Hasegawa, T.; Takahashi, T.; Inada, Y.; Yamada, C.; Tanaka, Y. Reparative effects of sodium alginate (Alloid G) on radiation stomatitis. Nihon Igaku Hoshasen Gakkai Zasshi. Nippon Acta Radiol. 1989, 49, 1047–1051. [Google Scholar]
- Katayama, S.; Ohshita, J.; Sugaya, K.; Hirano, M.; Momose, Y.; Yamamura, S. New medicinal treatment for severe gingivostomatitis. Int. J. Mol. Med. 1998, 2, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Jones, K.S. Effect of alginate on innate immune activation of macrophages. J. Biomed. Mater. Res. A 2009, 90, 411–418. [Google Scholar] [CrossRef]
- Son, E.H.; Moon, E.Y.; Rhee, D.K.; Pyo, S. Stimulation of various functions in murine peritoneal macrophages by high mannuronic acid-containing alginate (HMA) exposure in vivo. Int. Immunopharmacol. 2001, 1, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Otterlei, M.; Ostgaard, K.; Skjåk-Braek, G.; Smidsrød, O.; Soon-Shiong, P.; Espevik, T. Induction of cytokine production from human monocytes stimulated with alginate. J. Immunother. 1991, 10, 286–291. [Google Scholar] [CrossRef]
- Takahashi, K.; Watanuki, Y.; Yamazaki, M.; Abe, S. Local induction of a cytotoxic factor in a murine tumor by systemic administration of an antitumor polysaccharide, MGA. Br. J. Cancer 1988, 57, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Mirshafiey, A.; Rehm, B.H. Alginate and its comonomer mannuronic acid: Medical relevance as drugs. In Alginates: Biology and Applications, 1st ed.; Rehm, B.H., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 229–260. [Google Scholar]
- Mirshafiey, A.; Khodadadi, A.; Rehm, B.H.; Khorramizadeh, M.R.; Eslami, M.B.; Razavi, A.; Saadat, F. Sodium alginate as a novel therapeutic option in experimental colitis. Scand. J. Immunol. 2005, 64, 316–321. [Google Scholar] [CrossRef]
- Razavi, A.; Khodadadi, A.; Eslami, M.B.; Eshraghi, S.; Mirshafiey, A. Therapeutic effect of sodium alginate in experimental chronic ulcerative colitis. Iran J. Allergy Asthma Immunol. 2008, 7, 13–18. [Google Scholar]
- Mirshafiey, A.; Borzooy, Z.; Abhari, R.S.; Razavi, A.; Tavangar, M.; Rehm, B.H. Treatment of experimental immune complex glomerulonephritis by sodium alginate. Vascul. Pharmacol. 2005, 43, 30–35. [Google Scholar] [CrossRef]
- De la Coba, F.; Aguilera, J.; Figueroa, F.L.; De Gálvez, M.V.; Herrera, E. Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. J. Appl. Phycol. 2009, 21, 161–169. [Google Scholar] [CrossRef]
- Sarithakumari, C.H.; Renju, G.L.; Kurup, G.M. Anti-inflammatory and antioxidant potential of alginic acid isolated from the marine algae, Sargassum wightii on adjuvant-induced arthritic rats. Inflammopharmacology 2013, 21, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.J.; Son, E.W.; Rhee, D.K.; Pyo, S. Modulation of tnf-α-induced icam-1 expression, no and h 2 0 2 production by alginate, allicin and ascorbic acid in human endothelial cells. Arch. Pharm. Res. 2003, 26, 244–251. [Google Scholar] [CrossRef]
- Asada, M.; Sugie, M.; Inoue, M.; Nakagomi, K.; Hongo, S.; Murata, K.; Irie, S.; Takeuchi, T.; Tomizuka, N.; Oka, S. Inhibitory effect of alginic acids on hyaluronidase and on histamine release from mast cells. Biosci. Biotechnol. Biochem. 1997, 61, 1030–1032. [Google Scholar] [CrossRef]
- Jeong, H.J.; Lee, S.A.; Moon, P.D.; Na, H.J.; Park, R.K.; Um, J.Y.; Kim, H.M.; Hong, S.H. Alginic acid has anti-anaphylactic effects and inhibits inflammatory cytokine expression via suppression of nuclear factor-κB activation. Clin. Exp. Allergy 2006, 36, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Ménard, M.; Dusseault, J.; Langlois, G.; Baille, W.E.; Tam, S.K.; Yahia, L.H.; Zhu, X.X.; Hallé, J.P. Role of protein contaminants in the immunogenicity of alginates. J. Biomed. Mater. 2010, 93, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.; Saez, C.A.; Brown, M.T.; Bitonti, M.B. A simple and effective method for high quality co-extraction of genomic DNA and total RNA from low biomass Ectocarpus siliculosus, the model brown alga. PLoS ONE 2014, 9, e96470. [Google Scholar] [CrossRef] [PubMed]
- Skjak-Brek, G.; Martinsen, A. Applications of some algal polysaccharides in biotechnology. In Seaweed Resources in Europe: Uses and Potentials, 1st ed.; Guiry, L., Bluden, G., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 1991; pp. 219–259. [Google Scholar]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef] [PubMed]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, A.M.; Rocha, N.F.; Vasconcelos, L.F.; Rios, E.R.; Dias, M.L.; Silva, M.I.; de França Fonteles, M.M.; Filho, J.M.; Gutierrez, S.J.; de Sousa, F.C. Evaluation of the anti-inflammatory activity of riparin II (O-methil-N-2-hidroxi-benzoyl tyramine) in animal models. Chem. Biol. Interact. 2013, 205, 165–172. [Google Scholar] [CrossRef] [PubMed]


| Sample | ExtractionYield (%) | Neutral Sugars (%, w/w) | Uronic Acid (%, w/w) | Sulfates (%, w/w) | Total Polyphenols (%) | Protein (%) |
|---|---|---|---|---|---|---|
| C. crinita alginate | 20.18 ± 1.72 | 19.66 ± 1.05 | 50.14 ± 1.12 | 0.63 ± 0.02 | <0.10 | <0.04 |
| Fraction | FG | FM | FGG | FGM or FMG | FMM | M/G |
|---|---|---|---|---|---|---|
| Alginate | 0.495 | 0.505 | 0.280 | 0.216 | 0.289 | 1.018 |
| Time Point | 5th Minute | 15th Minute | 30th Minute | 60th Minute | 90th Minute | 120th Minute | |
|---|---|---|---|---|---|---|---|
| Groups | |||||||
| Controls | Mean (%) | 31.94 | 50.72 | 59.94 | 52.32 | 43.44 | 38.58 |
| SEM | 2.23 | 4.05 | 3.85 | 2.98 | 4.52 | 5.03 | |
| diclofenac 25 mg/kg | Mean (%) | 12.57 ** | 19.25 *** | 20.48 *** | 18.05 *** | 15.24 *** | 12.90 *** |
| SEM | 1.73 | 1.50 | 2.13 | 1.91 | 2.40 | 2.22 | |
| alginate standard 100 mg/kg | Mean (%) | 19.77 | 28.73 ** | 27.72 *** | 18.19 *** | 15.53 *** | 15.23 *** |
| SEM | 2.09 | 4.26 | 3.34 | 2.79 | 3.01 | 3.46 | |
| alginate test 25 mg/kg | Mean (%) | 17.39 * | 21.23 *** | 24.64 *** | 10.73 *** | 7.04 *** | 6.10 *** |
| SEM | 4.68 | 3.57 | 3.43 | 1.70 | 1.47 | 1.85 | |
| alginate test 100 mg/kg | Mean (%) | 21.31 | 28.99 ** | 25.46 *** | 12.16 *** | 10.37 *** | 8.27 *** |
| SEM | 4.49 | 3.75 | 5.46 | 2.83 | 3.06 | 2.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokova, V.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Delattre, C.; Molinié, R.; Petit, E.; Elboutachfaiti, R.; Murdjeva, M.; Apostolova, E. Extraction, Structural Characterization, and In Vivo Anti-Inflammatory Effect of Alginate from Cystoseira crinita (Desf.) Borry Harvested in the Bulgarian Black Sea. Mar. Drugs 2023, 21, 245. https://doi.org/10.3390/md21040245
Kokova V, Lukova P, Baldzhieva A, Katsarov P, Delattre C, Molinié R, Petit E, Elboutachfaiti R, Murdjeva M, Apostolova E. Extraction, Structural Characterization, and In Vivo Anti-Inflammatory Effect of Alginate from Cystoseira crinita (Desf.) Borry Harvested in the Bulgarian Black Sea. Marine Drugs. 2023; 21(4):245. https://doi.org/10.3390/md21040245
Chicago/Turabian StyleKokova, Vesela, Paolina Lukova, Alexandra Baldzhieva, Plamen Katsarov, Cédric Delattre, Roland Molinié, Emmanuel Petit, Redouan Elboutachfaiti, Marianna Murdjeva, and Elisaveta Apostolova. 2023. "Extraction, Structural Characterization, and In Vivo Anti-Inflammatory Effect of Alginate from Cystoseira crinita (Desf.) Borry Harvested in the Bulgarian Black Sea" Marine Drugs 21, no. 4: 245. https://doi.org/10.3390/md21040245
APA StyleKokova, V., Lukova, P., Baldzhieva, A., Katsarov, P., Delattre, C., Molinié, R., Petit, E., Elboutachfaiti, R., Murdjeva, M., & Apostolova, E. (2023). Extraction, Structural Characterization, and In Vivo Anti-Inflammatory Effect of Alginate from Cystoseira crinita (Desf.) Borry Harvested in the Bulgarian Black Sea. Marine Drugs, 21(4), 245. https://doi.org/10.3390/md21040245











