Overexpression of Global Regulator SCrp Leads to the Discovery of New Angucyclines in Streptomyces sp. XS-16
Abstract
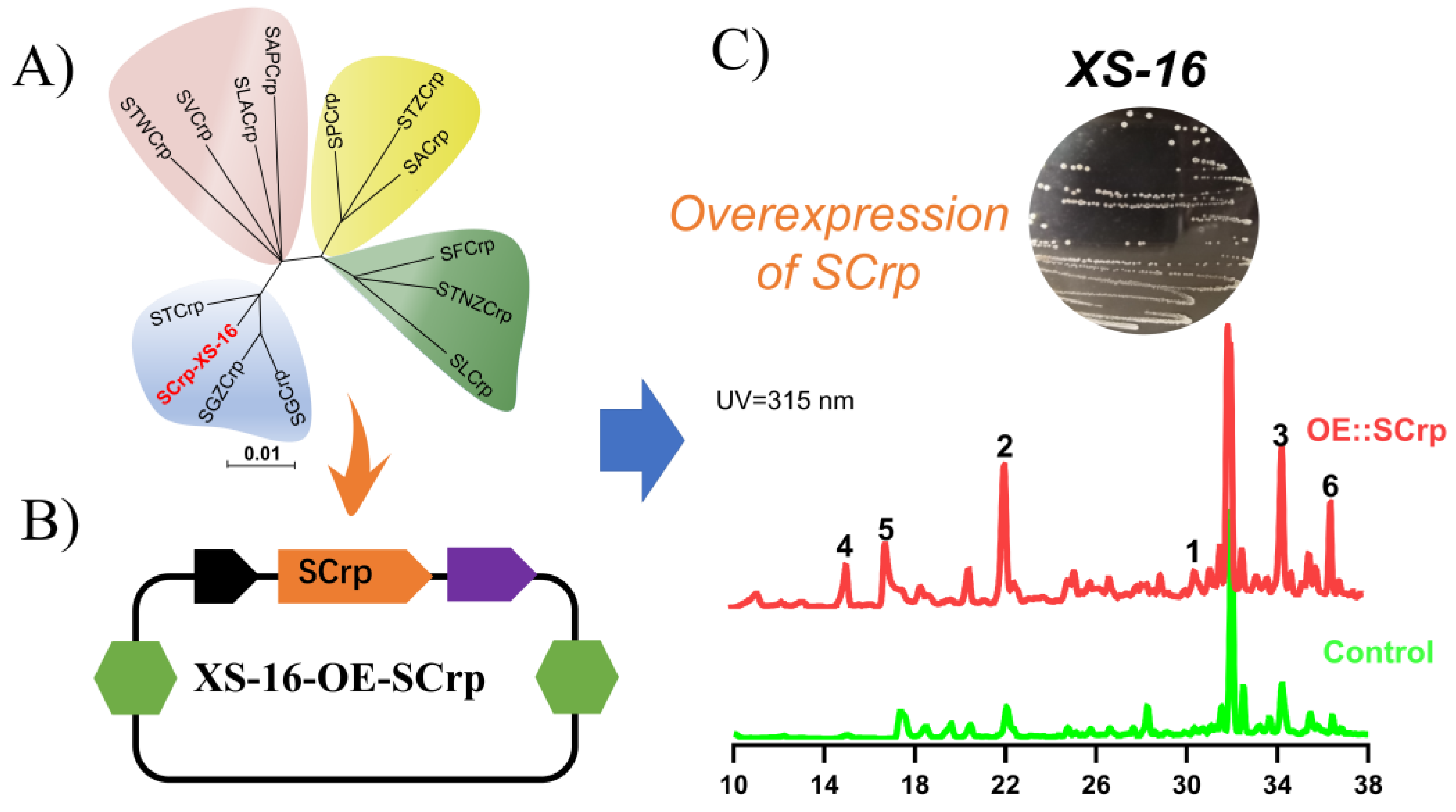
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Materials and Culture Conditions
3.3. Sequence Analysis of the SCrp Gene
3.4. Construction of the SCrp Expression Vector
3.5. Overexpression, Fermentation, and Isolation of Compounds 1–7 in Streptomyces sp. XS-16
3.6. Transformants Screening
3.7. Fermentation
3.8. Extraction and Purification
- Angumycinone E (1): pale yellowish powder; + 22.5 (c 0.03, MeOH); UV (DAD) λmax 210 nm, 235 nm, 364 nm; CD (MeOH) λmax (∆ε) 230 (16.32), 267 (−15.82), 324 (7.68); IR (KBr) νmax 3392, 2935, 1699, 1655, 1603, 1578, 1455, 1381, 1358, 1291, 1253, 1205, 1182, 1131, 1106, 1048, 1027, 1005, 976, 898, 801, 767, 730 cm−1. 1H and 13C NMR data, see Table 1; HRESIMS m/z 355.0824 [M − H]− (calcd. for C19H15O7, 355.0823).
- Angumycinone F (2): reddish powder; + 19.5 (c 0.03, MeOH); UV (DAD) λmax 221 nm, 270 nm, 421 nm; CD (MeOH) λmax (∆ε) 245 (33.27), 282 (−5.17), 301 (−3.54); IR (KBr) νmax 2922, 2852.87,1683, 1209, 1187,1134, 1079, 1033 cm−1. 1H and 13C NMR data, see Table 1; HRESIMS m/z 375.1066 [M + H]+ (calcd. for C19H19O8, 375.1074).
- Kanglemycin E (3): reddish powder; − 15.2 (c 0.03, MeOH); UV (DAD) λmax 205 nm, 234 nm, 363 nm; IR (KBr) νmax 3415.88, 2921.14, 1683.06, 1639.65, 1457.12, 1384.38, 1283.86, 1207.35, 1139.29, 1027.47, 838.46, 801.60, 722.46 cm−1. 1H and 13C NMR data, see Table 1; HRESIMS m/z 323.0908 [M + H]+ (calcd. for C19H15O5, 323.0914).
3.9. Computational Section
3.9.1. NMR Calculations
3.9.2. ECD Calculations
3.10. Cytotoxicity Assay
3.11. Antimicrobial Activity
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- De Lima Procópio, R.E.; da Silva, I.R.; Martins, M.K.; de Azevedo, J.L.; de Araújo, J.M. Antibiotics produced by Streptomyces. Braz. J. Infect. Dis. 2012, 16, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Alam, K.; Mazumder, A.; Sikdar, S.; Zhao, Y.M.; Hao, J.; Song, C.; Wang, Y.; Sarkar, R.; Islam, S.; Zhang, Y.; et al. Streptomyces: The biofactory of secondary metabolites. Front. Microbiol. 2022, 13, 968053–968073. [Google Scholar] [CrossRef] [PubMed]
- Doroghazi, J.R.; Metcalf, W.W. Comparative genomics of actinomycetes with a focus on natural product biosynthetic genes. BMC Genom. 2013, 14, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Seyedsayamdost, M.R. High-throughput platform for the discovery of elicitors of silent bacterial gene clusters. Proc. Natl. Acad. Sci. USA 2014, 111, 7266–7271. [Google Scholar] [CrossRef]
- Ye, S.; Brian, M.; Braña, A.F.; Daniel, Z.; Carlos, O.; Jesús, C.; Francisco, M.; Salas, J.A.; Carmen, M. Identification by genome mining of a Type I Polyketide Gene Cluster from Streptomyces argillaceus Involved in the Biosynthesis of Pyridine and Piperidine Alkaloids Argimycins P. Front. Microbiol. 2017, 8, 194–211. [Google Scholar] [CrossRef]
- Chen, R.; Wong, H.; Burns, B. New approaches to detect biosynthetic gene clusters in the Environment. Medicines 2019, 6, 32. [Google Scholar] [CrossRef]
- Bode, H.B.; Bethe, B.; Höfs, R.; Zeeck, A. Big effects from small changes: Possible ways to explore nature’s chemical diversity. ChemBioChem 2002, 3, 619–627. [Google Scholar] [CrossRef]
- Ochi, K.; Hosaka, T. New strategies for drug discovery: Activation of silent or weakly expressed microbial gene clusters. Appl. Microbiol. Biotechnol. 2013, 97, 87–98. [Google Scholar] [CrossRef]
- Lee, N.; Hwang, S.; Lee, Y.; Cho, S.; Palsson, B.; Cho, B.K. Synthetic biology tools for novel secondary metabolite discovery in Streptomyces. J. Microbiol. Biotechnol. 2019, 29, 667–686. [Google Scholar] [CrossRef]
- Kang, H.S.; Kim, E.S. Recent advances in heterologous expression of natural product biosynthetic gene clusters in Streptomyces hosts. Curr. Opin. Biotechnol. 2021, 69, 118–127. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, N.; Hwang, S.; Kim, W.; Lee, Y.; Cho, S.; Palsson, B.O.; Cho, B.K. Discovery of novel secondary metabolites encoded in actinomycete genomes through coculture. J. Ind. Microbiol. Biot. 2021, 48, kuaa001. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, Y.; Huang, C.; Luo, Y. Recent advances in silent gene cluster activation in Streptomyces. Front. Bioeng. Biotechnol. 2021, 9, 632230. [Google Scholar] [CrossRef] [PubMed]
- Bibb, M.J. Regulation of secondary metabolism in Streptomycetes. Curr. Opin. Microbiol. 2005, 8, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Zhan, X.; Mao, X.M.; Li, Y.Q. The regulatory cascades of antibiotic production in Streptomyces. J. Microbiol. Biotechnol. 2020, 36, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Li, X.; Li, Z.; Zhan, X.; Mao, X.; Li, Y. The application of regulatory cascades in Streptomyces: Yield enhancement and metabolite mining. Front. Microbiol. 2020, 11, 406–419. [Google Scholar] [CrossRef]
- Shimada, T.; Fujita, N.; Yamamoto, K.; Ishihama, A. Novel roles of cAMP receptor protein (CRP) in regulation of transport and metabolism of carbon sources. PLoS ONE 2011, 6, e20081–e20092. [Google Scholar] [CrossRef]
- Derouaux, A.; Dehareng, D.; Lecocq, E.; Halici, S.; Nothaft, H.; Giannotta, F.; Moutzourelis, G.; Dusart, J.; Devreese, B.; Titgemeyer, F.; et al. Crp of Streptomyces coelicolor is the third transcription factor of the large CRP-FNR superfamily able to bind cAMP. Biochem. Biophys. Res. Commun. 2004, 325, 983–990. [Google Scholar] [CrossRef]
- Derouaux, A.; Halici, S.; Nothaft, H.; Neutelings, T.; Moutzourelis, G.; Dusart, J.; Titgemeyer, F.; Rigali, S. Deletion of a cyclic AMP receptor protein homologue diminishes germination and affects morphological development of Streptomyces coelicolor. J. Bacteriol. 2004, 186, 1893–1897. [Google Scholar] [CrossRef]
- Piette, A.; Derouaux, A.; Gerkens, P.; Noens, E.E.E.; Mazzucchelli, G.; Vion, S.; Koerten, H.K.; Titgemeyer, F.; De Pauw, E.; Leprince, P.; et al. From dormant to germinating spores of Streptomyces coelicolor A3(2): New perspectives from the Crp null mutant. J. Proteome Res. 2005, 4, 1699–1708. [Google Scholar] [CrossRef]
- Gao, C.; Hindra; Mulder, D.; Yin, C.; Elliot, M.A. Crp is a global regulator of antibiotic production in Streptomyces. mBio 2012, 3, e00407–e00412. [Google Scholar] [CrossRef]
- Lin, C.Y.; Zhang, Y.; Wu, J.H.; Xie, R.H.; Qiao, J.; Zhao, G.R. Regulatory patterns of Crp on monensin biosynthesis in Streptomyces cinnamonensis. Microorganisms 2020, 8, 271. [Google Scholar] [CrossRef]
- Wu, J.; Chen, D.; Wu, J.; Chu, X.; Yang, Y.; Fang, L.; Zhang, W. Comparative transcriptome analysis demonstrates the positive effect of the cyclic AMP receptor protein Crp on daptomycin biosynthesis in Streptomyces roseosporus. Front. Bioeng. Biotechnol. 2021, 9, 618029. [Google Scholar] [CrossRef] [PubMed]
- Su, B.N.; Chang, L.C.; Park, E.J.; Cuendet, M.; Santarsiero, B.D.; Mesecar, A.D.; Mehta, R.G.; Fong, H.H.S.; Pezzuto, J.M.; Kinghorn, A.D. Bioactive constituents of the seeds of Brucea javanica. Planta Med. 2002, 68, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Z.; Yan, G.H.; Chen, W.H.; Yang, X.M. Microwave-assisted efficient synthesis of 2-hydroxydeoxybenzoins from the alkali degradation of readily prepared 3-Aryl-4-hydroxycoumarins in water. Chem. Pharm. Bull. 2013, 61, 1166–1172. [Google Scholar] [CrossRef]
- Matilla, M.A.; Velando, F.; Martín-Mora, D.; Monteagudo-Cascales, E.; Krell, T. A catalogue of signal molecules that interact with sensor kinases, chemoreceptors and transcriptional regulators. FEMS Microbiol. Rev. 2021, 46, fuab04. [Google Scholar] [CrossRef]
- Huffman, J.L.; Brennan, R.G. Prokaryotic transcription regulators: More than just the helix-turn-helix motif. Curr. Opin. Struct. Biol. 2002, 12, 98–106. [Google Scholar] [CrossRef]
- Rohr, J.; Thiericke, R. Angucycline group antibiotics. Nat. Prod. Rep. 1992, 9, 103–137. [Google Scholar] [CrossRef]
- Künzel, E.; Faust, B.; Oelkers, C.; Weissbach, U.; Bearden, D.W.; Weitnauer, G.; Westrich, L.; Bechthold, A.; Rohr, J. Inactivation of the urdGT2 Gene, which encodes a glycosyltransferase responsible for the C-glycosyltransfer of activated D-olivose, leads to formation of the novel urdamycins I, J, and K. J. Am. Chem. Soc. 1999, 121, 11058–11062. [Google Scholar] [CrossRef]
- Brown, P.M.; Thomson, R.H. Naturally occurring quinones. Part XXVI. A synthesis of tetrangulol (1,8-dihydroxy-3-methylbenz[a]anthracene-7,12-quinone). J. Chem. Soc. 1976, 9, 997–1000. [Google Scholar]
- Dann, M.; Lefemine, D.V.; Barbatschi, F.; Shu, P.; Kunstmann, M.P.; Mitscher, L.A.; Bohonos, N. Tetrangomycin, a new quinone antibiotic. Antimicrob. Agents Chemother 1965, 5, 832–835. [Google Scholar]
- Kaliappan, K.P.; Ravikumar, V. Angucyclinone antibiotics: Total syntheses of YM-181741, (+)-ochromycinone, (+)-rubiginone B2, (−)-tetrangomycin, and MM-47755. J. Org. Chem 2007, 72, 6116–6126. [Google Scholar] [CrossRef] [PubMed]
- Lombó, F.; Abdelfattah, M.S.; Braña, A.F.; Salas, J.A.; Rohr, J.; Méndez, C. Elucidation of oxygenation steps during oviedomycin biosynthesis and generation of derivatives with increased antitumor activity. ChemBioChem 2009, 10, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Voitsekhovskaia, I.; Paulus, C.; Dahlem, C.; Rebets, Y.; Nadmid, S.; Zapp, J.; Axenov-Gribanov, D.; Rückert, C.; Timofeyev, M.; Kalinowski, J.; et al. Baikalomycins A-C, new aquayamycin-type angucyclines isolated from Lake Baikal derived Streptomyces sp. IB201691-2A. Microorganisms 2020, 8, 680. [Google Scholar] [CrossRef]
- Sakai, K.; Koyama, N.; Fukuda, T.; Mori, Y.; Onaka, H.; Tomoda, H. Search method for inhibitors of staphyloxanthin production by methicillin-resistant Staphylococcus aureus. Biol. Pharm. Bull. 2012, 35, 48–53. [Google Scholar] [CrossRef]
- Wu, Q.; Chang, Y.; Che, Q.; Li, D.; Zhang, G.; Zhu, T. Citreobenzofuran D-F and phomenone A-B: Five novel sesquiterpenoids from the mangrove-derived fungus Penicillium sp. HDN13-494. Mar. Drugs 2022, 20, 137. [Google Scholar] [CrossRef]
- Mcwilliam, H.; Valentin, F.; Wallace, I.M.; Chenna, R.; Lopez, R.; Brown, N.P.; Mcgettigan, P.A.; Larkin, M.A.; Wilm, A.; Blackshields, G. Clustal W and clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar]
- Sudhir, K.; Glen, S.; Koichiro, T. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar]
- Mitchell, A.L.; Attwood, T.K.; Babbitt, P.C.; Blum, M.; Bork, P.; Bridge, A.; Brown, S.D.; Chang, H.Y.; El-Gebali, S.; Fraser, M.I.; et al. InterPro in 2019: Improving coverage, classification and access to protein sequence annotations. Nucleic Acids Res. 2019, 47, D351–D360. [Google Scholar] [CrossRef]
- Kieser, T.; Bibb, M.J.; Buttner, M.J.; Chater, K.F.; Hopwood, D.A.; Charter, K.; Bib, M.J.; Bipp, M.; Keiser, T.; Butner, M. Practical Streptomyces Genetics; John Innes Foundation: Norwich, UK, 2000. [Google Scholar]
- Marcarino, M.O.; Cicetti, S.; Zanardi, M.M.; Sarotti, A.M. A critical review on the use of DP4+ in the structural elucidation of natural products: The good, the bad and the ugly. A practical guide. Nat. Prod. Rep. 2022, 39, 58–76. [Google Scholar] [CrossRef]
- Yu, G.; Zhou, G.; Zhu, M.; Wang, W.; Zhu, T.; Gu, Q.; Li, D. Neosartoryadins A and B, fumiquinazoline alkaloids from a mangrove-derived fungus Neosartorya udagawae HDN13-313. Org. Lett. 2015, 18, 244–247. [Google Scholar] [CrossRef]
- Gao, C.; Guo, Z.; Lu, X.; Chen, H.; Liu, L.; Yu, Z.; Chen, Y. Hexaricins, pradimicin-like polyketides from a marine sediment-derived Streptosporangium sp. and their antioxidant effects. J. Nat. Prod. 2018, 81, 2069–2074. [Google Scholar] [CrossRef] [PubMed]
- Metsä-Ketelä, M.; Palmu, K.; Kunnari, T.; Ylihonko, K.; Mäntsälä, P. Engineering anthracycline biosynthesis toward angucyclines. Antimicrob. Agents Chemother. 2003, 47, 1291–1296. [Google Scholar] [CrossRef] [PubMed]




| No. | 1 a | 2 b | 3 b | |||
|---|---|---|---|---|---|---|
| δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | |
| 1 | 189.8, C | – | 192.8, C | – | 183.8, C | – |
| 1a | 131.9, C | – | 133.0, C | – | 133.5, C | – |
| 2 | 118.8, CH | 7.41, d, (7.6, 1.0) | 118.8, CH | 7.61, dd, (7.5, 1.1) | 118.4, CH | 7.48, dd, (7.5, 1.0) |
| 3 | 136.7, CH | 7.71, dd (8.4, 7.6) | 136.2, CH | 7.76, dd (8.4, 7.5) | 136.2, CH | 7.71, dd, (8.4, 7.5) |
| 4 | 124.3, CH | 7.34, dd (8.4, 1.0) | 123.6, CH | 7.35, dd (8.4, 1.1) | 123.2, CH | 7.31, dd, (8.4, 1.0) |
| 5 | 159.8, C | – | 159.9, C | – | 159.8, C | – |
| 5a | 114.3, C | – | 116.3, C | – | 114.6, C | – |
| 6 | 194.0, C | – | 194.1, C | – | 188.2, C | – |
| 6a | 72.8, C | – | 77.6, C | – | 145.3, C | – |
| 7α | 17.4, CH2 | 2.80, m | 21.6, CH2 | 2.28, m | 21.0, CH2 | 2.63, m |
| 7β | 2.27, m | 1.77, m | ||||
| 8α | 27.5, CH2 | 1.82, m | 28.2, CH2 | 2.01, m | 19.8, CH2 | 2.52, m |
| 8β | 1.49, m | 1.61, m | ||||
| 8a | 63.6, C | – | 75.4, C | – | 147.6, C | – |
| 9α | 41.5, CH2 | 2.70, m | 41.8, CH2 | 2.90, d, (18.0) | 139.2, C | – |
| 9β | 2.24, m | 2.07, d (18.0) | ||||
| 10 | 159.2, C | – | 158.2, C | – | 127.8, C | – |
| 11 | 121.7, CH | 5.91, s | 122.8, CH | 5.72, s | 115.7, CH | 6.58, s |
| 12 | 195.5, C | – | 200.5, C | – | 129.2, C | – |
| 12a | 109.5, C | – | 109.5 C | – | 121.2, C | – |
| 12b | 75.2, C | – | 76.2, C | – | 142.9, C | – |
| 13 | 23.4, CH3 | 1.86, s | 23.6, CH3 | 1.94, s | 22.4, CH3 | 1.98, s |
| 5-OH | – | 11.16, s | – | 11.11, s | – | 12.04, s |
| 9-OH | – | – | – | – | – | 9.80, s |
| 10-OH | – | – | – | – | – | 8.40, s |
| Compounds | Inhibition Ratio | IC50 (μM) | ||||
|---|---|---|---|---|---|---|
| L-02 | MDA-MB-231 | K562 | ASPC-1 | H69AR | H69 | |
| 1 | 10.01% | 4.91 | 4.85 | 5.33 | 0.59 | 0.32 |
| 2 | 3.83% | >50 | >25 | >50 | >50 | >50 |
| 3 | 0.15% | >50 | >50 | >50 | >50 | >50 |
| Adriamycin | 89.62% | 0.31 | 0.24 | 0.22 | 0.52 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Zhang, F.; Zhou, L.; Chang, Y.; Che, Q.; Zhu, T.; Li, D.; Zhang, G. Overexpression of Global Regulator SCrp Leads to the Discovery of New Angucyclines in Streptomyces sp. XS-16. Mar. Drugs 2023, 21, 240. https://doi.org/10.3390/md21040240
Xu X, Zhang F, Zhou L, Chang Y, Che Q, Zhu T, Li D, Zhang G. Overexpression of Global Regulator SCrp Leads to the Discovery of New Angucyclines in Streptomyces sp. XS-16. Marine Drugs. 2023; 21(4):240. https://doi.org/10.3390/md21040240
Chicago/Turabian StyleXu, Xiao, Falei Zhang, Luning Zhou, Yimin Chang, Qian Che, Tianjiao Zhu, Dehai Li, and Guojian Zhang. 2023. "Overexpression of Global Regulator SCrp Leads to the Discovery of New Angucyclines in Streptomyces sp. XS-16" Marine Drugs 21, no. 4: 240. https://doi.org/10.3390/md21040240
APA StyleXu, X., Zhang, F., Zhou, L., Chang, Y., Che, Q., Zhu, T., Li, D., & Zhang, G. (2023). Overexpression of Global Regulator SCrp Leads to the Discovery of New Angucyclines in Streptomyces sp. XS-16. Marine Drugs, 21(4), 240. https://doi.org/10.3390/md21040240








