Echinochrome A Prevents Diabetic Nephropathy by Inhibiting the PKC-Iota Pathway and Enhancing Renal Mitochondrial Function in db/db Mice
Abstract
1. Introduction
2. Results
2.1. EchA Reduced Blood Urea Nitrogen and Creatinine Levels and Improved Blood Glucose Tolerance in db/db Mice
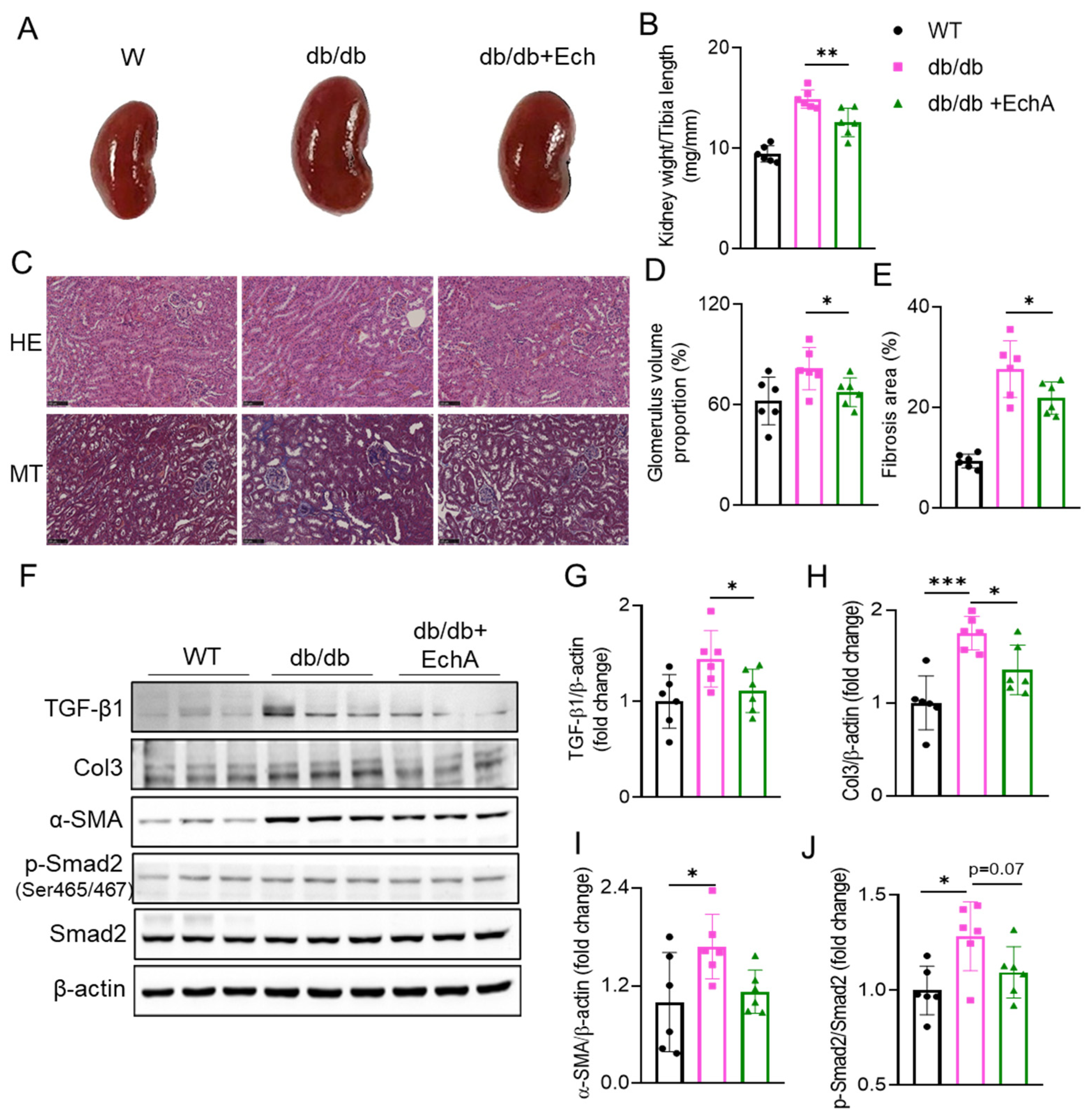
2.2. EchA Treatment Attenuated Renal Hypertrophy and Fibrosis in db/db Mice
2.3. EchA Ameliorated Renal Oxidative Stress in Diabetic Mice
2.4. EchA Activated AMPK Phosphorylation and the NRF2/HO-1 Pathway to Improve Mitochondrial Function and Increase ATP Production in Diabetic Kidneys
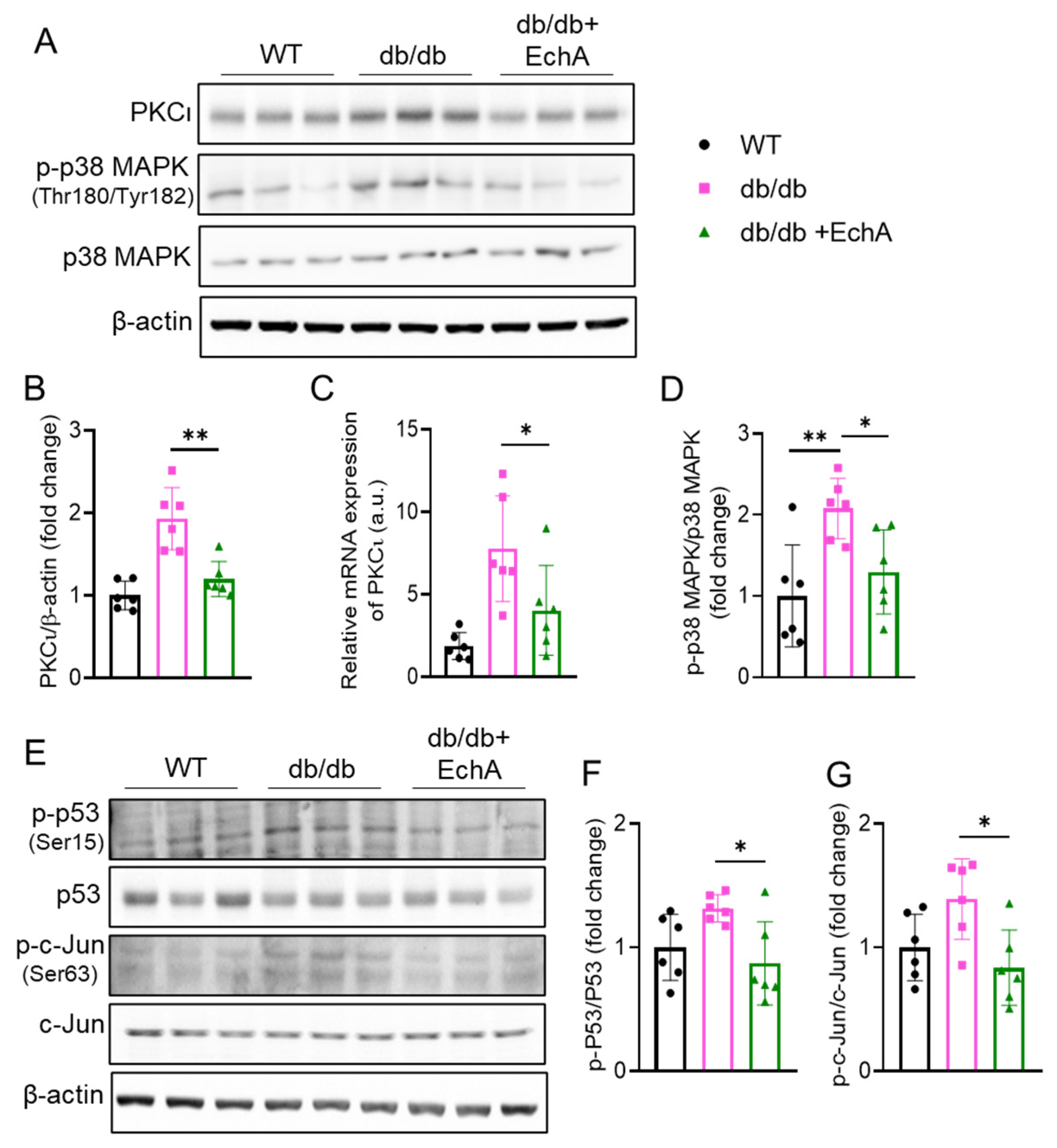
2.5. EchA Inhibited PKCι/p38MAPK Activation and Downregulated Both p53 and c-Jun Phosphorylation in Diabetic Kidneys
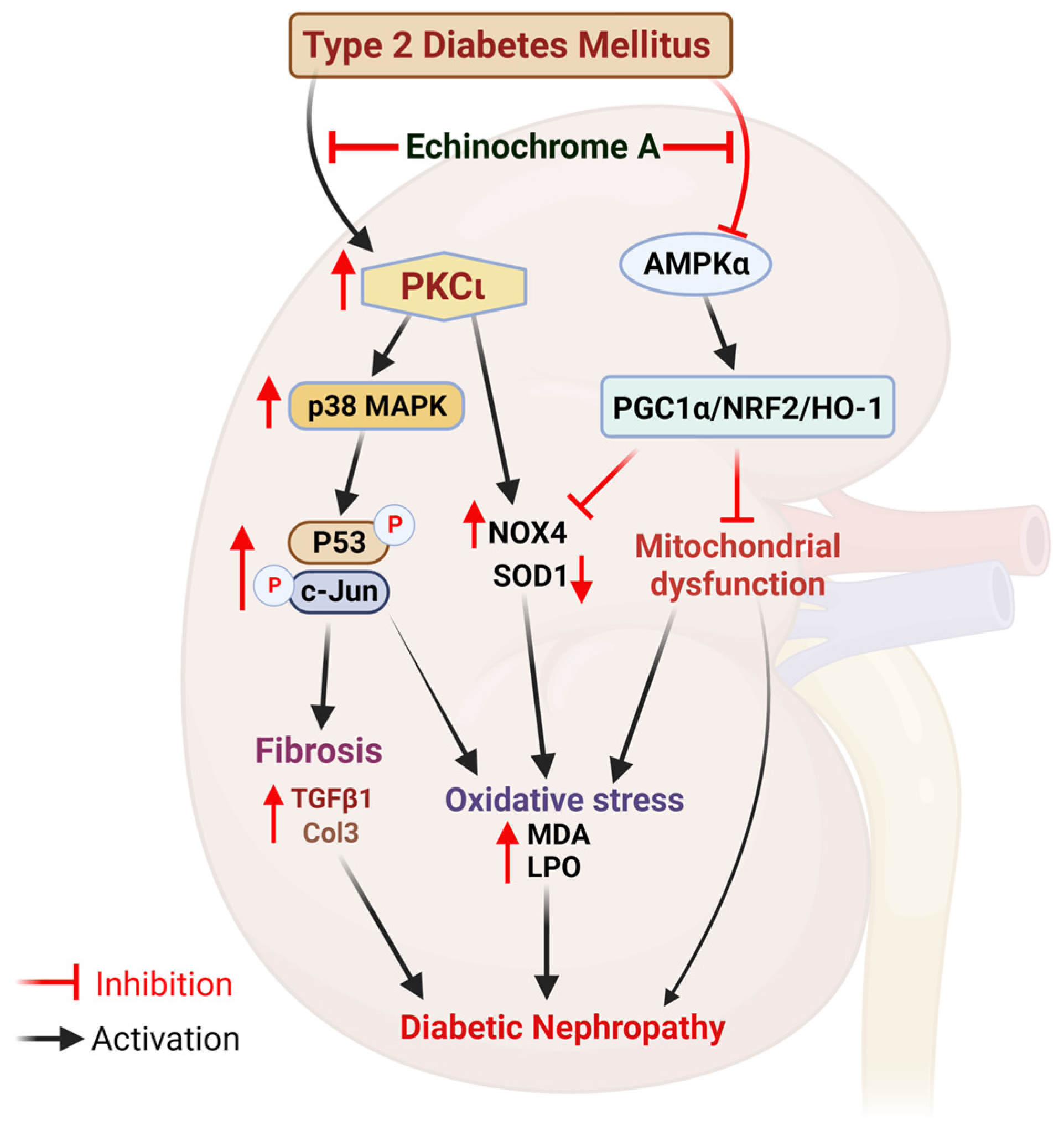
3. Discussion
4. Materials and Methods
4.1. Preparation of Echinochrome A
4.2. Animal Experiments
4.3. Fasting Blood Glucose and Glucose Tolerance Test
4.4. Measurement of Blood Creatinine, BUN, and Insulin Levels
4.5. Histological Analyses
4.6. Measurement of Kidney ATP Levels
4.7. Measurement of Kidney Malondialdehyde (MDA) and Lipid Hydroperoxide (LPO) Levels
4.8. Western Blotting Analysis
4.9. Quantitative Real-time PCR (RT-PCR)
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martin, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Ghaderian, S.B.; Hayati, F.; Shayanpour, S.; Beladi Mousavi, S.S. Diabetes and end-stage renal disease; a review article on new concepts. J. Ren. Inj. Prev. 2015, 4, 28–33. [Google Scholar]
- Afkarian, M.; Sachs, M.C.; Kestenbaum, B.; Hirsch, I.B.; Tuttle, K.R.; Himmelfarb, J.; de Boer, I.H. Kidney disease and increased mortality risk in type 2 diabetes. J. Am. Soc. Nephrol. JASN 2013, 24, 302–308. [Google Scholar] [CrossRef]
- Zhao, L.; Zou, Y.; Liu, F. Transforming Growth Factor-Beta1 in Diabetic Kidney Disease. Front. Cell Dev. Biol. 2020, 8, 187. [Google Scholar] [CrossRef]
- Umanath, K.; Lewis, J.B. Update on Diabetic Nephropathy: Core Curriculum 2018. Am. J. Kidney Dis. J. Natl. Kidney Found. 2018, 71, 884–895. [Google Scholar] [CrossRef]
- Tonneijck, L.; Muskiet, M.H.; Smits, M.M.; van Bommel, E.J.; Heerspink, H.J.; van Raalte, D.H.; Joles, J.A. Glomerular Hyperfiltration in Diabetes: Mechanisms, Clinical Significance, and Treatment. J. Am. Soc. Nephrol. JASN 2017, 28, 1023–1039. [Google Scholar] [CrossRef]
- Lovisa, S.; Zeisberg, M.; Kalluri, R. Partial Epithelial-to-Mesenchymal Transition and Other New Mechanisms of Kidney Fibrosis. Trends Endocrinol. Metab. 2016, 27, 681–695. [Google Scholar] [CrossRef]
- Sagoo, M.K.; Gnudi, L. Diabetic nephropathy: Is there a role for oxidative stress? Free Radic. Biol. Med. 2018, 116, 50–63. [Google Scholar] [CrossRef]
- Singh, D.K.; Winocour, P.; Farrington, K. Oxidative stress in early diabetic nephropathy: Fueling the fire. Nat. Rev. Endocrinol. 2011, 7, 176–184. [Google Scholar] [CrossRef]
- Firidin, G. Oxidative Stress Parameters, Induction of Lipid Peroxidation, and ATPase Activity in the Liver and Kidney of Oreochromis niloticus Exposed to Lead and Mixtures of Lead and Zinc. Bull. Environ. Contam. Toxicol. 2018, 100, 477–484. [Google Scholar] [CrossRef]
- Chen, X.; Fang, M. Oxidative stress mediated mitochondrial damage plays roles in pathogenesis of diabetic nephropathy rat. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5248–5254. [Google Scholar]
- Ozbek, E. Induction of oxidative stress in kidney. Int. J. Nephrol. 2012, 2012, 465897. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; Lee, K.; Liu, B.; Deng, Y.; Chen, Y.; Zhang, X.; He, J.C.; Zhong, Y. Puerarin attenuates diabetic kidney injury through the suppression of NOX4 expression in podocytes. Sci. Rep. 2017, 7, 14603. [Google Scholar] [CrossRef]
- Palicz, A.; Foubert, T.R.; Jesaitis, A.J.; Marodi, L.; McPhail, L.C. Phosphatidic acid and diacylglycerol directly activate NADPH oxidase by interacting with enzyme components. J. Biol. Chem. 2001, 276, 3090–3097. [Google Scholar] [CrossRef]
- Barnes, J.L.; Gorin, Y. Myofibroblast differentiation during fibrosis: Role of NAD(P)H oxidases. Kidney Int. 2011, 79, 944–956. [Google Scholar] [CrossRef]
- Xia, L.; Wang, H.; Goldberg, H.J.; Munk, S.; Fantus, I.G.; Whiteside, C.I. Mesangial cell NADPH oxidase upregulation in high glucose is protein kinase C dependent and required for collagen IV expression. Am. J. Physiol. Ren. Physiol. 2006, 290, F345–F356. [Google Scholar] [CrossRef]
- Pan, D.; Xu, L.; Guo, M. The role of protein kinase C in diabetic microvascular complications. Front. Endocrinol. 2022, 13, 973058. [Google Scholar] [CrossRef]
- Qin, J.; Peng, Z.; Yuan, Q.; Li, Q.; Peng, Y.; Wen, R.; Hu, Z.; Liu, J.; Xia, X.; Deng, H.; et al. AKF-PD alleviates diabetic nephropathy via blocking the RAGE/AGEs/NOX and PKC/NOX Pathways. Sci. Rep. 2019, 9, 4407. [Google Scholar] [CrossRef]
- Xiao, Y.H.; He, X.Y.; Han, Q.; Yang, F.; Zhou, S.X. Atorvastatin prevents glomerular extracellular matrix formation by interfering with the PKC signaling pathway. Mol. Med. Rep. 2018, 17, 6441–6448. [Google Scholar] [CrossRef]
- Meier, M.; Park, J.-K.; Overheu, D.; Kirsch, T.; Lindschau, C.; Gueler, F.; Leitges, M.; Menne, J.; Haller, H. Deletion of Protein Kinase C-β Isoform In Vivo Reduces Renal Hypertrophy but Not Albuminuria in the Streptozotocin-Induced Diabetic Mouse Model. Diabetes 2007, 56, 346–354. [Google Scholar] [CrossRef]
- Li, J.; Gobe, G. Protein kinase C activation and its role in kidney disease. Nephrology 2006, 11, 428–434. [Google Scholar] [CrossRef]
- Kim, H.K.; Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A.; Han, J. Multifaceted Clinical Effects of Echinochrome. Mar. Drugs 2021, 19, 412. [Google Scholar] [CrossRef]
- Artyukov, A.A.; Zelepuga, E.A.; Bogdanovich, L.N.; Lupach, N.M.; Novikov, V.L.; Rutckova, T.A.; Kozlovskaya, E.P. Marine Polyhydroxynaphthoquinone, Echinochrome A: Prevention of Atherosclerotic Inflammation and Probable Molecular Targets. J. Clin. Med. 2020, 9, 1494. [Google Scholar] [CrossRef]
- Mohamed, A.S.; Sadek, S.A.; Hassanein, S.S.; Soliman, A.M. Hepatoprotective Effect of Echinochrome Pigment in Septic Rats. J. Surg. Res. 2019, 234, 317–324. [Google Scholar] [CrossRef]
- Kim, R.; Hur, D.; Kim, H.K.; Han, J.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Chang, W. Echinochrome A Attenuates Cerebral Ischemic Injury through Regulation of Cell Survival after Middle Cerebral Artery Occlusion in Rat. Mar. Drugs 2019, 17, 501. [Google Scholar] [CrossRef]
- Lebedev, A.V.; Ivanova, M.V.; Levitsky, D.O. Echinochrome, a naturally occurring iron chelator and free radical scavenger in artificial and natural membrane systems. Life Sci. 2005, 76, 863–875. [Google Scholar] [CrossRef]
- Buĭmov, G.A.; Maksimov, I.V.; Perchatkin, V.A.; Repin, A.N.; Afanas’ev, S.A.; Markov, V.A.; Karpov, R.S. Effect of the bioantioxidant histochrome on myocardial injury in reperfusion therapy on patients with myocardial infarction. Ter. Arkh. 2002, 74, 12–16. [Google Scholar]
- Egorov, E.A.; Alekhina, V.A.; Volobueva, T.M.; Fedoreev, S.A.; Mishchenko, N.P.; Kol’tsova, E.A. Histochrome, a new antioxidant, in the treatment of ocular diseases. Vestn Oftalmol. 1999, 115, 34–35. [Google Scholar]
- Yun, H.R.; Ahn, S.W.; Seol, B.; Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Han, J.; Ko, K.S.; Rhee, B.D.; et al. Echinochrome A Treatment Alleviates Atopic Dermatitis-like Skin Lesions in NC/Nga Mice via IL-4 and IL-13 Suppression. Mar. Drugs 2021, 19, 622. [Google Scholar] [CrossRef]
- Fedoreyev, S.A.; Krylova, N.V.; Mishchenko, N.P.; Vasileva, E.A.; Pislyagin, E.A.; Iunikhina, O.V.; Lavrov, V.F.; Svitich, O.A.; Ebralidze, L.K.; Leonova, G.N. Antiviral and Antioxidant Properties of Echinochrome A. Mar. Drugs 2018, 16, 509. [Google Scholar] [CrossRef]
- Cui, H.; Liu, J.; Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Zhang, Y. Echinochrome A Reverses Kidney Abnormality and Reduces Blood Pressure in a Rat Model of Preeclampsia. Mar. Drugs 2022, 20, 722. [Google Scholar] [CrossRef]
- Kim, H.K.; Cho, S.W.; Heo, H.J.; Jeong, S.H.; Kim, M.; Ko, K.S.; Rhee, B.D.; Mishchenko, N.P.; Vasileva, E.A.; Fedoreyev, S.A.; et al. A Novel Atypical PKC-Iota Inhibitor, Echinochrome A, Enhances Cardiomyocyte Differentiation from Mouse Embryonic Stem Cells. Mar. Drugs 2018, 16, 192. [Google Scholar] [CrossRef]
- Lee, S.R.; An, E.J.; Kim, J.; Bae, Y.S. Function of NADPH Oxidases in Diabetic Nephropathy and Development of Nox Inhibitors. Biomol. Ther. 2020, 28, 25–33. [Google Scholar] [CrossRef]
- Clark, A.J.; Parikh, S.M. Targeting energy pathways in kidney disease: The roles of sirtuins, AMPK, and PGC1α. Kidney Int. 2021, 99, 828–840. [Google Scholar] [CrossRef]
- Fukuda, R.; Suico, M.A.; Kai, Y.; Omachi, K.; Motomura, K.; Koga, T.; Komohara, Y.; Koyama, K.; Yokota, T.; Taura, M.; et al. Podocyte p53 Limits the Severity of Experimental Alport Syndrome. J. Am. Soc. Nephrol. JASN 2016, 27, 144–157. [Google Scholar] [CrossRef]
- Ying, Y.; Kim, J.; Westphal, S.N.; Long, K.E.; Padanilam, B.J. Targeted deletion of p53 in the proximal tubule prevents ischemic renal injury. J. Am. Soc. Nephrol. JASN 2014, 25, 2707–2716. [Google Scholar] [CrossRef]
- Overstreet, J.M.; Samarakoon, R.; Meldrum, K.K.; Higgins, P.J. Redox control of p53 in the transcriptional regulation of TGF-β1 target genes through SMAD cooperativity. Cell Signal. 2014, 26, 1427–1436. [Google Scholar] [CrossRef]
- Rayego-Mateos, S.; Morgado-Pascual, J.L.; Opazo-Ríos, L.; Guerrero-Hue, M.; García-Caballero, C.; Vázquez-Carballo, C.; Mas, S.; Sanz, A.B.; Herencia, C.; Mezzano, S.; et al. Pathogenic Pathways and Therapeutic Approaches Targeting Inflammation in Diabetic Nephropathy. Int. J. Mol. Sci. 2020, 21, 3798. [Google Scholar] [CrossRef]
- Sawaf, H.; Thomas, G.; Taliercio, J.J.; Nakhoul, G.; Vachharajani, T.J.; Mehdi, A. Therapeutic Advances in Diabetic Nephropathy. J. Clin. Med. 2022, 11, 378. [Google Scholar] [CrossRef]
- Selby, N.M.; Taal, M.W. An updated overview of diabetic nephropathy: Diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes. Metab. 2020, 22 (Suppl. 1), 3–15. [Google Scholar] [CrossRef]
- Thomas, M.C.; Brownlee, M.; Susztak, K.; Sharma, K.; Jandeleit-Dahm, K.A.M.; Zoungas, S.; Rossing, P.; Groop, P.-H.; Cooper, M.E. Diabetic kidney disease. Nat. Rev. Dis. Prim. 2015, 1, 15018. [Google Scholar] [CrossRef]
- Anderson, H.A.; Mathieson, J.W.; Thomson, R.H. Distribution of spinochrome pigments in echinoids. Comp. Biochem. Physiol. 1969, 28, 333–345. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, D.; Kang, X.; Zhou, R.; Sun, Y.; Lian, F.; Tong, X. Signaling Pathways Involved in Diabetic Renal Fibrosis. Front. Cell Dev. Biol. 2021, 9, 696542. [Google Scholar] [CrossRef]
- Song, B.W.; Kim, S.; Kim, R.; Jeong, S.; Moon, H.; Kim, H.; Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; et al. Regulation of Inflammation-Mediated Endothelial to Mesenchymal Transition with Echinochrome a for Improving Myocardial Dysfunction. Mar. Drugs 2022, 20, 756. [Google Scholar] [CrossRef]
- Park, G.T.; Yoon, J.W.; Yoo, S.B.; Song, Y.C.; Song, P.; Kim, H.K.; Han, J.; Bae, S.J.; Ha, K.T.; Mishchenko, N.P.; et al. Echinochrome A Treatment Alleviates Fibrosis and Inflammation in Bleomycin-Induced Scleroderma. Mar. Drugs 2021, 19, 237. [Google Scholar] [CrossRef]
- Lebed’ko, O.A.; Ryzhavskii, B.Y.; Demidova, O.V. Effect of Antioxidant Echinochrome A on Bleomycin-Induced Pulmonary Fibrosis. Bull. Exp. Biol. Med. 2015, 159, 351–354. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Jha, J.C.; Gray, S.P.; Barit, D.; Okabe, J.; El-Osta, A.; Namikoshi, T.; Thallas-Bonke, V.; Wingler, K.; Szyndralewiez, C.; Heitz, F.; et al. Genetic targeting or pharmacologic inhibition of NADPH oxidase nox4 provides renoprotection in long-term diabetic nephropathy. J. Am. Soc. Nephrol. JASN 2014, 25, 1237–1254. [Google Scholar] [CrossRef]
- Li, L.; Wang, C.; Yang, H.; Liu, S.; Lu, Y.; Fu, P.; Liu, J. Metabolomics reveal mitochondrial and fatty acid metabolism disorders that contribute to the development of DKD in T2DM patients. Mol. Biosyst. 2017, 13, 2392–2400. [Google Scholar] [CrossRef]
- Eid, A.A.; Ford, B.M.; Block, K.; Kasinath, B.S.; Gorin, Y.; Ghosh-Choudhury, G.; Barnes, J.L.; Abboud, H.E. AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J. Biol. Chem. 2010, 285, 37503–37512. [Google Scholar] [CrossRef]
- Bullon, P.; Marin-Aguilar, F.; Roman-Malo, L. AMPK/Mitochondria in Metabolic Diseases. Exp. Suppl. 2016, 107, 129–152. [Google Scholar]
- Wan, Z.; Root-McCaig, J.; Castellani, L.; Kemp, B.E.; Steinberg, G.R.; Wright, D.C. Evidence for the role of AMPK in regulating PGC-1 alpha expression and mitochondrial proteins in mouse epididymal adipose tissue. Obesity 2014, 22, 730–738. [Google Scholar] [CrossRef]
- Younis, N.N.; Elsherbiny, N.M.; Shaheen, M.A.; Elseweidy, M.M. Modulation of NADPH oxidase and Nrf2/HO-1 pathway by vanillin in cisplatin-induced nephrotoxicity in rats. J. Pharm. Pharm. 2020, 72, 1546–1555. [Google Scholar] [CrossRef]
- Aladaileh, S.H.; Hussein, O.E.; Abukhalil, M.H.; Saghir, S.A.M.; Bin-Jumah, M.; Alfwuaires, M.A.; Germoush, M.O.; Almaiman, A.A.; Mahmoud, A.M. Formononetin Upregulates Nrf2/HO-1 Signaling and Prevents Oxidative Stress, Inflammation, and Kidney Injury in Methotrexate-Induced Rats. Antioxidants 2019, 8, 430. [Google Scholar] [CrossRef]
- Teng, B.; Duong, M.; Tossidou, I.; Yu, X.; Schiffer, M. Role of protein kinase C in podocytes and development of glomerular damage in diabetic nephropathy. Front. Endocrinol. 2014, 5, 179. [Google Scholar] [CrossRef]
- Tossidou, I.; Starker, G.; Kruger, J.; Meier, M.; Leitges, M.; Haller, H.; Schiffer, M. PKC-alpha modulates TGF-beta signaling and impairs podocyte survival. Cell Physiol. Biochem. 2009, 24, 627–634. [Google Scholar] [CrossRef]
- Meier, M.; Menne, J.; Park, J.K.; Holtz, M.; Gueler, F.; Kirsch, T.; Schiffer, M.; Mengel, M.; Lindschau, C.; Leitges, M.; et al. Deletion of protein kinase C-epsilon signaling pathway induces glomerulosclerosis and tubulointerstitial fibrosis in vivo. J. Am. Soc. Nephrol. JASN 2007, 18, 1190–1198. [Google Scholar] [CrossRef]
- Ma, F.Y.; Flanc, R.S.; Tesch, G.H.; Han, Y.; Atkins, R.C.; Bennett, B.L.; Friedman, G.C.; Fan, J.H.; Nikolic-Paterson, D.J. A pathogenic role for c-Jun amino-terminal kinase signaling in renal fibrosis and tubular cell apoptosis. J. Am. Soc. Nephrol. JASN 2007, 18, 472–484. [Google Scholar] [CrossRef]
- Chuang, L.Y.; Guh, J.Y.; Liu, S.F.; Hung, M.Y.; Liao, T.N.; Chiang, T.A.; Huang, J.S.; Huang, Y.L.; Lin, C.F.; Yang, Y.L. Regulation of type II transforming-growth-factor-beta receptors by protein kinase C iota. Biochem. J. 2003, 375 Pt 2, 385–393. [Google Scholar] [CrossRef]
- Jha, J.C.; Thallas-Bonke, V.; Banal, C.; Gray, S.P.; Chow, B.S.; Ramm, G.; Quaggin, S.E.; Cooper, M.E.; Schmidt, H.H.; Jandeleit-Dahm, K.A. Podocyte-specific Nox4 deletion affords renoprotection in a mouse model of diabetic nephropathy. Diabetologia 2016, 59, 379–389. [Google Scholar] [CrossRef]
- Mischenko, N.P.; Fedoreyev, S.A.; Pokhilo, N.D.; Anufriev, V.P.; Denisenko, V.A.; Glazunov, V.P. Echinamines A and B, first aminated hydroxynaphthazarins from the sea urchin Scaphechinus mirabilis. J. Nat. Prod. 2005, 68, 1390–1393. [Google Scholar] [CrossRef]
- Vasileva, E.A.; Mishchenko, N.P.; Tran, V.T.T.; Vo, H.M.N.; Fedoreyev, S.A. Spinochrome Identification and Quantification in Pacific Sea Urchin Shells, Coelomic Fluid and Eggs Using HPLC-DAD-MS. Mar. Drugs 2021, 19, 21. [Google Scholar] [CrossRef]
- Mishchenko, N.P.; Fedoreev, S.A.; Glazunov, V.P.; Denisenko, V.A.; Krasovskaya, N.P.; Glebko, L.I.; Maslov, L.G.; Dmitrenok, P.S.; Bagirova, V.L. Isolation and Identification of Impurities in the Parent Substance of Echinochrome and in the Drug Histochrome. Pharm. Chem. J. 2004, 38, 50–53. [Google Scholar] [CrossRef]
- Mishchenko, N.P.; Fedoreev, S.A.; Bagirova, V.L. Histochrome: A New Original Domestic Drug. Pharm. Chem. J. 2003, 37, 48–52. [Google Scholar] [CrossRef]
- Vinué, Á.; González-Navarro, H. Glucose and Insulin Tolerance Tests in the Mouse. Methods Mol. Biol. 2015, 1339, 247–254. [Google Scholar]



| Gene | Primer Sequences (5′-3′) | |
|---|---|---|
| Forward | Reverse | |
| PKCι | GTTTGAGCAGGCGTCCAATCAC | CAGGAAGTTTTCTCTGTCGCTGC |
| β-Actin | CATTGCTGACAGGATGCAGAAGG | TGCTGGAAGGTGGACAGTGAGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, T.K.; Nguyen, T.H.T.; Yun, H.R.; Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Vu, T.T.; Nguyen, H.Q.; Cho, S.W.; et al. Echinochrome A Prevents Diabetic Nephropathy by Inhibiting the PKC-Iota Pathway and Enhancing Renal Mitochondrial Function in db/db Mice. Mar. Drugs 2023, 21, 222. https://doi.org/10.3390/md21040222
Pham TK, Nguyen THT, Yun HR, Vasileva EA, Mishchenko NP, Fedoreyev SA, Stonik VA, Vu TT, Nguyen HQ, Cho SW, et al. Echinochrome A Prevents Diabetic Nephropathy by Inhibiting the PKC-Iota Pathway and Enhancing Renal Mitochondrial Function in db/db Mice. Marine Drugs. 2023; 21(4):222. https://doi.org/10.3390/md21040222
Chicago/Turabian StylePham, Trong Kha, To Hoai T. Nguyen, Hyeong Rok Yun, Elena A. Vasileva, Natalia P. Mishchenko, Sergey A. Fedoreyev, Valentin A. Stonik, Thu Thi Vu, Huy Quang Nguyen, Sung Woo Cho, and et al. 2023. "Echinochrome A Prevents Diabetic Nephropathy by Inhibiting the PKC-Iota Pathway and Enhancing Renal Mitochondrial Function in db/db Mice" Marine Drugs 21, no. 4: 222. https://doi.org/10.3390/md21040222
APA StylePham, T. K., Nguyen, T. H. T., Yun, H. R., Vasileva, E. A., Mishchenko, N. P., Fedoreyev, S. A., Stonik, V. A., Vu, T. T., Nguyen, H. Q., Cho, S. W., Kim, H. K., & Han, J. (2023). Echinochrome A Prevents Diabetic Nephropathy by Inhibiting the PKC-Iota Pathway and Enhancing Renal Mitochondrial Function in db/db Mice. Marine Drugs, 21(4), 222. https://doi.org/10.3390/md21040222








