Abstract
Crude anionic polysaccharides extracted from the Pacific starfish Lethasterias fusca were purified by anion-exchange chromatography. The main fraction LF, having MW 14.5 kDa and dispersity 1.28 (data of gel-permeation chromatography), was solvolytically desulfated and giving rise to preparation LF-deS with a structure of dermatan core [→3)-β-d-GalNAc-(1→4)-α-l-IdoA-(1→]n, which was identified according to NMR spectroscopy data. Analysis of the NMR spectra of the parent fraction LF led to identification of the main component as dermatan sulfate LF-Derm →3)-β-d-GalNAc4R-(1→4)-α-l-IdoA2R3S-(1→ (where R was SO3 or H), bearing sulfate groups at O-3 or both at O-2 and O-3 of α-l-iduronic acid, as well as at O-4 of some N-acetyl-d-galactosamine residues. The minor signals in NMR spectra of LF were assigned as resonances of heparinoid LF-Hep composed of the fragments →4)-α-d-GlcNS3S6S-(1→4)-α-l-IdoA2S3S-(1→. The 3-O-sulfated and 2,3-di-O-sulfated iduronic acid residues are very unusual for natural glycosaminoglycans, and further studies are needed to elucidate their possible specific influence on the biological activity of the corresponding polysaccharides. To confirm the presence of these units in LF-Derm and LF-Hep, a series of variously sulfated model 3-aminopropyl iduronosides were synthesized and their NMR spectra were compared with those of the polysaccharides. Preparations LF and LF-deS were studied as stimulators of hematopoiesis in vitro. Surprisingly, it was found that both preparations were active in these tests, and hence, the high level of sulfation is not necessary for hematopoiesis stimulation in this particular case.
1. Introduction
Marine invertebrates belonging to the classes Asteroidea (sea stars, starfishes) and Holothuroidea (sea cucumbers) are known for their peculiar carbohydrate metabolism [1,2,3]. They produce unique sialoglycolipids (gangliosides) [4,5] and complex glycosides containing steroidal or triterpene aglycons and mono- or oligosaccharide moieties, which are usually sulfated. These glycosides possess various biological activities, such as cytotoxic, hemolytic, antibacterial, anti-inflammatory, antitumor and cancer-preventing effects, and are currently being intensely investigated in order to find potential applications in drug development for human and veterinary medicine [6,7]. The body walls of holothuria contain two types of specific sulfated fucose-rich polysaccharides: sulfated fucans (resembling the fucoidans of brown algae) and fucosylated chondroitin sulfates (related to the chondroitin sulfates of vertebrates) [8,9,10,11]. These polysaccharides were shown to be very promising biologically active polymers having various preventive or therapeutic effects on medical conditions [12,13,14,15].
Meanwhile, data on the chemical structures of the corresponding polysaccharides isolated from starfishes are very scarce. A very unusual polysaccharide (composed of pentasaccharide repeating units containing xylose, galactose, fucose and sulfate) was found as to be the acrosome reaction-inducing substance in the starfish Asterias amurensis [16], but similar components in the egg jelly coat of other species were apparently not investigated. Crude polysaccharides obtained by extraction of the body walls were mainly characterized as biologically active preparations, but the chemical structures of the polysaccharides were not elucidated. For example, it was shown that polysaccharide samples obtained from Asterias rollestoni have neuroprotective and immunostimulating effects [17,18], a polysaccharide from Asterina pacifica possess chemopreventive activity against breast cancer and HT-29 human colon adenocarcinoma cells [19,20,21,22], and a polysaccharide from the brittle star Ophiocoma erinaceus was found to promote apoptosis [23]. A rather unusual branched α-glucan was isolated recently from the brittle star Trichaster palmiferus [24]. According to preliminary evidence [25], structural sulfated glycosaminoglycans may have widespread occurrence in the body walls of starfishes. This suggestion was confirmed by the detection of highly sulfated chondroitin sulfates/dermatan sulfates in several brittle stars [26,27]. In addition, the presence of oversulfated dermatan sulfate and heparinoid in the body walls of the starfish Lysastrosoma anthosticta was described in our previous work [28].
The present paper is devoted to the isolation and characterization of sulfated polysaccharides from the starfish Lethasterias fusca. This species was used previously as the source of gangliosides [29], asterosaponins, polyhydroxysteroids, as well as their sulfates and glycosides [30,31]. Many representatives of these unique metabolites have been isolated and structurally characterized [32]. Moreover, the distribution of polar steroids and glycoconjugates in different organs of the starfish was analyzed in order to understand the biological functions of these compounds [33], but polysaccharide composition was not investigated.
We have isolated a highly sulfated polysaccharide preparation LF from the body walls of L. fusca. Desulfated derivative LF-deS was prepared from LF by solvolytic procedure. The structures of LF and LF-deS were elucidated using chemical analysis and NMR spectroscopy data. It was shown that LF is a mixture of two components, highly sulfated dermatan sulfate LF-Derm and heparinoid LF-Hep in a ratio of about 4:1, with both polysaccharides containing unusual 2,3-di-O-sulfated α-l-iduronic acid residues. In the structure of LF-Derm, another unusual structural fragment, namely 3-O-sulfated α-l-iduronic acid residue, was observed. Polysaccharide preparations LF and LF-deS were studied as stimulators of hematopoiesis.
2. Results and Discussion
The body walls of the starfish Lethasterias fusca were extracted in the presence of papain [34] to obtain a crude preparation of sulfated polysaccharides LF-SP. Then the polysaccharide mixture was fractionated by anion-exchange chromatography on DEAE-Sephacel column. The main fraction LF (yield 40.7%) was obtained by elution with 1.0 M NaCl. According to the composition of LF (high levels of galactosamine, uronic acid and sulfate, moderate amount of glucosamine, see Section 3.2 and Figure S1), the preparation might be preliminarily regarded as a sulphated polysaccharide belonging to CS-DS group with possible admixture with other GAGs.
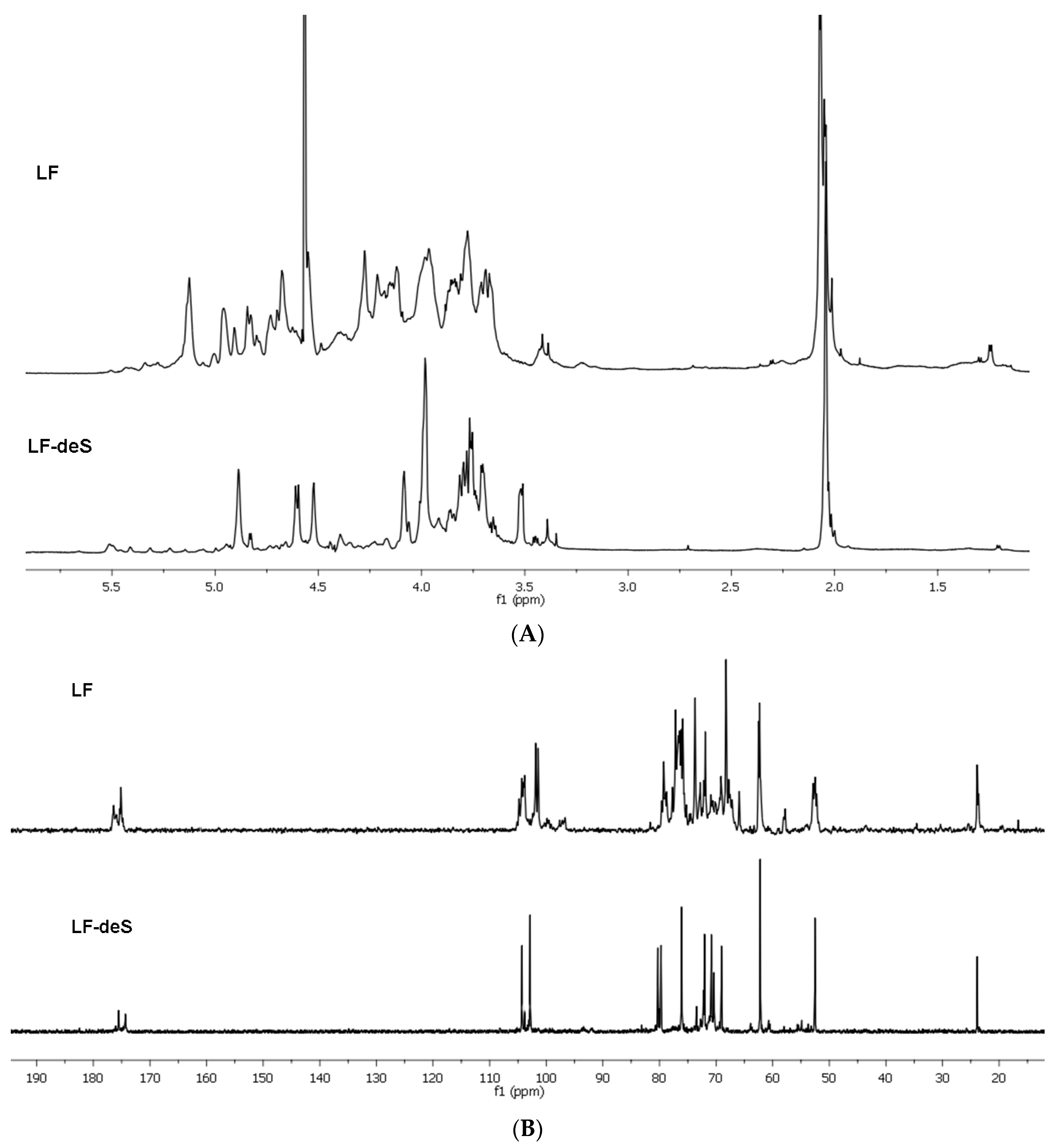
The NMR spectra of preparation LF contain several signals with different intensity in the anomeric region (Figure 1). Desulfation of LF under solvolytic conditions led to product LF-deS giving NMR spectra which were easier to interpret. Two main signals were observed in the anomeric region of its 13C NMR spectrum (Figure 1). Application of the 2D NMR techniques (COSY, ROESY and HSQC, see the HSQC spectrum in Figure S2) allowed us to assign the signals of the spin systems of units A and B (Figure 2) both in the 1H and 13C NMR spectra (Table 1). Comparing these data to those for the desulfated derivative LA-F1-DS, obtained previously by modification of the polysaccharide from the starfish Lysastrosoma anthosticta [28], revealed the identity of these polysaccharides. It was shown that polysaccharide LF-deS, similarly to LA-F1-DS, was dermatan with the regular structure [→3)-β-d-GalNAc-(1→4)-α-l-IdoA-(1→]n (Figure 2, the absolute configurations of monosaccharide residues were ascribed by analogy with other natural dermatan sulfates).
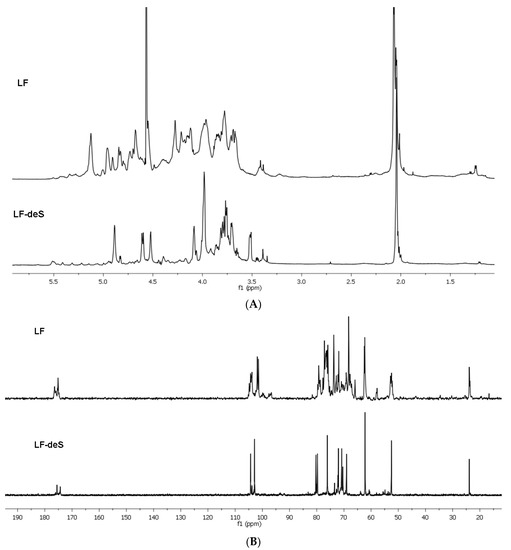
Figure 1.
The 1H NMR (A) and 13C NMR (B) spectra of preparations LF and LF-deS.
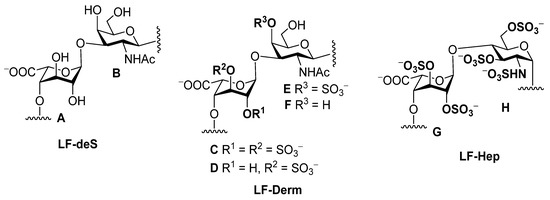
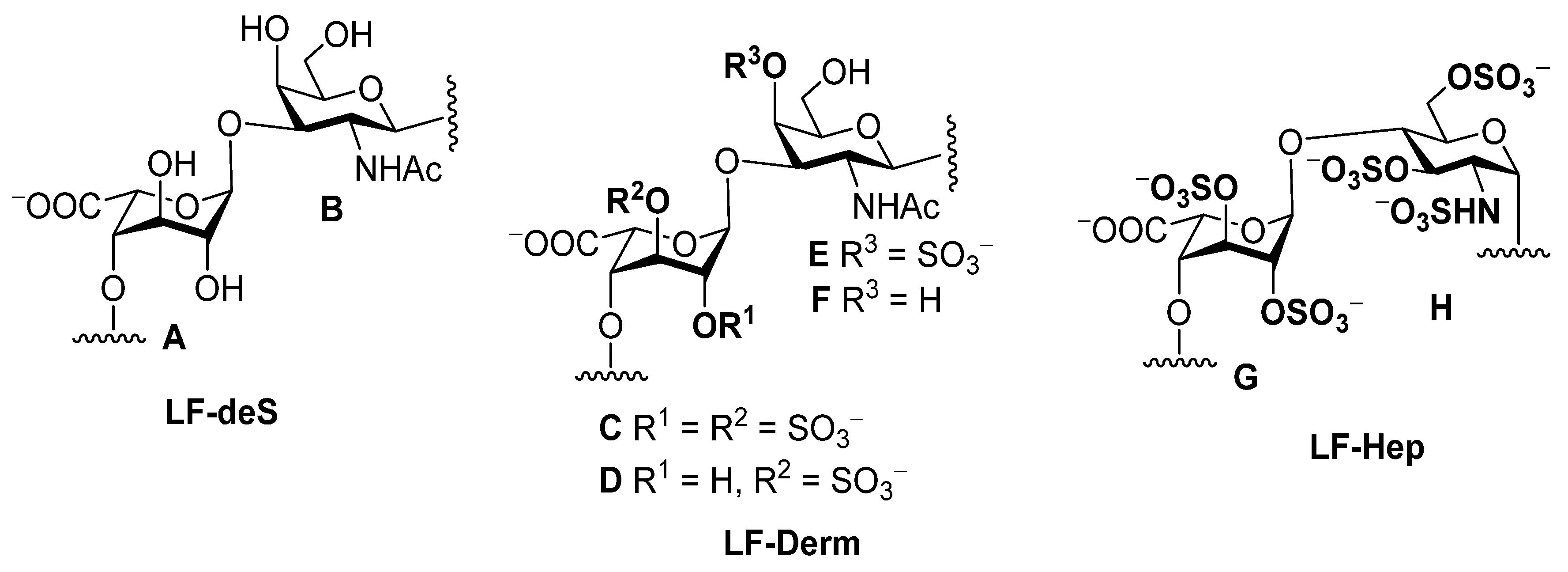
Figure 2.
The structures of polysaccharides LF-deS, LF-Derm and LF-Hep.

Table 1.
Chemical shifts of signals in the 1H and 13C NMR spectra of polysaccharides LF-deS (units A and B), LF-Derm (units C–F), LF-Hep (units G,H) and monosaccharide derivatives 7–11 (the bold numerals indicate the positions of sulfate).
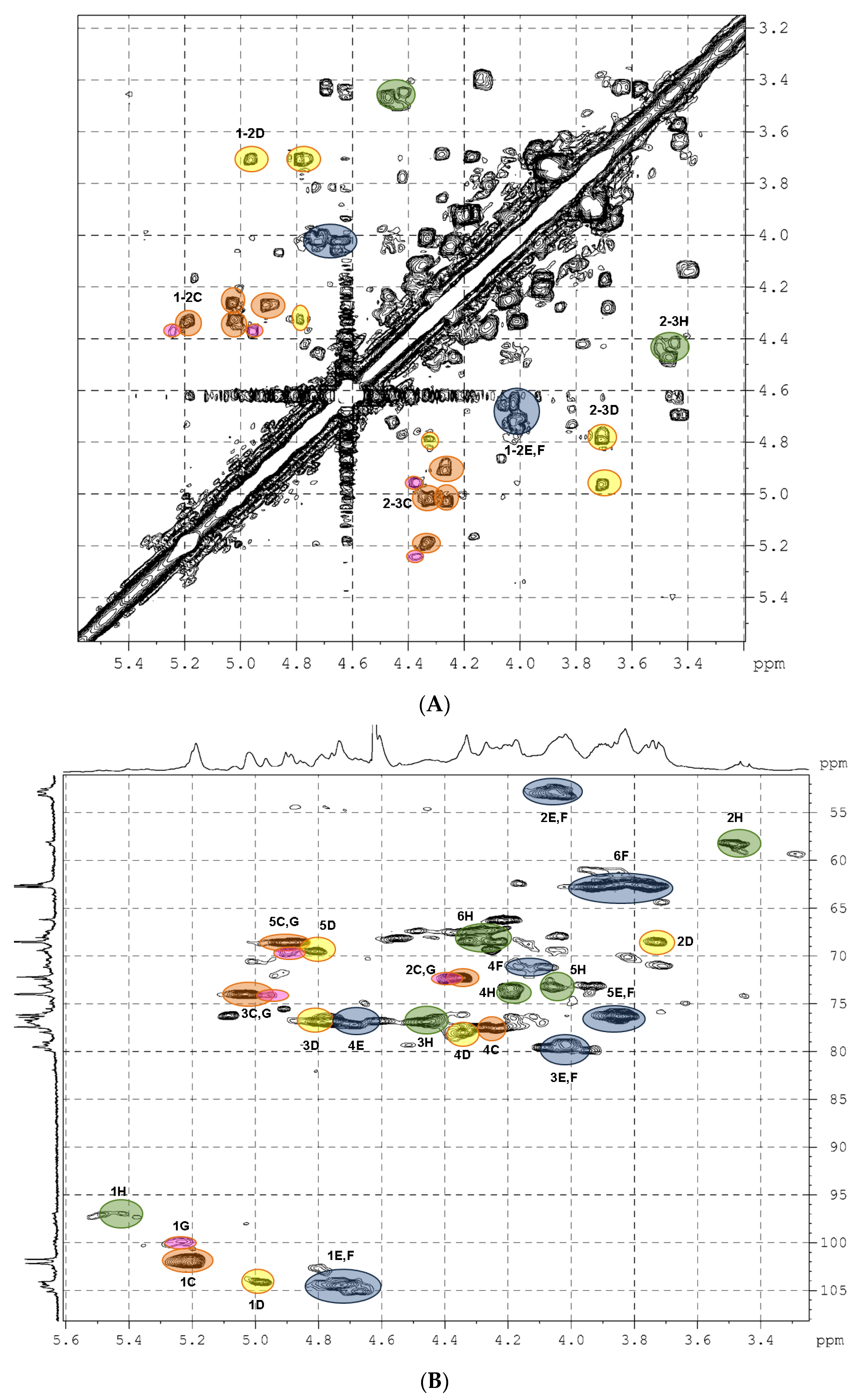
Then, the detailed assignment of the signals in the NMR spectra of the parent preparation LF was performed using the 2D COSY, TOCSY, ROESY, HSQC experiments (Figure 3, Figures S3 and S4, Table 1). It was found that the main component of LF was dermatan sulfate bearing sulfate groups at positions 2 and 3 of α-l-iduronic acid (unit C), as well as at O-4 of most parts of N-acetyl-d-galactosamine residues (unit E). A similar polysaccharide LA-Derm was determined previously in the starfish L. anthosticta [28]. These structural fragments were found earlier in several oversulfated dermatans isolated from ascidians [35,36] and in a polysaccharide prepared by chemical sulfation of pig intestine dermatan sulfate [37]. We observed a good coincidence in the NMR data of our sample and these oversulfated polysaccharides described previously.
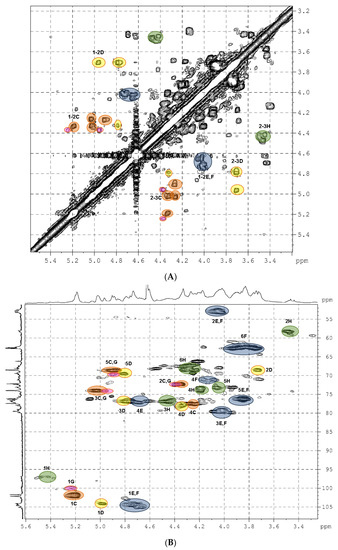
Figure 3.
The COSY (A) and HSQC (B) NMR spectra of preparation LF.
In addition to the IdoA2S3S unit, there was an IdoA3S fragment (unit D) in preparation LF. The signals of the anomeric H-1 (δ 4.97 ppm) and C-1 (δ 103.8 ppm) of this unit differed significantly from those of IdoA2S3S (Figure 3, Table 1). The presence of a sulfate group at O-3 was confirmed by the downfield chemical shift of the respective H-3 signal. Attachment of IdoA3S to O-3 of GalNAc unit was confirmed by the presence of the correlation peak H1(D)-H3(E,F) in the ROESY spectrum (Figure S3). Therefore, the dermatan sulfate LF-Derm from L. fusca can be described by the formula [→3)-β-d-GalNAc4R-(1→4)-α-l-IdoA2R3S-(1→]n (where R was SO3– or H) and should be considered as a more complex polysaccharide than that from L. anthosticta.
Dermatan sulfate was not the only component of preparation LF. The correlation H2-C2 (δ 3.47 ppm/57.9 ppm) in the HSQC spectrum (Figure 3B) indicated the presence of glucosamine residue sulfated at N-2 GlcNS (unit H) [38]. This unit was also sulfated at O-3 and at O-6 (Table 1). Unit IdoA2S3S (G) was found to be connected to O-4 of GlcNS (H) (see ROESY spectrum, Figure S3). Therefore, the minor component of LF should be considered as heparinoid LF-Hep described by the formula [→4)-α-d-GlcNS3S6S-(1→4)-α-l-IdoA2S3S-(1→]n. A similar structural fragment was found in an oversulfated polysaccharide obtained by chemical sulfation of heparin [39]. Comparing the chemical shift values of the corresponding signals showed a good coincidence. Nevertheless, there are inevitable differences in the data for polysaccharides, since LF-Hep contains the IdoA2S3S residue at O-4 of unit H, whereas in the oversulfated heparin, this position is taken up by a sulfated β-glucuronic acid substituent. Previously, a similar heparinoid was determined in the starfish L. anthosticta in significant quantities [28]. The ratio of GalNAc and GlcNS residues in LF calculated as a ratio between the integral intensities of the respective H2-C2 cross peaks in the HSQC NMR spectrum was found to be 4:1, which practically coincides with the ratio between galactosamine and glucosamine determined by chemical monosaccharide analysis (Figure S1).
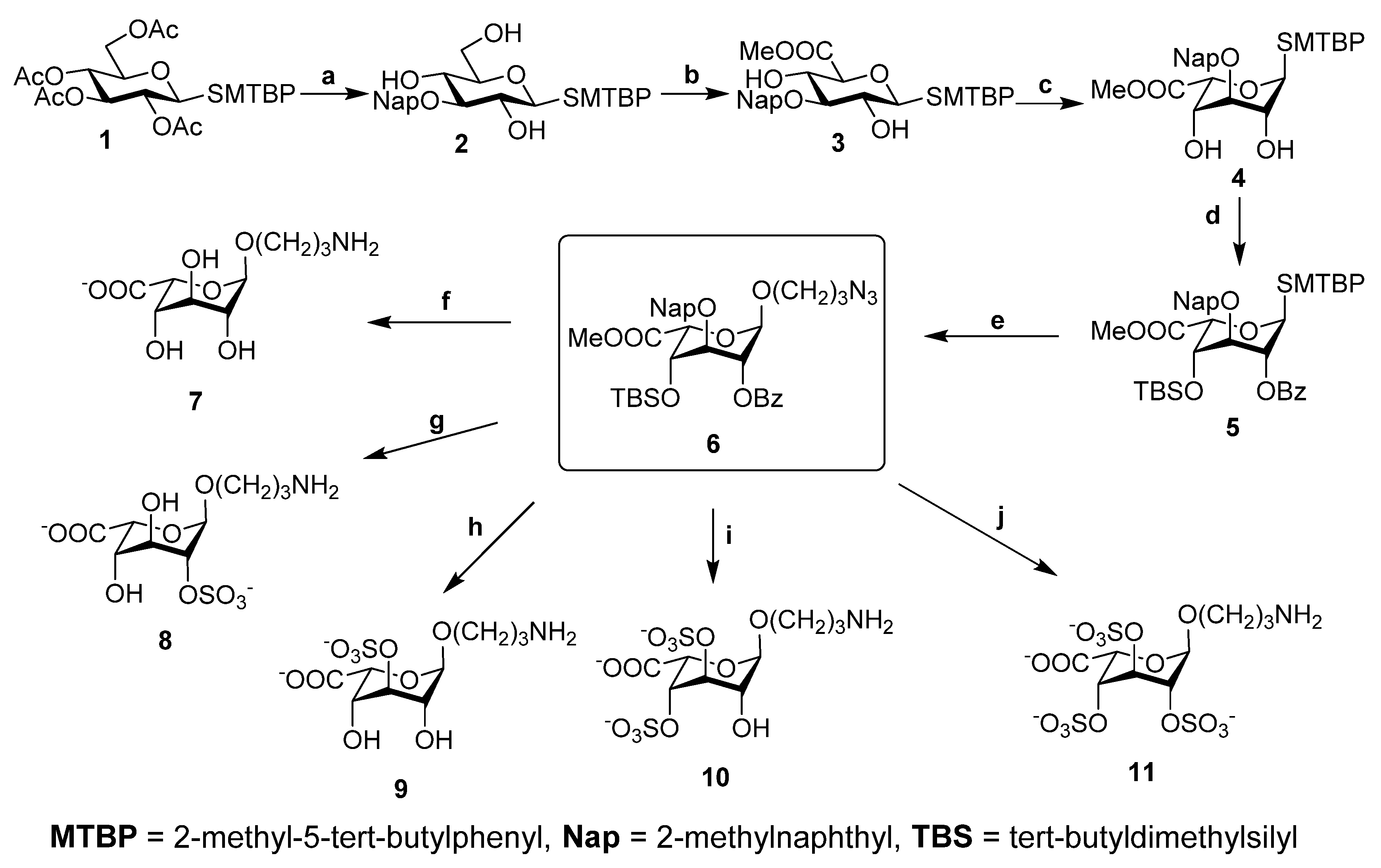
The monosaccharide residues IdoA3S and IdoA2S3S are unusual for natural polysaccharides. Model iduronic acid aminopropyl glycosides 7–11 (Scheme 1) with different sulfation patterns were synthesized to aid the detailed analysis of the NMR spectra of LF. All model compounds were obtained from fully protected intermediate 6. Readily available thioglycoside 1 [40] was synthesized from glucose pentaacetate, subjected to acetyl cleavage, 4,6-O-p-methoxybenzylidene protection and DBTO-promoted regioselective introduction of 2-methylnaphthyl at O-3. Subsequent removal of the acetal protection yielded 3-O-protected compound 2. C-6-oxidation using the TEMPO/BAIB system resulted in glucuronic thioglycoside 3, which was then subjected to direct epimerization under basic conditions [41] to give iduronic compound 4 in a yield of 50%. Selective 2-O-benzoylation using DBTO and silylation with tert-butyldimethylsilyl trifluoromethanesulfonate in the presence of triethylamine gave fully protected thioglycoside 5, which was then oxidized by m-chloroperbenzoic acid to yield a mixture of diastereomeric sulfoxide glycosyl donors. Those were used in a Tf2O-promoted glycosylation reaction with 3-azido-1-propanol as acceptor to obtain compound 6, which was then subjected to selective deprotection and sulfation, followed by final deprotection, to give the desired glycosides 7–11 (Scheme 1).
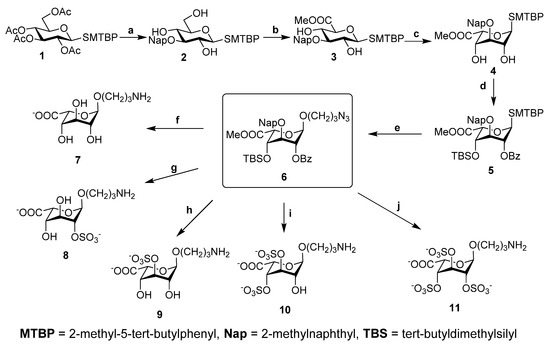
Scheme 1.
Synthesis of uronic acid derivatives 7-11. Reagents and conditions: (a) i: MeONa, MeOH, ii: p-Anisaldehyde, CH(OMe)3, CSA, CH3CN, iii: Bu2SnO, MeOH, reflux, iv: 2-bromomethyl naphthalene, CsF, DMF, v: TFA (90% aq.); (b) i: BAIB, TEMPO, DCM/H2O, 0 °C, ii: MeI, K2CO3, DMF; (c) MeONa, MeOH; (d) i: Bu2SnO, Tol., reflux, ii: BzCl, iii: TBSOTf, NEt3, DCM; (e) i: 3-chloroperbenzoic acid, DCM, −30 °C, ii: N3(CH2)3OH, Tf2O, DTBMP, AW300, DCM, rt. (f) i: TBAF, THF, 50 °C, ii: LiOH 2 M(aq.), H2O, iii: NaOH 1 M(aq.), iv: H2, Pd(OH)2/C, THF/H2O; (g) i: K2CO3, MeOH, ii: Py·SO3, DMF, iii: TBAF, THF, 50 °C, iv: LiOH 2 M(aq.), H2O, v: H2, Pd(OH)2/C, THF/H2O; (h) i: DDQ, DCM/H2O, ii: TBAF, THF, 50 °C; iii: LiOH 2 M(aq.), H2O; iv: NaOH 1 M(aq.), v: H2, Pd(OH)2/C, THF/H2O; (i) i: TBAF, THF, 50 °C, ii: DDQ, DCM/H2O, iii: Py·SO3, DMF, iv: LiOH 2 M(aq.), H2O, v: NaOH 1 M(aq.), vi: H2, Pd(OH)2/C, THF/H2O; (j) i: TBAF, THF, 50 °C, ii: DDQ, DCM/H2O, iii: K2CO3, MeOH, iv: Py·SO3, DMF, v:. LiOH 2 M(aq.), H2O, vi: H2, Pd(OH)2/C, THF/H2O.
Analysis of the NMR spectra of model compounds 7–11 (see Supplementary data) revealed that sulfation led to significant (0.6–1.5 ppm) downfield shift of the signals of the respective protons and in some cases of the respective carbons (compounds 8 and 9, Table 1). A similar effect was observed in polysaccharides LF-Derm and LF-Hep for the units IdoA2S3S (C,G) and IdoA3S (D) in comparison to non-sulfated IdoA (unit A). Additionally, 2-O-sulfation influences the signals of the anomeric protons (downfield shift on 0.25–0.30 ppm) and carbons (upfield shift on 1.7–2.1 ppm) (see compounds 8 and 11). This effect was found for unit IdoA2S3S (C), compare δ 5.20 ppm for H-1 and 101.8 ppm for C-1 (units C) to 4.94 ppm for H-1 and 105.0 for C-1 (unit A). Interestingly 3-O-sulfation also slightly influences the value of anomeric proton chemical shift (see compound 9). Additional introduction of a bulky substituent at neighbor O-4 (see compound 10) increases the downfield shift of H-1 up to 0.1 ppm. A similar observation was made for unit IdoA3S (D) in the polysaccharide LF-Derm (compare δ 4.97 ppm for H-1 of units D to 4.94 ppm for H-1 of unit A).
Notably the presence of IdoA3S units in a glycosaminoglycan molecule was observed for the first time. As this manuscript was being prepared for publication, a paper appeared [42] describing IdoA3S residues found in the carbohydrate moiety of some unusual glycoproteins isolated from the halophilic archaeon Halobacterium salinarum. The assignments of the IdoA3S signals in the NMR spectra given by Notaro et al. [42] slightly differ from our data, and this inconsistency may be explained by the influence of the varying monosaccharide residues surrounding IdoA3S in the two different biopolymers.
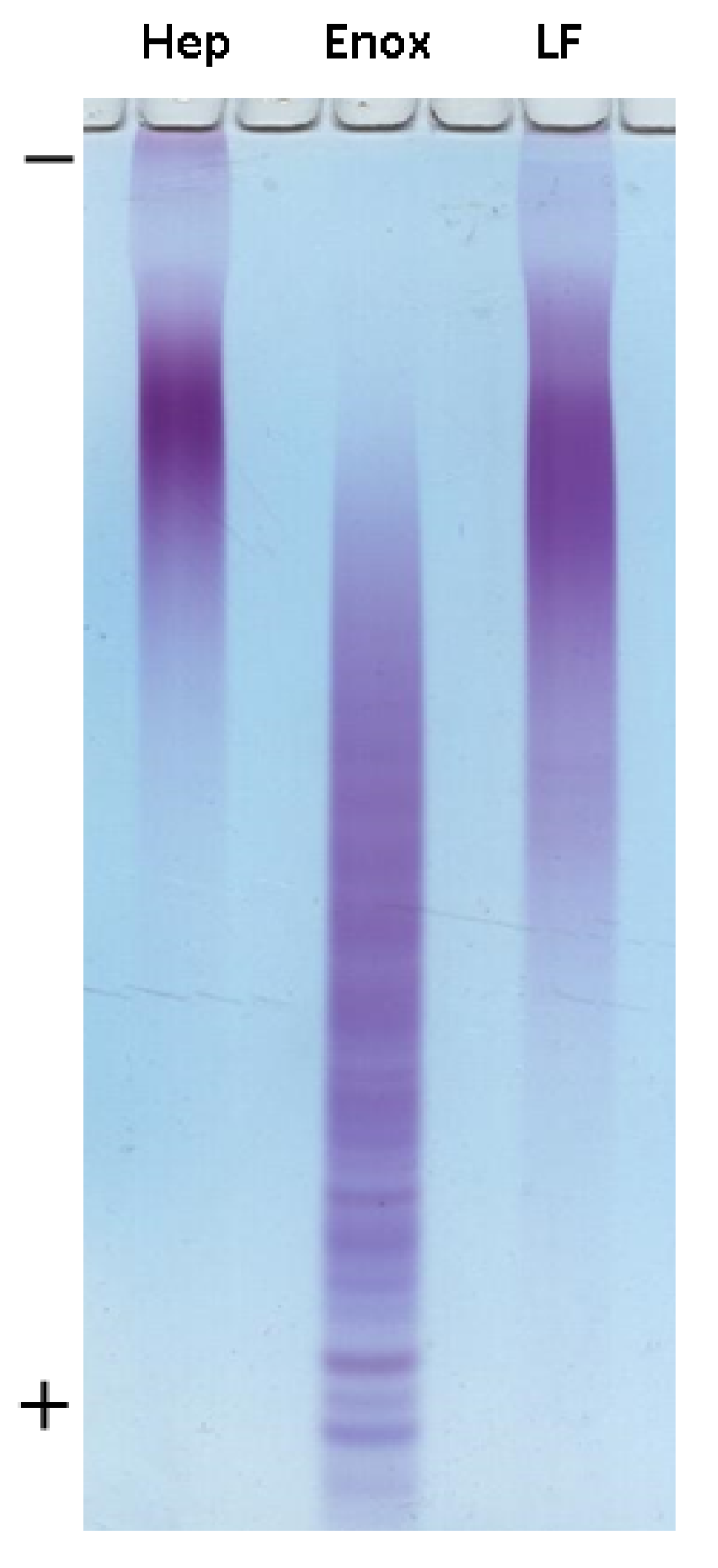
For the preliminary assessment of the molecular weight of preparation LF, gel electrophoresis was performed using heparin (Sigma-Aldrich, St. Louis, MO, USA) and enoxaparin (Clexan®, Sanofi, Paris, France) with defined MW as a standard (Figure 4). Based on mobility of samples, it could be concluded that MW value of LF was quite similar to that of heparin (15 kDa). Further detailed MW determination was performed using gel chromatography. As a result, almost equal values of MW were found for heparin and preparation LF (14.5 kDa, dispersity 1.28, Figure S5).
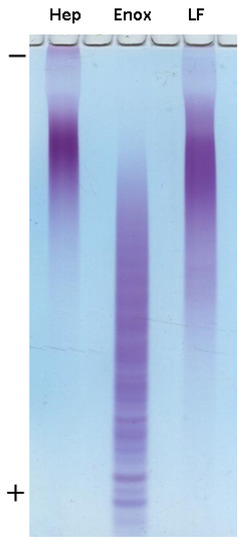
Figure 4.
Electrophoresis in polyacrylamide gel of Hep (heparin Sigma, MW 15 kDa), Enox (enoxaparin, Clexane®, Sanofi, MW 4.5 kDa) and LF.
Sulfated polysaccharides isolated from marine plants and animals, such as fucoidans and fucosylated chondroitin sulfates, were shown to stimulate hematopoiesis in mice [43,44,45,46,47]. This type of activity was found to be connected with the ability of sulfated polysaccharides to stimulate the adhesion of bone marrow cells and their further proliferation [28]. Previously, we have shown that fucoidans and fucosylated chondroitin sulfates demonstrated significant activity in concentrations 10–50 µg/mL [43,47]. As we did not know the level of this type of activity for glycosaminoglycans from the starfishes, we have studied the samples from L. fusca in the concentration 50 µg/mL, and additionally, in a concentration of 250 µg/mL, which was five times higher.
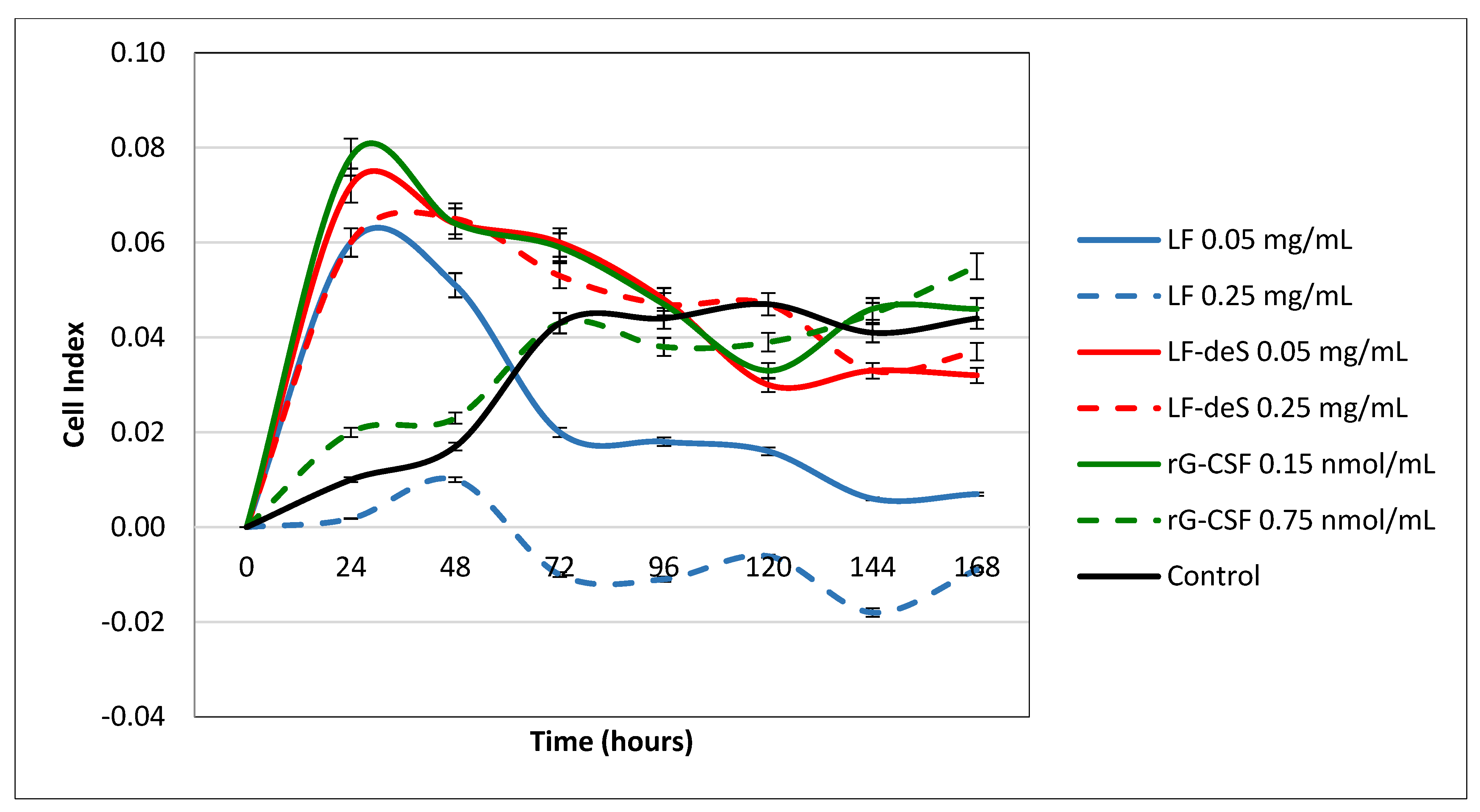
To study the influence of glycosaminoglycans from the starfish L. fusca on hematopoiesis, samples of LF and LF-deS were incubated with bone marrow cells for 7 days ex vivo. Recombinant granulocyte colony-stimulating factor (rG-CSF) was used as a positive control. The parameter Cell Index (CI) was measured using the Agilent xCELLigence real-time cell analysis multiple plates system (Figure 5). The value of CI is correlated with the ability of the cells to adhere and consequently proliferate. It was found that preparation LF in a concentration of 50 µg/mL led to an increase in CI during the first day, but further on a significant decrease in this value was observed, which could be connected with the induction of apoptosis or some toxic effects of LF. It was confirmed by the experiment with a five times higher concentration of LF (250 µg/mL), where almost no attached cells were detected. On the contrary, preparation LF-deS in both tested concentrations led to an increase in CI. The stimulation curve profiles for LF-deS were quite similar to that of rG-CSF in lower concentration, indicating the ability of LF-deS to stimulate cell adhesion and proliferation. Interestingly, a higher concentration of rG-CSF also led to a slower increase in CI.
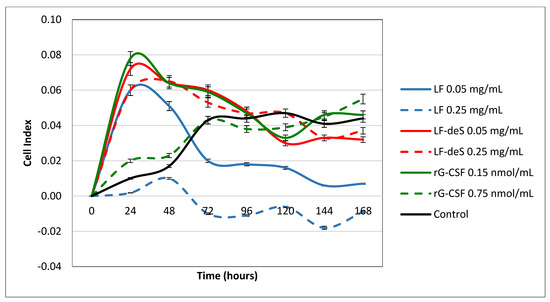
Figure 5.
Influence of preparations LF and LF-deS on bone marrow cell proliferation. rG-CSF was used as a positive control. Intact cells were considered as a control.
3. Materials and Methods
3.1. General Methods
Acid hydrolysis of polysaccharide samples followed by preparation of alditol acetates was described previously [28,48,49]. Gas-liquid chromatography was performed using Agilent 8860 GC System equipped with flame-ionization detector and HP-5 chromatographic column 30 m × 0.320 mm × 0.25 μm working at 160–290 °C with nitrogen as carrier gas (Figure S1). Quantitative determination of monosaccharides with myo-inositol acetate as internal standard was carried out using OpenLab CDS 2 program. Estimations of sulfate [50], uronic aids [51] and proteins [52] was accomplished using Ultrospec 4050 spectrophotometer (LKB Biochrom, Bromma, Sweden).
The NMR spectra were recorded using the facilities of Zelinsky Institute Shared Center. Preparation of polysaccharide solutions, as well as conditions used to record 1D and 2D proton and carbon-13 COSY, TOCSY, ROESY and HSQC spectra were described earlier [28,53,54]. For synthetic monosaccharide derivatives chemical shifts are reported in ppm referenced either to the solvent residual peaks as standard for chloroform (δ 7.26 1H, δ 77.16 13C) or methanol as internal standard for D2O (δ 3.34 1H, δ 49.50 for 13C).
Optical rotations were measured using a JASCO P-2000 polarimeter at ambient temperature (22–25 °C).
High-resolution mass spectra (HR MS) were measured on a Bruker micrOTOF II instrument using electrospray ionization (ESI). The measurements were performed in a positive ion mode (interface capillary voltage −4500 V) or in a negative ion mode (3200 V); mass range from m/z 50 to m/z 3000 Da; external or internal calibration was made with Electrospray Calibrant Solution (Fluka). A syringe injection was used for solutions in a mixture of acetonitrile and water (50:50 v/v, flow rate 3 μL/min). Nitrogen was applied as a dry gas; interface temperature was set at 180 °C.
Commercial chemicals for the synthesis of model monosaccharide derivatives were used without purification unless noted. All solvents were distilled and dried if necessary, according to standard procedures (DCM, MeOH) or purchased as dry (DMF, THF, CH3CN, Sigma-Aldrich). All reactions involving air- or moisture-sensitive reagents were carried out using dry solvents under Ar atmosphere. Thin-layer chromatography (TLC) was carried out on aluminum sheets coated with silica gel 60 F254 (Merck, Rahway, NJ, USA). TLC plates were inspected by UV light (λ = 254 nm) and developed by treatment with a mixture of 15% H3PO4 and orcinol (1.8 g/L) in EtOH/H2O (95:5, v/v) followed by heating. Silica gel column chromatography was performed with Silica Gel 60 (40–63 μm, E. Merck, Darmstadt, Germany). Solvents for column chromatography and thin layer chromatography (TLC) are listed in volume-to-volume ratios. Gel-filtration of the synthetic compounds was performed on a Sephadex G-15 column (400 × 16 mm) by elution with water at a flow rate of 0.5 mL/min, or a Toyopearl HW-40S column (400 × 16 mm) eluted with a 0.1 M solution of AcOH at a flow rate of 0.5 mL/min.
Other experimental conditions were described in detail previously [53,54]. Heparin sodium salt was purchased from Sigma-Aldrich (H5515). Molecular weight of polysaccharides was determined using gel chromatography on an analytical TSK 2000 SWXL column (Toyo Soda, Tokyo, Japan, 7.5 × 300 mm) calibrated using pullulans (Fluka, Buchs, Switzerland) [55] at a flow rate of 0.75 mL/min by elution with 1 M NaCl in PBS with refractometer detection at 35 °C.
3.2. Isolation of Sulfated Polysaccharides
The starfish Lethasterias fusca (Djakonov, 1931) was collected in the littoral zone of the Peter the Great Bay (the Sea of Japan) and identified by the late Prof. V.S Levin (Pacific Institute of Bioorganic Chemistry, FEB RAS, Vladivostok, Russia). After removing the viscera, the body walls were treated several times with methanol and stored under methanol in a refrigerator. According to the conventional procedure [30], dried and minced biomass (227 g) was suspended in 300 mL of 0.1 M sodium acetate buffer (pH 6.0), containing papain (1 g), EDTA (0.4 g), and l-cysteine hydrochloride (0.2 g), and incubated at 45–50 °C for 24 h with occasional agitation. After centrifugation, an aqueous hexadecyl-trimethylammonium bromide solution (10%, 25 mL) was added to the supernatant, and the mixture was allowed to sit overnight. The resulting precipitate was isolated by centrifugation and washed successively with water and ethanol. Then, it was stirred with 20% ethanolic NaI solution (5 × 40 mL) for 2–3 days, washed with ethanol, dissolved in water and lyophilized to give the crude polysaccharide preparation LF-SP, yield 0.19%, composition: sulfate, 23.9%; uronic acids, 9.6%; galactosamine, 7.2%; glucosamine, 1.8%; galactose, 2.2%.
An aqueous solution of LF-SP (410 mg) was placed on a column (3 × 10 cm) with DEAE-Sephacel in Cl−-form and eluted with water followed by NaCl solution of increasing concentration (0.5, 1.0 and 1.5 M) each time until the eluate no longer gave a positive reaction for carbohydrates [56]. The main 1.0 M fraction was desalted on Sephadex G-15 column and lyophilized, giving rise to preparation LF, yield 150 mg, composition: sulfate, 36.7%; uronic acids, 28.3%; galactosamine, 12.2%; glucosamine, 3.3%; galactose, 2.7%; protein, 4.6%.
3.3. Chemical Modification of Polysaccharide LF
Solvolytic desulfation of LF (as pyridinium salt) was carried out as described earlier [48,49]. Briefly, a solution of LF (100 mg) in a DMSO-MeOH mixture (9:1, 10 mL) was heated at 80 °C for 5 h, diluted with water, made slightly alkaline (pH about 8) by addition of 3 M aqueous NaOH (0.2 mL); the precipitate formed was separated by centrifugation, dissolved in water and chromatographed on a column containing Sephadex G-15. A high-molecular-weight fraction was lyophilized to afford desulfated preparation LF-deS in a yield of 43 mg, composition: sulfate, 1.6%; uronic acids, 50.8%; galactosamine, 21.2%; glucosamine, 6.3%; galactose, 3.0%; protein, 8.2%.
3.4. Polyacrylamide Gel Electrophoresis (PAGE)
The polysaccharides LF, heparin and enoxaparin (15 μg) were investigated using PAGE under conditions described earlier [28].
3.5. Model Monosaccharide Synthesis
Detailed synthetic procedures for the preparation of the model monosaccharides are presented in the Supplementary section.
3.6. Cell Model
Bone marrow cells (BM cells) were isolated from the femoral bone of healthy Balb/c mice (male, weight 19 ± 1 g). BM cells were suspended in the complete growth medium based on Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich, St. Louis, MO, USA), supplemented with 10% fetal bovine serum (FBS; HyClon, Thermo Fisher Scientific, Waltham, Massachusetts, USA), 1% penicillin/streptomycin (PanEco, Moscow, Russia) and 4 mM L-glutamine (PanEco, Moscow, Russia) at 37 °C in atmosphere with 5% CO2 to a concentration of 500,000 cells/mL. To study cell activity, the suspension of BM cells (150 μL) was placed in E-plates 16 (ACEA Biosciences, San Diego, CA, USA). Solutions of LF, LF-deS, and rG-CSF in isotonic sodium chloride solution (50 μL) were added to the cells to a concentration of 50 µg/mL or 250 µg/mL for polysaccharides and 0.15 nmol/mL or 0.75 nmol/mL for rG-CSF. BM cells supplemented with 50 μL isotonic sodium chloride solution were used as an intact Control. The results were examined daily by assessing the change in the Cell Index in comparison with the Control, which was measured using the Agilent xCELLigence real-time cell analysis multiple plates system (xCELLigence RTCA DP, ACEA Biosciences, San Diego, CA, USA) during incubation for 7 days at 37 °C in atmosphere with 5% CO2. A fresh portion of the growth medium (50 μL) was added every two days in each well.
3.7. Statistical Analysis
Determinations of the biological activity mentioned in Section 3.6 were performed in quadruplicate (n = 4). The results are presented as Mean ± SD. Statistical significance was determined with Student’s t test. p values less than 0.05 were considered as significant.
4. Conclusions
This work represents the first investigation of glycosaminoglycans from L. fusca. Crude sulfated polysaccharides LF-SP were isolated from the defatted body walls of the starfish by a conventional procedure and purified further using anion-exchange chromatography to obtain the main heavily sulfated fraction LF. Both galactosamine and glucosamine were found in hydrolysate of this preparation, suggesting that it contained at least two different glycosaminoglycans. Their separation was not possible evidently due to their close similarity in molecular weight and sulfate content. Solvolytic treatment of LF gave rise to desulfated preparation LF-deS.
According to NMR spectroscopy data, the main component of LF-deS was the well-known dermatan [→3)-β-d-GalNAc-(1→4)-α-l-IdoA-(1→]n. Interpretation of the complex NMR spectra of LF was rather difficult and required synthesis of several model compounds. These models included 3-aminopropyl glycoside of α-L-idopyranosyluronic acid and four of its sulfated derivatives differed in number and position of sulfate groups. The signals in the NMR spectra of these models were carefully assigned and gathered together in Table 1, giving valuable information about the influence of sulfate position on chemical shifts of resonances in NMR spectra. Taking into account this evidence, the spectra of LF were interpreted. These spectra corresponded to a mixture of two components, an oversulfated dermatan sulfate LF-Derm →3)-β-d-GalNAc4R-(1→4)-α-l-IdoA2R3S-(1→ (where R was SO3− or H) and a heparinoid LF-Hep composed of the fragments →4)-α-d-GlcNS3S6S-(1→4)-α-l-IdoA2S3S-(1→. The ratio between LF-Derm and LF-Hep was calculated as about 4:1. It should be emphasized that α-l-IdoA3S is uncommon for known dermatan sulfates, and α-l-IdoA2S3S is also a rare component of dermatan sulfates and heparinoids.
In order to determine the biological activity of LF and LF-deS, both preparations were tested as possible stimulators of hematopoiesis. It was shown that LF in low concentration promoted the adhesion and further proliferation of bone marrow cells at the beginning of experiment, but further on, a significant decrease in this effect was observed, possibly linked with the induction of apoptosis or toxic effects of LF. It is very interesting that LF-deS demonstrated similar action, and hence, this influence on hematopoiesis does not depend directly on the sulfation of polysaccharides. Further investigations are highly desirable in order to explain these unusual properties and determine the structure-activity relationship of these biologically active compounds, as well as elucidating the actual mechanisms behind their activity. It is very probable that some other types of biological activity, characteristic for sulfated glycosaminoglycans, may be found for LF and LF-deS.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/md21040205/s1, Section 3.5. Model monosaccharide synthesis; Figure S1: GLC analysis of aminosugar composition of LF and LF-deS; Figure S2: The HSQC NMR spectrum of LF-deS; Figure S3: The ROESY NMR spectrum of LF; Figure S4. The TOCSY NMR spectrum of LF; Figure S5: The chromatograms of polysaccharide preparation LF on TSK 2000 SWXL column (Toyo Soda, Japan, 7.5 × 300 mm) calibrated using pullulans (Fluka) at flow rate of 0.75 mL/min by elution with 1 M NaCl in PBS.
Author Contributions
Conceptualization, A.I.U. and N.E.U.; methodology, M.I.B., N.E.U. and A.S.D.; synthesis, A.I.T. and D.Z.V.; structural analysis, S.P.N., M.I.B. and E.A.T.; biological activity, N.Y.A. and M.V.K.; resources, A.I.U.; writing—original draft preparation, N.E.U. and M.V.K.; writing—review and editing, M.I.B. and A.I.U.; supervision, A.I.U., M.V.K. and N.E.N.; project administration, N.E.N.; funding acquisition, N.E.N. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the Russian Science Foundation grant 19-73-20240.
Institutional Review Board Statement
The study was approved by the Institutional Ethics Committee of the N.N. Blokhin National Medical Research Center of Oncology (Protocol 12-2017, 24 May 2017).
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Vasconcelos, A.A.; Pomin, V.H. Marine carbohydrate-based compounds with medicinal properties. Mar. Drugs 2018, 16, 233. [Google Scholar] [CrossRef] [PubMed]
- Khotimchenko, Y. Pharmacological potential of sea cucumbers. Int. J. Mol. Sci. 2018, 19, 1342. [Google Scholar] [CrossRef]
- Lazzara, V.; Arizza, V.; Luparello, C.; Mauro, M.; Vazzana, M. Bright spots in the darkness of cancer: A review of starfishes-derived compounds and their anti-tumor action. Mar. Drugs 2019, 17, 617. [Google Scholar] [CrossRef] [PubMed]
- Kochetkov, N.K.; Smirnova, G.P. Glycolipids of marine invertebrates. Adv. Carbohydr. Chem. Biochem. 1986, 44, 387–438. [Google Scholar] [CrossRef]
- Smirnova, G.P. Gangliosides of starfishes: Variety and possible species specificity of structures. Russ. J. Mar. Biol. 1987, 1, 3–7. [Google Scholar]
- Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Separation procedures for complicated mixtures of sea cucumber triterpene glycosides, their comparison with HPLC/MS metabolomics approach and biosynthetic interpretation of the obtained structural data. In Studies in Natural Products Chemistry. Bioactive Natural Products; Atta-ur-Rahman, F.R.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 72, pp. 103–146. [Google Scholar] [CrossRef]
- Ivanchina, N.V.; Kicha, A.A.; Malyarenko, T.V.; Stonik, V.A. Recent studies of polar steroids from starfish: Structures, biological activities and biosynthesis. In Advances in Natural Product Discovery (Chemistry Research and Applications); Gomes, A.R., Rocha-Santos, T., Duarte, A.S., Eds.; Nova Publishers: New York, NY, USA, 2017. [Google Scholar]
- Pomin, V.H.; Mourão, P.A.S. Structure, biology, evolution and medical importance of sulfated fucans and galactans. Glycobiology 2008, 18, 1016–1027. [Google Scholar] [CrossRef]
- Pomin, V.H. Holothurian fucosylated chondroitin sulfate. Mar. Drugs 2014, 12, 232–254. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Nifantiev, N.E.; Usov, A.I. New insight on the structural diversity of holothurian fucosylated chondroitin sulfates. Pure Appl. Chem. 2019, 91, 1065–1071. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Nifantiev, N.E.; Usov, A.I. Structural analysis of holothurian chondroitin sulfates: Degradation versus non-destructive approach. Carbohydr. Res. 2019, 476, 8–11. [Google Scholar] [CrossRef]
- Mourão, P.A.S. Perspective on the use of sulfated polysaccharides from marine organisms as a source of new antithrombotic drugs. Mar. Drugs 2015, 13, 2770–2784. [Google Scholar] [CrossRef]
- Carvalhal, F.; Cristelo, R.R.; Resende, D.I.S.P.; Pinto, M.M.M.; Sousa, E.; Correia-da-Silva, M. Antithrombotics from the sea: Polysaccharides and beyond. Mar. Drugs 2019, 17, 170. [Google Scholar] [CrossRef]
- Salih, A.E.M.; Thissera, B.; Yaseen, M.; Hussane, A.S.I.; El-Seedi, H.R.; Sayed, A.M.; Rateb, M.E. Marine sulfated polysaccharides as promising antiviral agents: A comprehensive report and modeling study focusing on SARS CoV-2. Mar. Drugs 2021, 19, 406. [Google Scholar] [CrossRef]
- Li, Y.; Qin, J.; Cheng, Y.; Lv, D.; Li, M.; Qi, Y.; Lan, J.; Zhao, Q.; Li, Z. Marine sulfated polysaccharides: Preventive and therapeutic effects on metabolic syndrome: A review. Mar. Drugs 2021, 19, 608. [Google Scholar] [CrossRef]
- Koyota, S.; Wimalasiri, K.M.S.; Hoshi, M. Structure of the main saccharide chain in the acrosome reaction-inducing substance of the starfish, Asterias amurensis. J. Biol. Chem. 1997, 272, 10372–10376. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J.; Jin, W.; Zhang, Q. The antioxidant activities and neuroprotective effect of polysaccharides from the starfish Asterias rollestoni. Carbohydr. Polym. 2013, 95, 9–15. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X.; Wang, Y.; Jin, W.; Guo, Y. The immunoenhancement effects of starfish Asterias rollestoni polysaccharides in macrophages and cyclophosphamide-induced immunosuppression mouse models. Food Funct. 2020, 11, 10700–10708. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.-S.; Kim, C.-H.; Shon, Y.-H. Breast cancer chemopreventive activity of polysaccharides from starfish in vitro. J. Microbiol. Biotechnol. 2006, 16, 1405–1409. [Google Scholar]
- Nam, K.-S.; Shon, Y.-H. Chemopreventive effects of polysaccharide extract from Asterina pectinifera on HT-29 human colon adenocarcinoma cells. BMB Rep. 2009, 42, 277–280. [Google Scholar] [CrossRef]
- Lee, K.-S.; Shin, J.-S.; Nam, K.-S. Cancer chemopreventive effects of starfish polysaccharide in human breast cancer cells. Biotechnol. Bioprocess Eng. 2011, 16, 978–991. [Google Scholar] [CrossRef]
- Lee, K.-S.; Shin, J.-S.; Nam, K.-S. Starfish polysaccharides downregulate metastatic activity through the MAPK signaling pathway in MCF-7 human breast cancer cells. Mol. Biol. Rep. 2013, 40, 5959–5966. [Google Scholar] [CrossRef] [PubMed]
- Baharara, J.; Amini, E. The potential of brittle star extracted polysaccharide in promoting apoptosis via intrinsic signaling pathway. Avicenna J. Med. Biotechnol. 2015, 7, 151–158. [Google Scholar] [PubMed]
- Ma, H.; Yuan, Q.; Tang, H.; Tan, H.; Li, T.; Wei, S.; Huang, J.; Yao, Y.; Hu, Y.; Zhong, S.; et al. Structural elucidation of a glucan from Trichaster palmiferus by its degraded products and preparation of its sulfated derivative as an anticoagulant. Mar. Drugs 2023, 21, 148. [Google Scholar] [CrossRef]
- Medeiros, G.F.; Mendes, A.; Castro, R.A.B.; Baú, E.C.; Nader, H.B.; Dietrich, C.P. Distribution of sulfated glycosaminoglycans in the animal kingdom: Widespread occurrence of heparin-like compounds in invertebrates. Biochim. Biophys. Acta 2000, 1475, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, R.; Namburi, R.B.; Ortega-Martinez, O.; Shi, X.; Zaja, J.; Dupont, S.T.; Thorndyke, M.C.; Lindahl, U.; Spillmann, D. Brittlestars contain highly sulfated chondroitin sulfates/dermatan sulfates that promote fibroblast growth factor 2-induced cell signaling. Glycobiology 2014, 24, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, R.; Namburi, R.B.; Dupont, S.T.; Ortega-Martinez, O.; van Kuppevelt, T.H.; Lindahl, U.; Spillmann, D. A potential role for chondroitin sulfate/dermatan sulfate in arm regeneration in Amphiura filiformis. Glycobiology 2017, 27, 438–449. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Tsvetkova, E.A.; Nifantiev, N.E.; Usov, A.I. Oversulfated dermatan sulfate and heparinoid in the starfish Lysastrosoma anthosticta: Structures and anticoagulant activity. Carbohydr. Polym. 2021, 261, 117867. [Google Scholar] [CrossRef]
- Smirnova, G.P.; Glukhoded, I.S.; Kochetkov, N.K. Gangliosides of the starfish Lethasterias fusca. Russ. J. Bioorg. Chem. 1986, 12, 377–382. [Google Scholar]
- Ivanchina, N.V.; Malyarenko, T.V.; Kicha, A.A.; Kalinovskii, A.I.; Dmitrenok, P.S. Polar steroidal compounds from the Far-Eastern starfish Lethasterias fusca. Russ. Chem. Bull. 2008, 57, 204–208. [Google Scholar] [CrossRef]
- Ivanchina, N.V.; Kalinovsky, A.I.; Kicha, A.A.; Malyarenko, T.V.; Dmitrenok, P.S.; Ermakova, S.P.; Stonik, V.A. Two new asterosaponins from the Far Eastern starfish Lethasterias fusca. Nat. Prod. Commun. 2012, 7, 853–858. [Google Scholar] [CrossRef]
- Popov, R.S.; Ivanchina, N.V.; Kicha, A.A.; Malyarenko, T.V.; Dmitrenok, P.S. Structural characterization of polar steroid compounds of the Far Eastern starfish Lethasterias fusca by nanoflow liquid chromatography coupled to quadrupole time-of-flight tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 2019, 30, 743–764. [Google Scholar] [CrossRef]
- Popov, R.S.; Ivanchina, N.V.; Kicha, A.A.; Malyarenko, T.V.; Grebnev, B.B.; Stonik, V.A.; Dmitrenok, P.S. The distribution of asterosaponins, polyhydroxysteroids and related glycosides in different body components of the Far Eastern starfish Lethasterias fusca. Mar. Drugs 2019, 17, 523. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.P.; Mulloy, B.; Mourão, P.A.S. Structure of a fucose-branched chondroitin sulfate from sea cucumber. Evidence for the presence of 3-O-sulfo-β-D-glucuronosyl residues. J. Biol. Chem. 1991, 266, 13530–13536. [Google Scholar] [CrossRef] [PubMed]
- Pavão, M.S.G.; Aiello, K.R.M.; Werneck, C.C.; Silva, L.C.F.; Valente, A.-P.; Mulloy, B.; Colwell, N.S.; Tollefsen, D.M.; Mourão, P.A. Highly sulfated dermatan sulfates from Ascidians. Structure versus anticoagulant activity of these glycosaminoglycans. J. Biol. Chem. 1998, 273, 27848–27857. [Google Scholar] [CrossRef] [PubMed]
- Pavão, M.S.G.; Mourão, P.A.S.; Mulloy, B.; Tollefsen, D.M. A unique dermatan sulfate-like glycosaminoglycan from Ascidian. Its structure and effect of its unusual sulfation pattern on anticoagulant activity. J. Biol. Chem. 1995, 270, 31027–31036. [Google Scholar] [CrossRef]
- Bossennec, V.; Petitou, M.; Perly, B. 1H-NMR investigation of naturally occurring and chemically oversulphated dermatan sulphates. Identification of minor monosaccharide residues. Biochem. J. 1990, 267, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lin, L.; Agyecum, I.; Zhang, X.; Ange, K.S.; Yu, Y.; Linhardt, R.J. Structural analysis of heparin-derived 3-O-sulfated tetrasaccharides: Antithrombin binding site variants. J. Pharm. Sci. 2017, 106, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Yates, E.A.; Santini, F.; De Cristofano, B.; Payre, N.; Cosentino, C.; Guerrini, M.; Naggi, A.; Torri, G.; Hricovini, M. Effect of substitution pattern on 1H, 13C NMR chemical shifts and 1JCH coupling constants in heparin derivatives. Carbohydr. Res. 2000, 329, 239–247. [Google Scholar] [CrossRef]
- Martin, C.E.; Weishaupt, M.W.; Seeberger, P.H. Progress toward developing a carbohydrate-conjugate vaccine against Clostridium difficile ribotype 027: Synthesis of the cell-surface polysaccharide PS-I repeating unit. Chem. Commun. 2011, 47, 10260–10262. [Google Scholar] [CrossRef]
- Cao, X.; Lv, Q.; Li, D.; Ye, H.; Yan, X.; Yang, X.; Gan, H.; Zhao, W.; Jin, L.; Wang, P.; et al. Direct C5-isomerization approach to L-iduronic acid derivatives. Asian J. Org. Chem. 2015, 4, 899–902. [Google Scholar] [CrossRef]
- Notaro, A.; Vershinin, Z.; Guan, Z.; Eichler, J.; De Castro, C. An N-linked tetrasaccharide from Halobacterium salinarum presents a novel modification, sulfation of iduronic acid at the O-3 position. Carbohydr. Res. 2022, 521, 108651. [Google Scholar] [CrossRef]
- Anisimova, N.; Ustyuzhanina, N.; Bilan, M.; Donenko, F.; Usov, A.; Kiselevskiy, M.; Nifantiev, N. Fucoidan and fucosylated chondroitin sulfate stimulate hematopoiesis in cyclophosphamide-induced mice. Mar. Drugs 2017, 15, 301. [Google Scholar] [CrossRef] [PubMed]
- Anisimova, N.Y.; Ustyuzhanina, N.E.; Bilan, M.I.; Donenko, F.V.; Ushakova, N.A.; Usov, A.I.; Kiselevskiy, M.V.; Nifantiev, N.E. Influence of modified fucoidan and related sulfated oligosaccharides on hematopoiesis in cyclophosphamide-induced mice. Mar. Drugs 2018, 16, 333. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Niu, Q.; Li, S.; Zhang, X.; Liu, C.; Cai, C.; Li, G.; Yu, G. Fucoidan from sea cucumber Holothuria polii: Structural elucidation and stimulation of hematopoietic activity. Int. J. Biol. Macromol. 2020, 154, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Shashkov, A.S.; Ponce, N.M.A.; Stortz, C.A.; Nifantiev, N.E.; Usov, A.I. Fucosylated chondroitin sulfate from the sea cucumber Hemioedema spectabilis: Structure and influence on cell adhesion and tubulogenesis. Carbohydr. Polym. 2020, 234, 115895. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.E.; Anisimova, N.Y.; Bilan, M.I.; Donenko, F.V.; Morozevich, G.E.; Yashunskiy, D.V.; Usov, A.I.; Siminyan, N.G.; Kirgisov, K.I.; Varfolomeeva, S.R.; et al. Chondroitin sulfate and fucosylated chondroitin sulfate as stimulators of hematopoiesis in cyclophosphamide-induced mice. Pharmaceuticals 2021, 14, 1074. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus evanescens C.Ag. Carbohydr. Res. 2002, 337, 719–730. [Google Scholar] [CrossRef]
- Bilan, M.I.; Zakharova, A.N.; Grachev, A.A.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Polysaccharides of algae. 60. Fucoidan from the Pacific brown alga Analipus japonicus (Harv.) Winne (Ectocarpales, Scytosiphonaceae). Russ. J. Bioorg. Chem. 2007, 33, 38–46. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulfate content of sulfated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef]
- Usov, A.I.; Bilan, M.I.; Klochkova, N.G. Polysaccharides of algae. 48. Polysaccharide composition of several calcareous red algae: Isolation of alginate from Corallina pilulifera P. et R. (Rhodophyta, Corallinaceae). Bot. Mar. 1995, 38, 43–51. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Tsvetkova, E.A.; Shashkov, A.S.; Stonik, V.A.; Nifantiev, N.E.; Usov, A.I. Structural characterization of fucosylated chondroitin sulfates from sea cucumbers Apostichopus japonicus and Actinopyga mauritiana. Carbohydr. Polym. 2016, 153, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. The structure of a fucosylated chondroitin sulfate from the sea cucumber Cucumaria frondosa. Carbohydr. Polym. 2017, 165, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Condra, M.; Kimura, K.; Berth, G.; Dautzenberg, H.; Dubin, P.L. Determination of molecular weight of heparin by size exclusion chromatography with universal calibration. Anal. Biochem. 2003, 312, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).