A Novel, Highly Potent NADPH-Dependent Cytochrome P450 Reductase from Waste Liza klunzingeri Liver
Abstract
1. Introduction
2. Results and Discussion
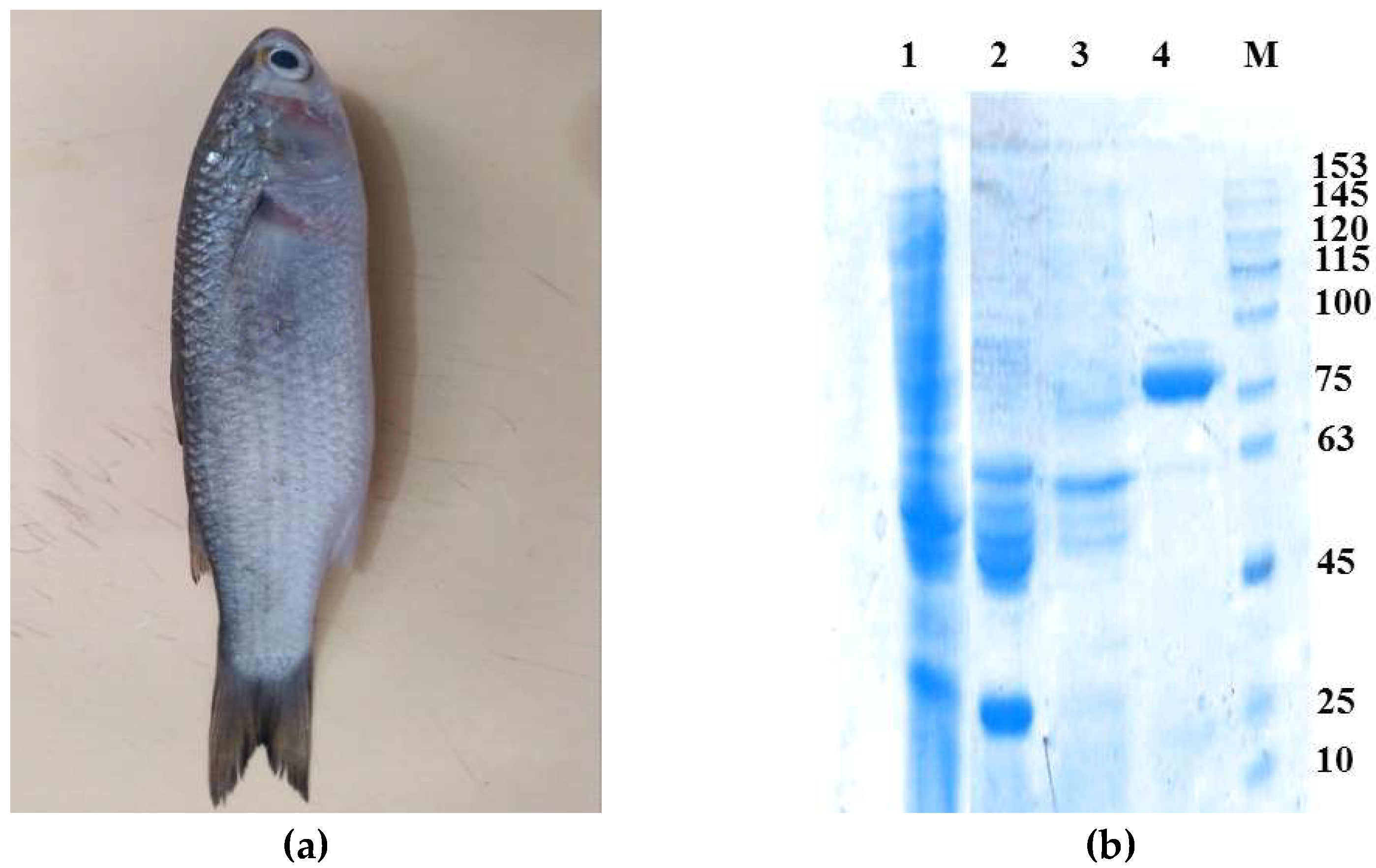
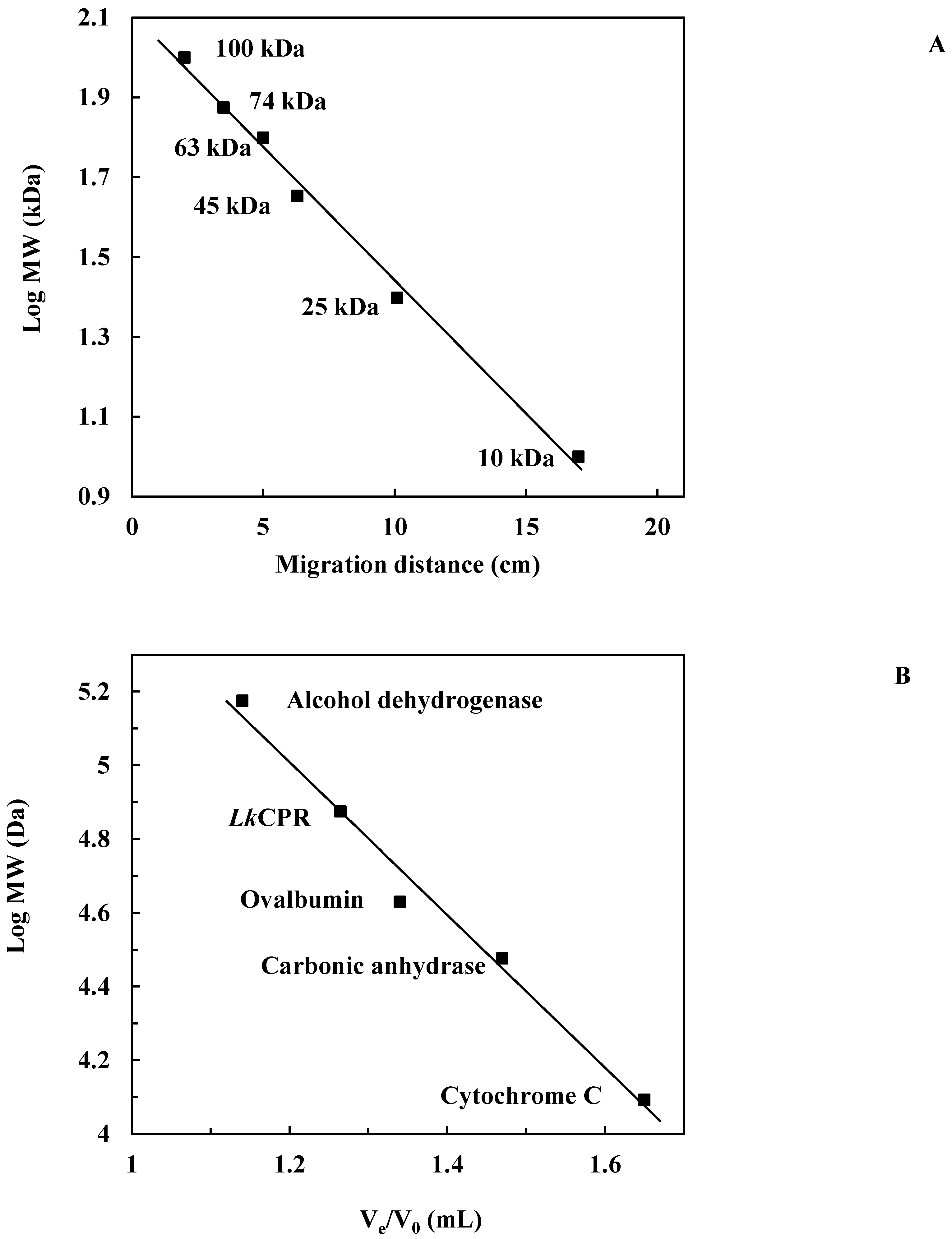
2.1. Isolation and Purification of the Enzyme
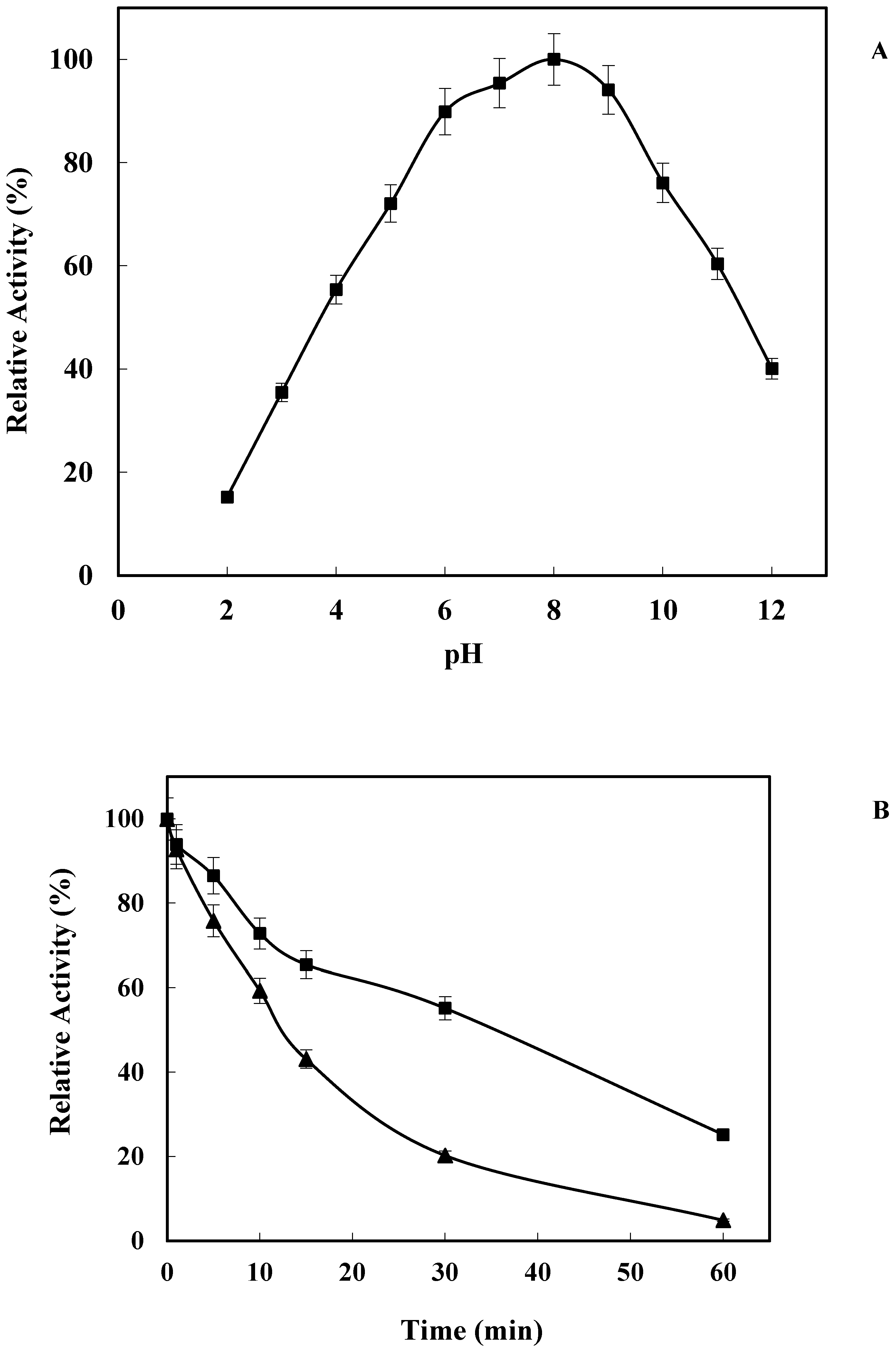
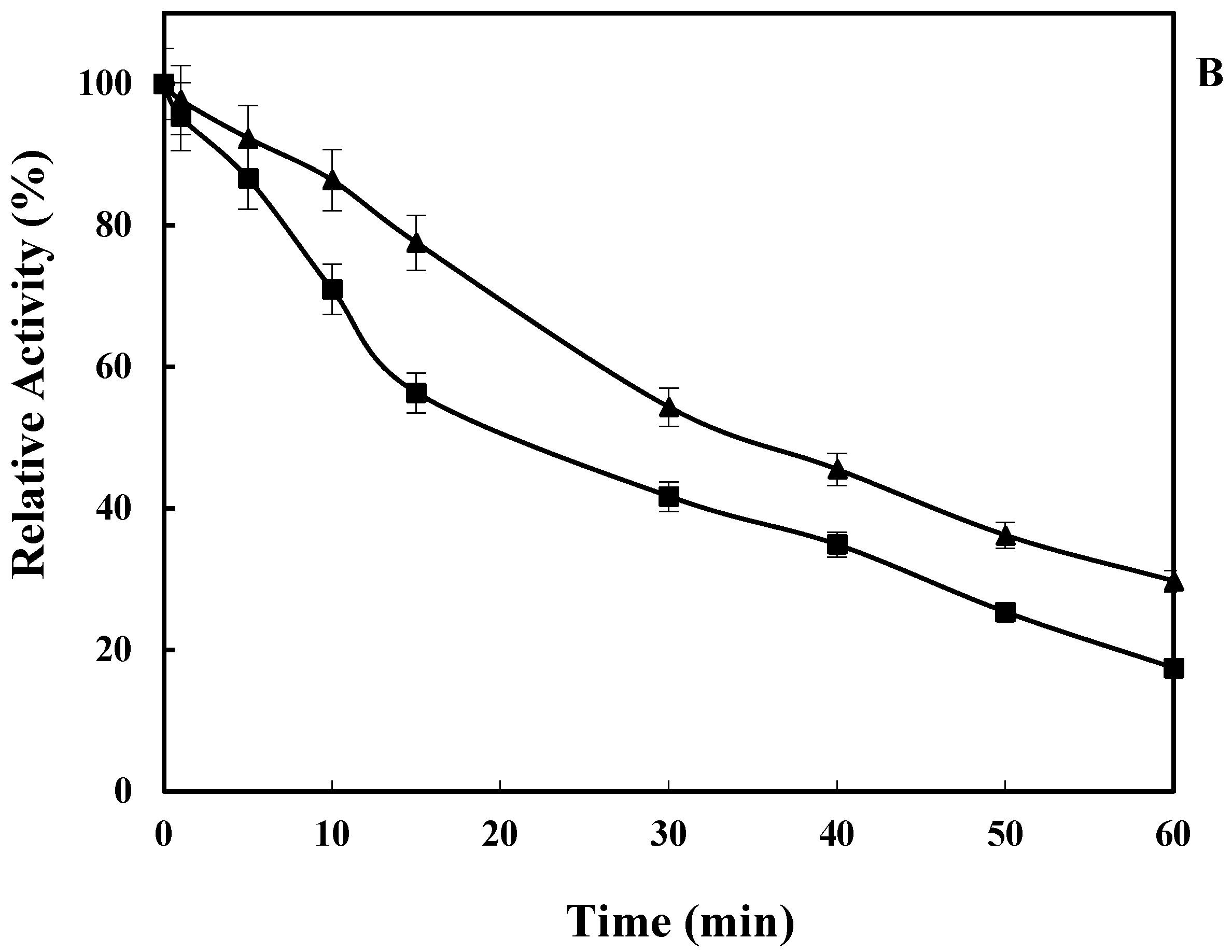
2.2. Effect of pH on LkCPR Activity and Stability
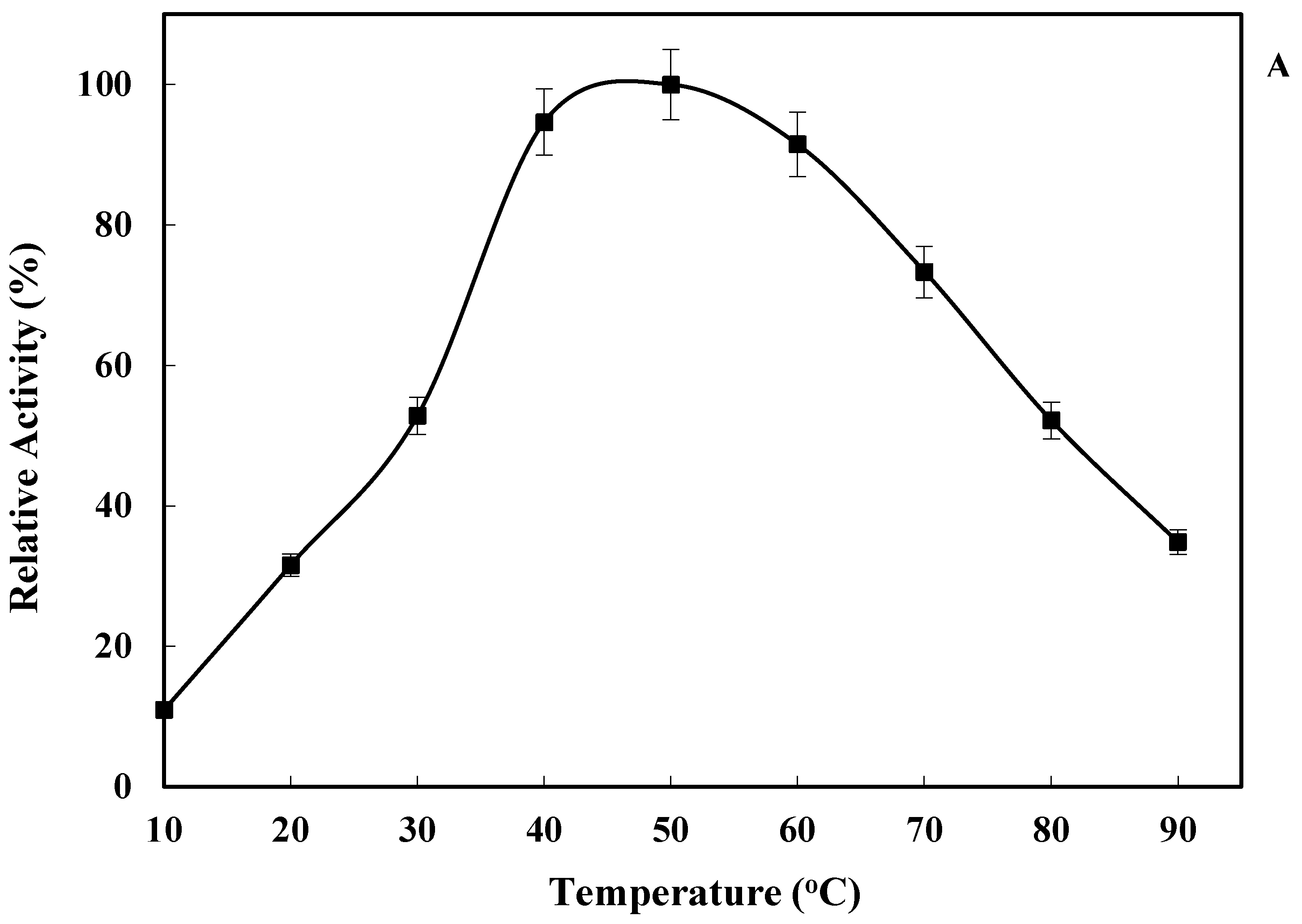
2.3. Effect of Temperature on LkCPR Activity and Stability
2.4. Steady-State Kinetics
3. Materials and Methods
3.1. Materials
3.2. Collecting Samples and Extraction of Protein Extracts from Liver Microsomal Cells
3.3. Isolation and Purification of LkCPR
3.3.1. Ammonium Sulfate Precipitation
3.3.2. Purification and Determination of Molecular Weight
3.4. Enzymatic Activity Assay
3.5. Biochemical Characterization of LkCPR
3.5.1. Effect of Temperature on Enzyme Activity and Stability
3.5.2. Effect of pH on Enzyme Activity and Stability
3.5.3. Determination of Kinetic Parameters
3.5.4. Calculation of Thermodynamic Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hale, S.S.; Buffum, H.W.; Hughes, M.M. Six decades of change in pollution and benthic invertebrate biodiversity in a southern New England estuary. Mar. Pollut. Bull. 2018, 133, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Gaur, N.; Narasimhulu, K.; PydiSetty, Y. Recent advances in the bio-remediation of persistent organic pollutants and its effect on environment. J. Clean. Prod. 2018, 198, 1602–1631. [Google Scholar] [CrossRef]
- Ismail, M.; Akhtar, K.; Khan, M.; Kamal, T.; Khan, M.A.; Asiri, A.M.; Seo, J.; Khan, S.B. Pollution, toxicity and carcinogenicity of organic dyes and their catalytic bio-remediation. Curr. Pharm. Des. 2019, 25, 3645–3663. [Google Scholar] [CrossRef]
- Razzaghi, M.; Homaei, A.; Vianello, F.; Azad, T.; Sharma, T.; Nadda, A.K.; Stevanato, R.; Bilal, M.; Iqbal, H. Industrial applications of immobilized nano-biocatalysts. Bioprocess Biosyst. Eng. 2022, 45, 237–256. [Google Scholar] [CrossRef] [PubMed]
- Ranjbari, N.; Razzaghi, M.; Fernandez-Lafuente, R.; Shojaei, F.; Satari, M.; Homaei, A. Improved features of a highly stable protease from Penaeus vannamei by immobilization on glutaraldehyde activated graphene oxide nanosheets. Int. J. Biol. Macromol. 2019, 130, 564–572. [Google Scholar] [CrossRef]
- Yan, H.; Wu, L.; Yu, J. The environmental impact analysis of hazardous materials and the development of green technology in the shipbreaking process. Ocean Eng. 2018, 161, 187–194. [Google Scholar] [CrossRef]
- Homaei, A. Purification and biochemical properties of highly efficient alkaline phosphatase from Fenneropenaeus merguiensis brain. J. Mol. Catal. B Enzym. 2015, 118, 16–22. [Google Scholar] [CrossRef]
- Ufarte, L.; Laville, E.; Duquesne, S.; Potocki-Veronese, G. Metagenomics for the discovery of pollutant degrading enzymes. Biotechnol. Adv. 2015, 33, 1845–1854. [Google Scholar] [CrossRef]
- Kadri, T.; Rouissi, T.; Brar, S.K.; Cledon, M.; Sarma, S.; Verma, M. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by fungal enzymes: A review. J. Environ. Sci. 2017, 51, 52–74. [Google Scholar] [CrossRef]
- Homaei, A. Immobilization of Penaeus merguiensis alkaline phosphatase on gold nanorods for heavy metal detection. Ecotoxicol. Environ. Saf. 2017, 136, 1–7. [Google Scholar] [CrossRef]
- Ahuja, S.K.; Ferreira, G.M.; Moreira, A.R. Utilization of enzymes for environmental applications. Crit. Rev. Biotechnol. 2004, 24, 125–154. [Google Scholar] [CrossRef]
- Nikolaivits, E.; Dimarogona, M.; Fokialakis, N.; Topakas, E. Marine-derived biocatalysts: Importance, accessing, and application in aromatic pollutant bioremediation. Front. Microbiol. 2017, 8, 265. [Google Scholar] [CrossRef] [PubMed]
- Menzorova, N.I.; Seitkalieva, A.V.; Rasskazov, V.A. Enzymatic methods for the determination of pollution in seawater using salt resistant alkaline phosphatase from eggs of the sea urchin Strongylocentrotus intermedius. Mar. Pollut. Bull. 2014, 79, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Seitkalieva, A.V.; Menzorova, N.I.; Rasskazov, V.A. Application of different enzyme assays and biomarkers for pollution monitoring of the marine environment. Environ. Monit. Assess. 2016, 188, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ducharme, J.; Auclair, K. Use of bioconjugation with cytochrome P450 enzymes. Biochim. Et Biophys. Acta (BBA) Proteins Proteom. 2018, 1866, 32–51. [Google Scholar] [CrossRef] [PubMed]
- Rudeck, J.; Bert, B.; Marx-Stoelting, P.; Schönfelder, G.; Vogl, S. Liver lobe and strain differences in the activity of murine cytochrome p450 enzymes. Toxicology 2018, 404, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Brummund, J.; Müller, M.; Schmitges, T.; Kaluzna, I.; Mink, D.; Hilterhaus, L.; Liese, A. Process development for oxidations of hydrophobic compounds applying cytochrome P450 monooxygenases in-vitro. J. Biotechnol. 2016, 233, 143–150. [Google Scholar] [CrossRef]
- Sharma, B.; Dangi, A.K.; Shukla, P. Contemporary enzyme based technologies for bioremediation: A review. J. Environ. Manag. 2018, 210, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Chen, B.; Qiu, X.; Lin, K.; Yu, X. Three novel cytochrome P450 genes identified in the marine polychaete Perinereis nuntia and their transcriptional response to xenobiotics. Aquat. Toxicol. 2013, 134, 11–22. [Google Scholar] [CrossRef]
- Han, J.; Won, E.-J.; Kang, H.-M.; Lee, M.-C.; Jeong, C.-B.; Kim, H.-S.; Hwang, D.-S.; Lee, J.-S. Marine copepod cytochrome P450 genes and their applications for molecular ecotoxicological studies in response to oil pollution. Mar. Pollut. Bull. 2017, 124, 953–961. [Google Scholar] [CrossRef]
- Basilone, G.; Gargano, A.; Corriero, A.; Zupa, R.; Santamaria, N.; Mangano, S.; Ferreri, R.; Pulizzi, M.; Mazzola, S.; Bonanno, A. Liver melanomacrophage centres and CYP1A expression as response biomarkers to environmental pollution in European anchovy (Engraulis encrasicolus) from the western Mediterranean Sea. Mar. Pollut. Bull. 2018, 131, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Mundle, S.O.; Spain, J.C.; Lacrampe-Couloume, G.; Nishino, S.F.; Lollar, B.S. Branched pathways in the degradation of cDCE by cytochrome P450 in Polaromonas sp. JS666. Sci. Total Environ. 2017, 605, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, V.; Donmez, E.; Girhard, M.; Urlacher, V.B.; Constantí, M. Biodegradation of fuel oxygenates and their effect on the expression of a newly identified cytochrome P450 gene in Achromobacter xylosoxidans MCM2/2/1. Process Biochem. 2014, 49, 124–129. [Google Scholar] [CrossRef]
- Wu, R.-R.; Dang, Z.; Yi, X.-Y.; Yang, C.; Lu, G.-N.; Guo, C.-L.; Liu, C.-Q. The effects of nutrient amendment on biodegradation and cytochrome P450 activity of an n-alkane degrading strain of Burkholderia sp. GS3C. J. Hazard. Mater. 2011, 186, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.V.; Flück, C.E. NADPH P450 oxidoreductase: Structure, function, and pathology of diseases. Pharmacol. Ther. 2013, 138, 229–254. [Google Scholar] [CrossRef] [PubMed]
- Klotz, A.V.; Stegeman, J.J.; Walsh, C. An aryl hydrocarbon hydroxylating hepatic cytochrome P-450 from the marine fish Stenotomus chrysops. Arch. Biochem. Biophys. 1983, 226, 578–592. [Google Scholar] [CrossRef]
- Sen, A.; Arinc, E. Purification and characterization of cytochrome P450 reductase from liver microsomes of feral leaping mullet (Liza saliens). J. Biochem. Mol. Toxicol. 1998, 12, 103–113. [Google Scholar] [CrossRef]
- Arinç, E. Characterization of cytochrome P450 dependent mixed-function oxidase system of gilthead seabream (Sparus aurata; Sparidae) liver. Comp. Biochem. Physiol. Part B Comp. Biochem. 1993, 104, 133–139. [Google Scholar] [CrossRef]
- Kojima, H.; Takahashi, K.; Sakane, F.; Koyama, J. Purification and characterization of NADPH-cytochrome c reductase from porcine polymorphonuclear leukocytes. J. Biochem. 1987, 102, 1083–1088. [Google Scholar] [CrossRef]
- Kubota, S.; Yoshida, Y.; Kumaoka, H.; Furumichi, A. Studies on the microsomal electron-transport system of anaerobically grown yeast: V. Purification and characterization of NADPH-cytochrome c reductase. J. Biochem. 1977, 81, 197–205. [Google Scholar] [CrossRef]
- Shen, A.L.; Porter, T.; Wilson, T.; Kasper, C. Structural analysis of the FMN binding domain of NADPH-cytochrome P-450 oxidoreductase by site-directed mutagenesis. J. Biol. Chem. 1989, 264, 7584–7589. [Google Scholar] [CrossRef] [PubMed]
- Kuzu, M.; Ciftci, M. Purification and characterization of NADPH-cytochrome P450 reductase from Lake Van fish liver microsomes and investigation of some chemical and metals’ effects on the enzyme activity. Turk. J. Chem. 2015, 39, 149–158. [Google Scholar] [CrossRef]
- Kuwahara, T.; White, R.A., Jr.; Agosin, M. A cytosolic FAD-containing enzyme catalyzing cytochrome c reduction in Trypanosoma cruzi. I. Purification and some properties. Arch. Biochem. Biophys. 1985, 239, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Tsou, C.-Y.; Matsunaga, S.; Okada, S. Molecular cloning and functional characterization of NADPH-dependent cytochrome P450 reductase from the green microalga Botryococcus braunii, B race. J. Biosci. Bioeng. 2018, 125, 30–37. [Google Scholar] [CrossRef]
- Milhim, M.; Gerber, A.; Neunzig, J.; Hannemann, F.; Bernhardt, R. A Novel NADPH-dependent flavoprotein reductase from Bacillus megaterium acts as an efficient cytochrome P450 reductase. J. Biotechnol. 2016, 231, 83–94. [Google Scholar] [CrossRef]
- Lee, G.-Y.; Kim, H.M.; Ma, S.H.; Park, S.H.; Joung, Y.H.; Yun, C.-H. Heterologous expression and functional characterization of the NADPH-cytochrome P450 reductase from Capsicum annuum. Plant Physiol. Biochem. 2014, 82, 116–122. [Google Scholar] [CrossRef]
- Takahashi, N.; Saito, T.; Goda, Y.; Tomita, K. Characterization of microsomal NADPH-dependent aldehyde reductase from rat brain. J. Biochem. 1986, 99, 513–519. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Guengerich, F.P.; Martin, M.V.; Sohl, C.D.; Cheng, Q. Measurement of cytochrome P450 and NADPH–cytochrome P450 reductase. Nat. Protoc. 2009, 4, 1245. [Google Scholar] [CrossRef]
- Williams, J.; Kamin, H. The preparation and properties of microsomal TPNH-cytochrome c reductase from pig liver. J. Biol. Chem. 1962, 237, 587–595. [Google Scholar] [CrossRef]
- Yonetani, T. Studies on cytochrome c peroxidase II. Stoichiometry between enzyme, H2O2, and ferrocytochrome c and enzymic determination of extinction coefficients of cytochrome c. J. Biol. Chem. 1965, 240, 4509–4514. [Google Scholar] [CrossRef] [PubMed]
- Swift, M.L. GraphPad prism, data analysis, and scientific graphing. J. Chem. Inf. Comput. Sci. 1997, 37, 411–412. [Google Scholar] [CrossRef]
- Arrhenius, S. On the reaction velocity of the inversion of cane sugar by acids. J. Phys. Chem 1889, 4, 226. [Google Scholar]
- Copeland, R.A. Enzymes: A Practical Introduction to Structure, Mechanism, and Data Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Lamb, S.B.; Lamb, D.C.; Kelly, S.L.; Stuckey, D.C. Cytochrome P450 immobilisation as a route to bioremediation/biocatalysis. FEBS Lett. 1998, 431, 343–346. [Google Scholar] [CrossRef]
| Purification Steps | Total Protein (mg) | Total Activity (IU) | Specific Activity (IU/mg) | Purification Fold | Yield (%) |
|---|---|---|---|---|---|
| Cell extract | 1683 ± 156 | 14,732 ± 2160 | 8.70 ± 0.75 | 1 ± 0.00 | 100 ± 0.00 |
| (NH4)2SO4 | 1009 ± 128 | 12,316 ± 1983 | 12.16 ± 0.59 | 1.39 ± 0.05 | 83 ± 1.52 |
| DEAE-Sepharose I | 352 ± 43 | 10,663 ± 2351 | 30.01 ± 3.50 | 3.43 ± 0.12 | 71 ± 5.85 |
| DEAE-Sepharose II | 58 ± 13 | 8434 ± 2500 | 143.70 ± 11.62 | 16.51 ± 0.46 | 56 ± 9.07 |
| 2′,5′-ADP Sepharose 4B | 16 ± 6 | 6579 ± 2968 | 402.07 ± 35.10 | 46.20 ± 2.08 | 42 ± 14.74 |
| Parameters | Substrate | |
|---|---|---|
| Cytochrome C | NADPH | |
| Km (µM) | 8.83 ± 0.15 | 7.26 ± 0.20 |
| Vmax (µM·min−1) | 25.96 ± 0.30 | 26.73 ± 0.35 |
| kcat (s−1) | 202.93 ± 0.53 | 206.79 ± 33 |
| kcat/Km (s−1 µM−1) | 22.05 ± 0.59 | 27.57 ± 0.84 |
| Ea (kcal mol−1) | ΔH# (kcal mol−1) | ΔG# (kcal mol−1) | ΔS# (cal molK−1) | ΔG#E-S (kcal mol−1) | ΔG#E-T (kcal mol−1) |
|---|---|---|---|---|---|
| 16.33 ± 0.20 | 15.73 ± 0.20 | 14.40 ± 0.35 | 4.43 ± 0.51 | 1.36 ± 0.15 | −2 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahramian Nasab, S.; Homaei, A.; Fernandez-Lafuente, R.; Del Arco, J.; Fernández-Lucas, J. A Novel, Highly Potent NADPH-Dependent Cytochrome P450 Reductase from Waste Liza klunzingeri Liver. Mar. Drugs 2023, 21, 99. https://doi.org/10.3390/md21020099
Bahramian Nasab S, Homaei A, Fernandez-Lafuente R, Del Arco J, Fernández-Lucas J. A Novel, Highly Potent NADPH-Dependent Cytochrome P450 Reductase from Waste Liza klunzingeri Liver. Marine Drugs. 2023; 21(2):99. https://doi.org/10.3390/md21020099
Chicago/Turabian StyleBahramian Nasab, Soudeh, Ahmad Homaei, Roberto Fernandez-Lafuente, Jon Del Arco, and Jesús Fernández-Lucas. 2023. "A Novel, Highly Potent NADPH-Dependent Cytochrome P450 Reductase from Waste Liza klunzingeri Liver" Marine Drugs 21, no. 2: 99. https://doi.org/10.3390/md21020099
APA StyleBahramian Nasab, S., Homaei, A., Fernandez-Lafuente, R., Del Arco, J., & Fernández-Lucas, J. (2023). A Novel, Highly Potent NADPH-Dependent Cytochrome P450 Reductase from Waste Liza klunzingeri Liver. Marine Drugs, 21(2), 99. https://doi.org/10.3390/md21020099










