Bioprospecting of Targeted Phenolic Compounds of Dictyota dichotoma, Gongolaria barbata, Ericaria amentacea, Sargassum hornschuchii and Ellisolandia elongata from the Adriatic Sea Extracted by Two Green Methods
Abstract
1. Introduction
2. Results and Discussion
2.1. Solvent Selection
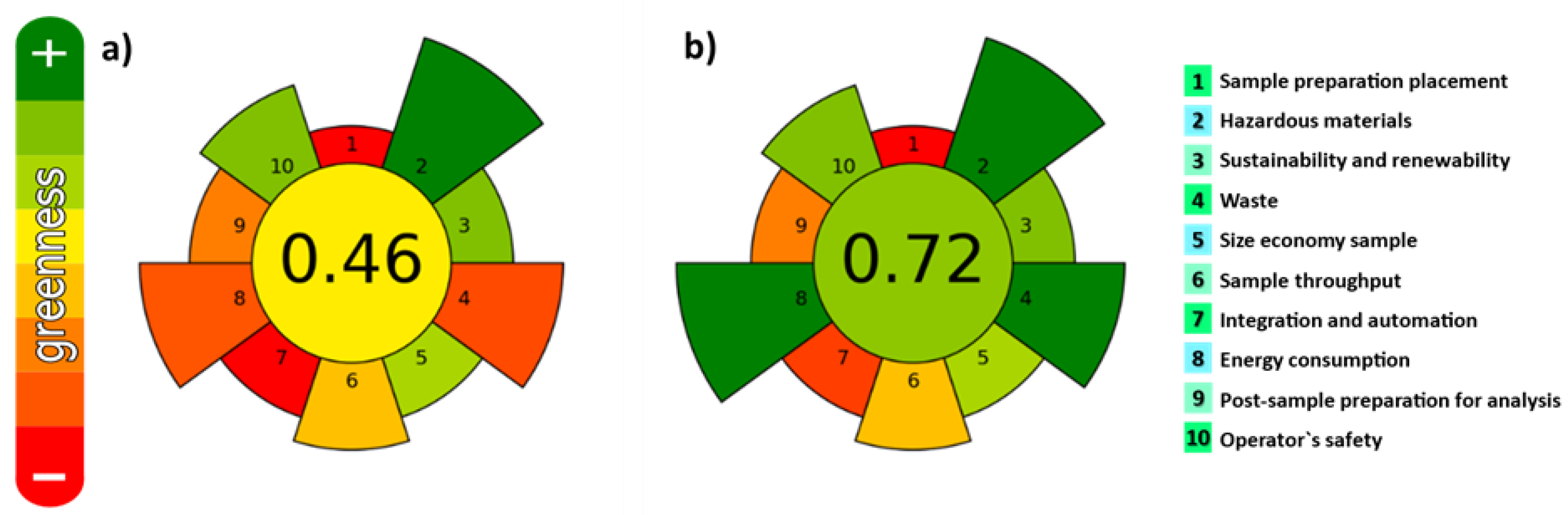
2.2. Comparison between UAE and MSPD to Extract Polyphenols
2.2.1. TPC and AA Analysis
2.2.2. IC50 Results
2.2.3. Quantification of Individual Polyphenols
3. Materials and Methods
3.1. Sampling
3.2. Standards, Reagents and Materials
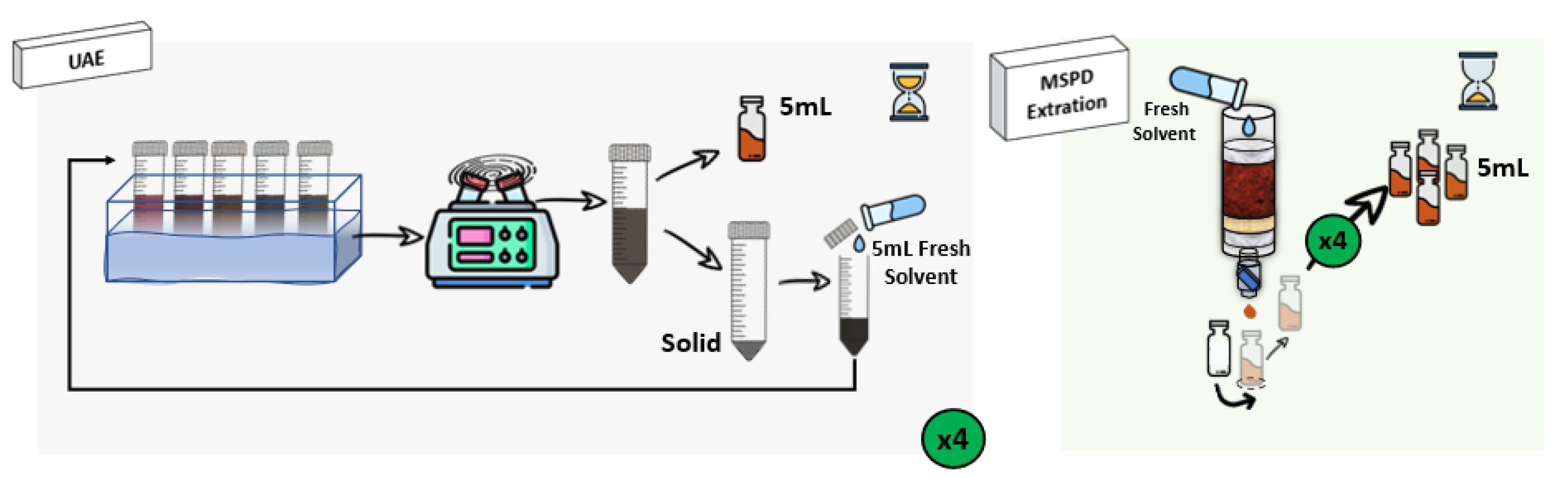
3.3. Extraction Procedures: UAE and MSPD
3.3.1. Ultrasound-Assisted Extraction (UAE)
3.3.2. Matrix Solid-Phase Dispersion (MSPD)
3.4. Total Polyphenolic Content (TPC)
3.5. Antioxidant Activity (AA) and IC50
3.6. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santos, S.N.; Félix, R.; Pais, A.C.S.; Rocha, S.M.; Silvestre, A.J.D. The quest for phenolic compounds from macroalgae: A review of extraction and identification methodologies. Biomolecules 2019, 9, 847. [Google Scholar] [CrossRef]
- Abdala-Diaz, R.T.; Cabello-Pasini, A.; Pérez-Rodriguez, E.; Alvarez, R.M.; Figueroa, F.L. Daily and seasonal variations of optimum quantum yield and phenolic compounds in Cystoseira tamariscifolia (Phaeophyta). Mar. Biol. 2006, 148, 459–465. [Google Scholar] [CrossRef]
- Martins, C.D.L.; Ramlov, F.; Nocchi Carneiro, N.P.; Gestinari, L.M.; dos Santos, B.F.; Bento, L.M.; Lhullier, C.; Gouvea, L.; Bastos, E.; Horta, P.A. Antioxidant properties and total phenolic contents of some tropical seaweeds of the Brazilian coast. J. Appl. Phycol. 2013, 25, 1179–1187. [Google Scholar] [CrossRef]
- Stiger-Pouvreau, V.R.; Jégou, C.; Cérantola, S.P.; Guerard, F.; Lann, K.L.; Bourgougnon, N. Chapter Thirteen—Phlorotannins in Sargassaceae Species from Brittany (France). In Interesting Molecules for Ecophysiological and Valorisation Purposes, Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2014; pp. 379–411. [Google Scholar]
- Kosanic, M.; Rankovic, B.; Stanojkovic, T. Brown macroalgae from the Adriatic Sea as a promising source of bioactive nutrients. J. Food Measur. Charact. 2019, 13, 330–338. [Google Scholar] [CrossRef]
- Bruno de Sousa, C.; Gangadhar, K.N.; Macridachis, J.; Pav, M.; Morais, T.R.; Campino, L.; Varela, J.; Lago, J. Cystoseira algae (Fucaceae): Update on their chemical entities and biological activities. Tetrahedron. Asym. 2017, 28, 1486–1505. [Google Scholar] [CrossRef]
- Mannino, A.M.; Micheli, C. Ecological function of phenolic compounds from mediterranean fucoid algae and seagrasses: An overview on the genus Cystoseira sensu lato and Posidonia oceanica (L.) Delile. J. Mar. Sci. Eng. 2020, 8, 19. [Google Scholar] [CrossRef]
- Generalic Mekinic, I.; Simat, V.; Botic, V.; Crnjac, A.; Smoljo, M.; Soldo, B.; Ljubenkov, I.; Cagalj, M.; Skroza, D. Bioactive phenolic metabolites from Adriatic brown algae Dictyota dichotoma and Padina pavonica (Dictyotaceae). Foods 2021, 10, 1187. [Google Scholar] [CrossRef]
- Farvin, K.H.S.; Jacobsen, C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef]
- Cadar, E.; Sirbu, R.; Ibram, A.; Ionescu, A.M. Evaluation of total phenolic content in relation to antioxidant activity of brown algae Cystoseira barbata from Black Sea. Rev. Chimie 2019, 70, 2684–2689. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Mohamed, H.; Mostafa, S.; Ibraheem, I. Controlling of microbial growth by using Cystoseira barbata extract. Egyp. J. Bot. 2017, 57, 469–477. [Google Scholar]
- Heffernan, N.; Brunton, N.P.; FitzGerald, R.J.; Smyth, T.J. Profiling of the molecular weight and structural isomer abundance of macroalgae-derived phlorotannins. Mar. Drugs 2015, 13, 509–528. [Google Scholar] [CrossRef]
- Montero, L.; Herrero, M.; Ibañez, E.; Cifuentes, A. Separation and characterization of phlorotannins from brown algae Cystoseira abies-marina by comprehensive two-dimensional liquid chromatography. Electrophoresis 2014, 35, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentao, P.C. Phlorotannin extracts from fucales characterized by HPLC-DAD-ESI-MS: Approaches to hyaluronidase inhibitory capacity and antioxidant properties. Mar. Drugs 2012, 10, 2766–2781. [Google Scholar] [CrossRef] [PubMed]
- Klejdus, B.; Lojková, L.; Plaza, M.; Snovoblá, M.; Stervobá, D. Hyphenated technique for the extraction and determination of isoflavones in algae: Ultrasound-assisted supercritical fluid extraction followed by fast chromatography with tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 7956–7965. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, R.; Chitra, L.; Penislusshiyan, S.; Palvannan, T. Exploring bioactive fraction of Sargassum wightii: In Vitro elucidation of angiotensin-I-converting enzyme inhibition and antioxidant potential. Int. J. Food Prop. 2018, 21, 674–684. [Google Scholar] [CrossRef]
- Rodrigues, D.; Sousa, S.; Silva, A.; Amorim, M.; Pereira, L.; Rocha-Santos, T.A.P.; Gomes, A.M.P.; Duarte, A.C.; Freitas, A.C. Impact of enzyme-and ultrasound-assisted extraction methods on biological properties of red, brown, and green seaweeds from the central west coast of Portugal. J. Agric. Food Chem. 2015, 63, 3177–3188. [Google Scholar] [CrossRef]
- Del Pilar Sanchez-Camargo, A.; Montero, L.; Stiger-Pouvreau, V.; Tanniou, A.; Cifuentes, A.; Herrero, M.; Ibañez, E. Considerations on the use of enzyme-assisted extraction in combination with pressurized liquids to recover bioactive compounds from algae. Food Chem. 2016, 192, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.S.; Ngo, D.H.; Kim, S.K. Brown Algal Polyphenol and Its Pharmaceutical Properties, Marine-Derived Biomaterials for Tissue Engineering Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 223–243. [Google Scholar]
- Aminina, N.M.; Karaulova, E.P.; Vishnevskaya, T.I.; Yakush, E.V.; Kim, Y.K.; Nam, K.H.; Son, K.T. Characteristics of polyphenolic content in brown algae of the Pacific Coast of Russia. Molecules 2020, 25, 3909. [Google Scholar] [CrossRef]
- Generalic-Mekinic, I.; Skroza, D.; Simat, V.; Hamed, I.; Sgalj, M.; Popovic Perkovic, Z. Phenolic content of brown algae (Pheophyceae) species: Extraction, identification, and quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef]
- Ktari, L.; Mdallel, C.; Aoun, B.; Chebil Ajjabi, L.; Sadok, S. Fucoxanthin and phenolic contents of six dictyotales from the Tunisian coasts with an emphasis for a green extraction using a supercritical CO2 method. Front. Mar. Sci. 2021, 8, 647159. [Google Scholar] [CrossRef]
- Korbee, N.; Navarro, N.P.; García-Sánchez, M.; Celis-Plá, P.S.M.; Quintano, E.; Copertino, M.; Pedersen, A.; Mariath, R.; Mangaiyarkarasi, N.; Pérez-Ruzafa, A. A novel in situ system to evaluate the effect of high CO2 on photosynthesis and biochemistry of seaweeds. Aquat. Biol. 2014, 22, 245–259. [Google Scholar] [CrossRef]
- Haddar, A.; Sellimi, S.; Ghannouchi, R.; Alvarez, O.M.; Nasri, M.; Bougatef, A. Functional, antioxidant and film-forming properties of tuna-skin gelatin with a brown algae extract. Int. J. Biol. Macromol. 2012, 51, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). Generally Recognized as Safe (GRAS). Available online: https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras (accessed on 28 November 2022).
- Kadam, S.U.; O’Donnell, C.P.; Rai, D.K.; Hossain, M.B.; Burgess, C.M.; Walsh, D.; Tiwari, B.K. Laminarin from Irish brown seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound assisted extraction, characterization and bioactivity. Mar. Drugs 2015, 13, 4270–4280. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.T.; Van Vuong, Q.; Schreider, M.J.; Bowyer, M.C.; Van Altena, L.A.; Scarlett, C.J. Optimisation of ultrasound-assisted extraction conditions for phenolic content and antioxidant activities of the alga Hormosira banksii using response surface methodology. J. Appl. Phycol. 2017, 29, 3161–3173. [Google Scholar] [CrossRef]
- Dang, T.T.; Bowyer, M.C.; Van Altena, L.A.; Scarlett, C.J. Comparison of chemical profile and antioxidant properties of the brown algae. Int. J. Food Sci. Technol. 2018, 53, 174–181. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Tavanandi, H.A.; Mantri, V.A.; Raghavarao, K. Ultrasound assisted methods for enhanced extraction of phycobiliproteins from marine macro-algae, Gelidium pusillum (Rhodophyta). Ultrason. Sonochem. 2017, 38, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and drawbacks of ultrasound-assisted extraction for the recovery of bioactive compounds from marine algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef]
- Castillo, A.; Pereira, S.; Otero, A.; Fiol, S.; Garcia-Jares, C.; Lores, M. Matrix solid-phase dispersion as a greener alternative to obtain bioactive extracts from Haematococcus pluvialis. Characterization by UHPLC-QToF. RSC Adv. 2020, 10, 27995–28006. [Google Scholar] [CrossRef]
- Hyotylainen, T.; Riekkola, M.L. Potential of effective extraction techniques and new analytical systems for profiling the marine environment. TrAC-Trends Anal. Chem. 2007, 26, 788–808. [Google Scholar] [CrossRef]
- Armenta, S.; Garrigues, S.; Esteve-Turrillas, F.A.; de la Guardia, M. Green extraction techniques in green analytical chemistry. TrAC-Trends Anal. Chem. 2019, 116, 248–253. [Google Scholar] [CrossRef]
- López-Lorente, A.I.; Pena-Pereira, F.; Pedersen-Bjergaard, S.; Zuin, V.G.; Ozkan, S.A.; Psillakis, E. The ten principles of green sample preparation. TrAC-Trends Anal. Chem. 2022, 148, 116530. [Google Scholar] [CrossRef]
- Wojnowski, W.; Tobiszewski, M.; Pena-Pereira, F.; Psillakis, E. AGREEprep-analytical greenness metric for sample preparation. TrAC-Trends Anal. Chem. 2022, 149, 116553. [Google Scholar] [CrossRef]
- Chronopoulou, L.; Dal Bosco, C.; Di Caprio, F.; Prosini, L.; Gentili, A.; Pagnanelli, F.; Palocci, C. Extraction of carotenoids and fat-soluble vitamins from Tetradesmus obliquus microalgae: An optimized approach by using supercritical CO2. Molecules 2019, 24, 2581. [Google Scholar] [CrossRef] [PubMed]
- De Melo Malinowski, M.H.; Maciel, P.K.; Chaves, M.J.S.; Barbosa, S.C.; Primel, E.G.; Duarte, F.A.; Soares, B.M. Vortex-assisted matrix solid-phase dispersion: An eco-friendly alternative for the determination of halogens in edible seaweed. Talanta 2022, 244, 123395. [Google Scholar] [CrossRef] [PubMed]
- Boonchum, W.; Peerapornpisal, Y.; Kanjanapothi, D.; Pekkoh, J.; Pumas, C.; Jamjai, U.; Amornlerdpison, D.; Noiraksar, T.; Vacharapiyasophon, P. Antioxidant activity of some seaweed from the Gulf of Thailand. Int. J. Agric. Biol. 2011, 13, 95–99. [Google Scholar]
- Anaelle, T.; Leon, E.S.; Laurent, V.; Elena, I.; Mendiola, J.A.; Stephane, C.; Nelly, K.; Luc, M.; Valerie, S.P. Green improved processes to extract bioactive phenolic compounds from brown macroalgae using Sargassum muticum as model. Talanta 2013, 104, 44–52. [Google Scholar] [CrossRef]
- Singh, I.P.; Bharate, S.B. Phloroglucinol compounds of natural origin. Nat. Prod. Rep. 2006, 23, 558–591. [Google Scholar] [CrossRef]
- Lee, S.H.; Jeon, Y.J. Anti-diabetic effects of brown algae derived phlorotannins, marine polyphenols through diverse mechanisms. Fitoterapia 2013, 86, 129–136. [Google Scholar] [CrossRef]
- Jegou, C.; Kervarec, N.; Cerantola, S.; Bihannic, I.; Stiger-Pouvreau, V. NMR use to quantify phlorotannins: The case of Cystoseira tamariscifolia, a phloroglucinol-producing brown macroalga in Brittany (France). Talanta 2015, 135, 1–6. [Google Scholar] [CrossRef]
- Zhao, K. An Overall Review on Salicylic Acid. Int. Core J. Eng. 2021, 7, 414–418. [Google Scholar]
- Velika, B.; Kron, I. Antioxidant properties of benzoic acid derivatives against Superoxide radical. Free Radic. Antiox. 2012, 2, 62–67. [Google Scholar] [CrossRef]
- Sellimi, S.; Benslima, A.; Barragan-Montero, V.; Hajji, M.; Nasri, M. Polyphenolic-protein-polysaccharide ternary conjugates from Cystoseira barbata Tunisian seaweed as potential biopreservatives: Chemical, antioxidant and antimicrobial properties. Int. J. Biol. Macromol. 2017, 105, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Chong, K.P.; Rossall, S.; Atong, M. In vitro antimicrobial activity and fungitoxicity of syringic acid, caffeic acid and 4-hydroxybenzoic acid against Ganoderma boninense. J. Agric. Sci. 2009, 1, 15. [Google Scholar] [CrossRef]
- Khadem, S.; Marles, R.J. Monocyclic phenolic acids; hydroxy-and polyhydroxybenzoic acids: Occurrence and recent bioactivity studies. Molecules 2010, 15, 7985–8005. [Google Scholar] [CrossRef] [PubMed]
- Cotas, J.O.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; da Silva, C.G.; Pereira, L. Seaweed phenolics: From extraction to applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Ozgen, S.; Kilinc, O.K.; Selamoglu, Z. Antioxidant activity of quercetin: A mechanistic review. Turk. J. Agric. Food Sci. Technol. 2016, 4, 1134–1138. [Google Scholar] [CrossRef]
- Al-Saif, S.S.A.; Abdel-Raouf, N.; El-Wazanani, H.A.; Aref, I.A. Antibacterial substances from marine algae isolated from Jeddah coast of Red sea, Saudi Arabia. Saud. J. Biol. Sci. 2014, 21, 57–64. [Google Scholar] [CrossRef]
- Rohn, S.; Buchner, N.; Driemel, G.; Rauser, M.; Kroh, L.W. Thermal degradation of onion quercetin glucosides under roasting conditions. J. Agric. Food Chem. 2007, 55, 1568–1573. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Amer. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhang, Q.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.A.; Barrow, C.J. A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2, 2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
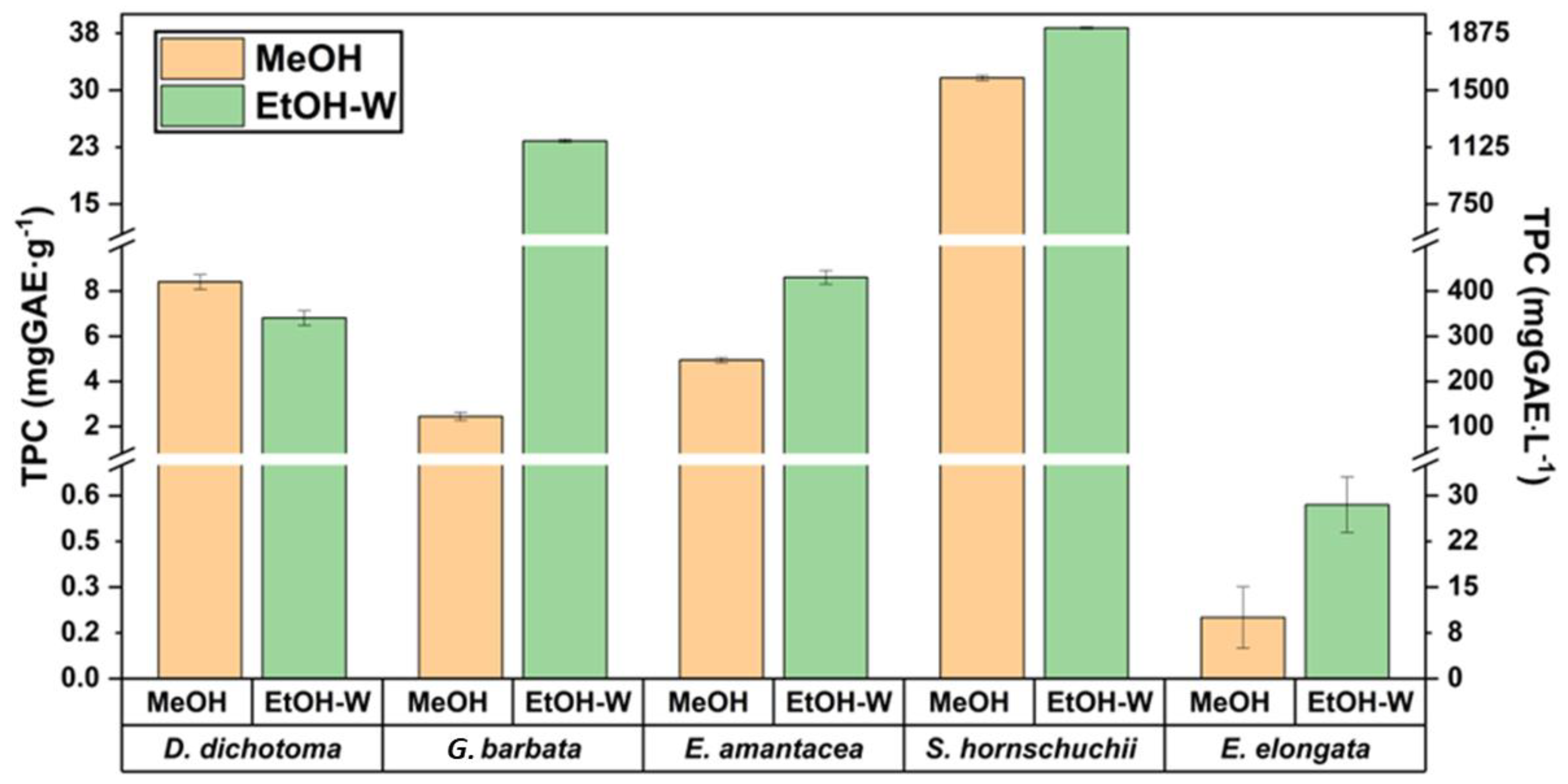
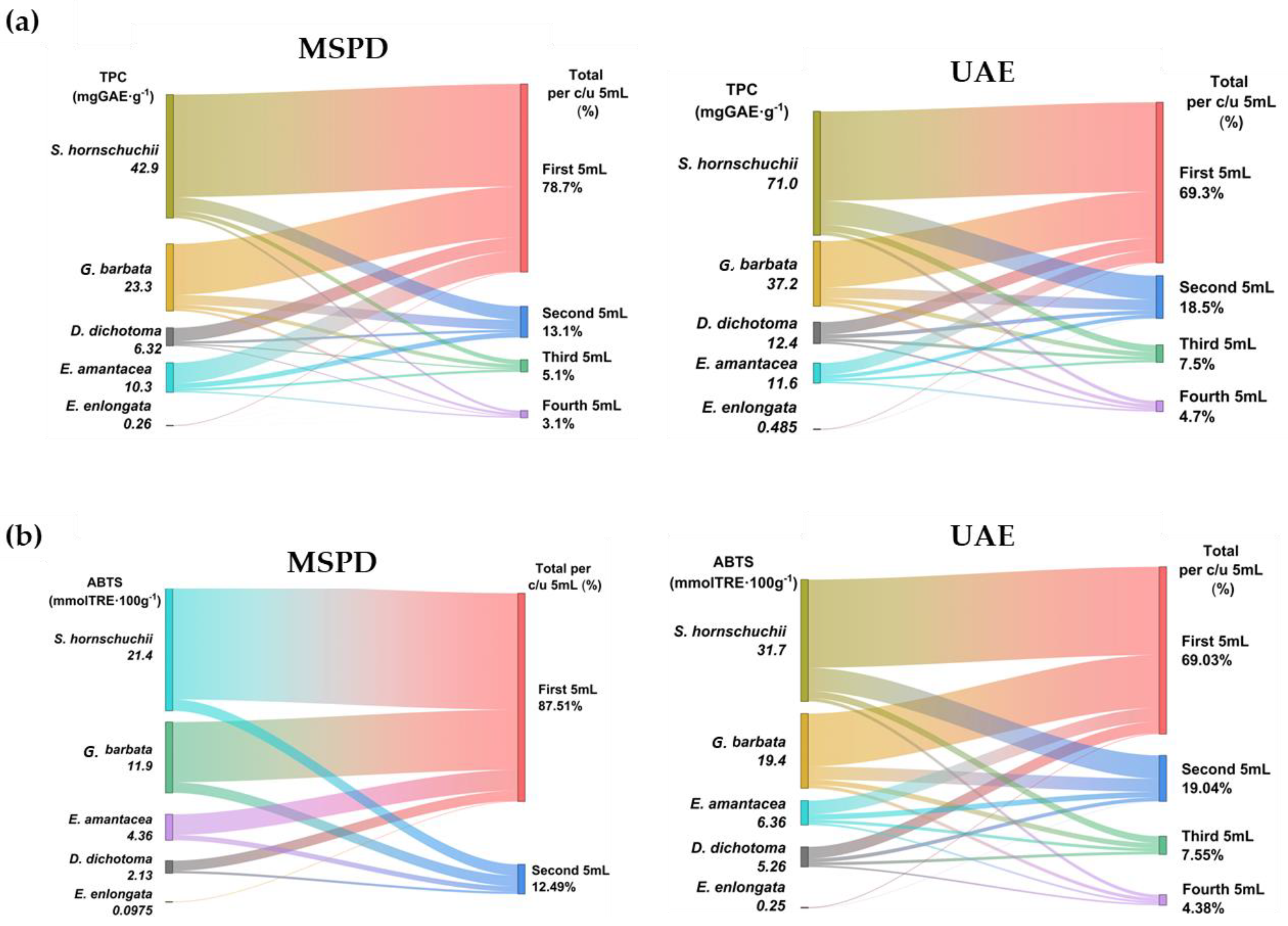


| Sample | Collected Aliquot | TPC (mgGAE/g) | TPC (mgGAE/L) | ABTS (mmolTRE/100g) | ABTS (mmolTRE/L) | IC50 (mg/L) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MSPD | UAE | MSPD | UAE | MSPD | UAE | MSPD | UAE | MSPD | UAE | ||
| D. dichotoma | 1st 5 mL | 4.7 | 7.0 | 938 | 1414 | 1.7 | 3.0 | 3.4 | 6.0 | 989 | 331 |
| 2nd 5 mL | 0.8 | 2.3 | 177 | 470 | 0.4 | 1.0 | 0.8 | 2.00 | 5310 | 796 | |
| 3rd 5 mL | 0.4 | 1.8 | 87 | 364 | <LDL | 0.7 | <LDL | 1.56 | >UDL | 1279 | |
| 4th 5 mL | 0.3 | 1.1 | 61 | 225 | <LDL | 0.4 | <LDL | 0.90 | >UDL | 1928 | |
| G. barbata | 1st 5 mL | 17 | 26 | 3546 | 5294 | 10 | 13 | 20 | 27.54 | 213 | 65 |
| 2nd 5 mL | 3.3 | 6.1 | 678 | 1231 | 1.7 | 3.4 | 3.6 | 6.85 | 1189 | 288 | |
| 3rd 5 mL | 1.3 | 2.6 | 268 | 539 | <LDL | 1.3 | <LDL | 2.73 | >UDL | 733 | |
| 4th 5 mL | 0.8 | 1.9 | 168 | 381 | <LDL | 0.8 | <LDL | 1.76 | >UDL | 1014 | |
| E. amentacea | 1st 5 mL | 7.1 | 6.8 | 1421 | 1361 | 3.4 | 3.5 | 6.9 | 7.13 | 251 | 240 |
| 2nd 5 mL | 1.8 | 2.2 | 361 | 444 | 0.8 | 1.4 | 1.77 | 2.91 | 955 | 689 | |
| 3rd 5 mL | 0.8 | 1.4 | 173 | 294 | <LDL | 0.8 | <LDL | 1.61 | >UDL | 1212 | |
| 4th 5 mL | 0.5 | 1.0 | 103 | 212 | <LDL | 0.5 | <LDL | 1.07 | >UDL | 1552 | |
| S. hornschuchii | 1st 5 mL | 38 | 51 | 7128 | 10240 | 19 | 23 | 59 | 46 | 55 | 44 |
| 2nd 5 mL | 4.7 | 13 | 954 | 2725 | 1.9 | 6.0 | 3.8 | 12 | 434 | 125 | |
| 3rd 5 mL | 1.6 | 4.0 | 324 | 810 | <LDL | 1.8 | <LDL | 3.6 | >UDL | 470 | |
| 4th 5 mL | 0.8 | 2.0 | 176 | 417 | <LDL | 0.9 | <LDL | 1.7 | >UDL | 951 | |
| E. enlongata | 1st 5 mL | 0.2 | 0.3 | 42 | 74 | 0.1 | 0.2 | 0.2 | 1.2 | 11040 | 5006 |
| 2nd 5 mL | 0.02 | 0.1 | <LDL | 23 | <LDL | 0.05 | <LDL | 0.26 | >UDL | 18,657 | |
| 3rd 5 mL | 0.01 | <LDL | <LDL | <LDL | <LDL | <LDL | <LDL | <LDL | >UDL | >UDL | |
| 4th 5 mL | 0.02 | <LDL | <LDL | <LDL | <LDL | <LDL | <LDL | <LDL | >UDL | >UDL | |
| Polyphenols | D. dichotoma | G. barbata | E. amentacea | S. hornschuchii | E. elongata | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| UAE | MSPD | UAE | MSPD | UAE | MSPD | UAE | MSPD | UAE | MSPD | |
| Non flavonoids | ||||||||||
| 3,4-dihydroxybenzoic acid | 14 ± 1 | 40 ± 10 | 0.42 ± 0.01 | 1.3 ± 0.4 | nd | nd | 0.24 ± 0.02 | 1.6 ± 0.2 | nd | nd |
| 2,4-dihydroxybenzoic acid + 2-5-dihydroxybenzoic acid | 9.3 ± 0.4 | 26 ± 4 | 0.29 ± 0.03 | 1.0 ± 0.1 | 0.05 ± 0.01 | 0.4 ± 0.1 | 0.11 ± 0.01 | 0.8 ± 0.1 | nd | nd |
| 2,5-dihydroxybenzaldehyde + 3,4-dihydroxybenzaldehyde | 0.16 ± 0.01 | 0.20 ± 0.02 | 0.17 ± 0.03 | 0.12 ± 0.02 | 0.14 ± 0.06 | 0.21 ± 0.04 | nd | nd | nd | nd |
| 3-hydroxybenzoic acid + 4-hydroxybenzoic acid | 12 ± 3 | 13 ± 5 | 393 ± 19 | 543 ± 75 | 8 ± 1 | 6 ± 2 | 6 ± 1 | 8 ± 3 | 15 ± 1 | 16 ± 1 |
| 2,6-dihydroxybenzoic acid + 3-5-dihydroxybenzoic acid | 11 ± 1 | 31 ± 5 | nd | 1.2 ± 0.1 | nd | nd | nd | nd | nd | nd |
| 3-hydroxybenzaldehyde + 4-hydroxybenzaldehyde | 1.8 ± 0.2 | 0.9 ± 0.2 | 1.1 ± 0.2 | 1.0 ± 0.2 | 2.1 ± 0.1 | 1.1 ± 0.3 | nd | nd | 6 ± 1 | 4 ± 1 |
| Flavonoids | ||||||||||
| Quercetin-3-glucuronide | 0.07 ± 0.01 | 0.3 ± 0.1 | 0.12 ± 0.02 | 0.3 ± 0.1 | nd | nd | nd | nd | nd | nd |
| Quercetin-3-glucoside | 0.12 ± 0.01 | 0.08 ± 0.01 | 0.09 ± 0.03 | 0.14 ± 0.01 | nd | nd | nd | nd | nd | nd |
| 3,5-dimethoxybenzaldehyde | 0.10 ± 0.03 | 2.5 ± 0.9 | nd | 1.8 ± 0.6 | nd | nd | nd | nd | nd | nd |
| Quercetin | 10 ± 1 | 4.3 ± 0.4 | 11 ± 3 | 3 ± 1 | 3 ± 1 | 1.8 ± 0.8 | 9 ± 1 | 1.9 ± 0.3 | 15 ± 2 | 1.9 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo, A.; Celeiro, M.; Lores, M.; Grgić, K.; Banožić, M.; Jerković, I.; Jokić, S. Bioprospecting of Targeted Phenolic Compounds of Dictyota dichotoma, Gongolaria barbata, Ericaria amentacea, Sargassum hornschuchii and Ellisolandia elongata from the Adriatic Sea Extracted by Two Green Methods. Mar. Drugs 2023, 21, 97. https://doi.org/10.3390/md21020097
Castillo A, Celeiro M, Lores M, Grgić K, Banožić M, Jerković I, Jokić S. Bioprospecting of Targeted Phenolic Compounds of Dictyota dichotoma, Gongolaria barbata, Ericaria amentacea, Sargassum hornschuchii and Ellisolandia elongata from the Adriatic Sea Extracted by Two Green Methods. Marine Drugs. 2023; 21(2):97. https://doi.org/10.3390/md21020097
Chicago/Turabian StyleCastillo, Aly, Maria Celeiro, Marta Lores, Kristina Grgić, Marija Banožić, Igor Jerković, and Stela Jokić. 2023. "Bioprospecting of Targeted Phenolic Compounds of Dictyota dichotoma, Gongolaria barbata, Ericaria amentacea, Sargassum hornschuchii and Ellisolandia elongata from the Adriatic Sea Extracted by Two Green Methods" Marine Drugs 21, no. 2: 97. https://doi.org/10.3390/md21020097
APA StyleCastillo, A., Celeiro, M., Lores, M., Grgić, K., Banožić, M., Jerković, I., & Jokić, S. (2023). Bioprospecting of Targeted Phenolic Compounds of Dictyota dichotoma, Gongolaria barbata, Ericaria amentacea, Sargassum hornschuchii and Ellisolandia elongata from the Adriatic Sea Extracted by Two Green Methods. Marine Drugs, 21(2), 97. https://doi.org/10.3390/md21020097











