Promising Antiparasitic Natural and Synthetic Products from Marine Invertebrates and Microorganisms
Abstract
1. Introduction
- When IC50 > 20 μM, the activity of the compounds was low or inactive; when 1 ≤ IC50 ≤ 20 μM, the compounds showed moderate activity. When IC50 < 1 μM, they showed good potent activity [34];
- When measured in μg/mL, if IC50 > 20 μM, the activity of the compounds was low or inactive; if 3 ≤ IC50 ≤ 10 μg/mL, the compound showed moderate activity. If IC50 < 3 μg/mL, the compound showed good potent activity [35].
2. Marine Invertebrate-Derived Antiparasitic Compounds
2.1. Alkaloid Compounds
2.2. Terpenoids, Sesquiterpenoids, and Diterpenoids Compounds
2.3. Steroids and Sterols Compounds
2.4. Other Compounds
3. Marine Microorganisms-Derived Antiparasitic Compounds
3.1. Steroids and Sterols Compounds
3.2. Marine Fungi
4. Cyanophyta
4.1. Linear Peptides
4.2. Cyclic Peptides
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mostafa, O.; Al-Shehri, M.; Moustafa, M. Promising antiparasitic agents from marine sponges. Saudi J. Biol. Sci. 2021, 29, 217–227. [Google Scholar] [CrossRef]
- Greenwood, B.; Mutabingwa, T. Malaria in 2002. Nature 2002, 415, 670. [Google Scholar] [CrossRef] [PubMed]
- della Torre, A.; Tu, Z.; Petrarca, V. On the distribution and genetic differentiation of Anopheles gambiae ss molecular forms. Insect Biochem. Mol. Biol. 2005, 35, 755–769. [Google Scholar] [CrossRef]
- Horn, D.; Duraisingh, M.T. Antiparasitic chemotherapy–from genomes to mechanisms. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 71. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.B.; du Plessis, L.H.; Viljoen, J.M. Solidification of Self-Emulsifying Drug Delivery Systems as a Novel Approach to the Management of Uncomplicated Malaria. Pharmaceuticals 2022, 15, 120. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Hotez, P.J.; Aksoy, S.; Brindley, P.J.; Kamhawi, S. What Constitutes a Neglected Tropical Disease? Public Library of Science: San Francisco, CA USA, 2020; Volume 14, p. e0008001. [Google Scholar]
- Engels, D.; Zhou, X.-N. Neglected tropical diseases: An effective global response to local poverty-related disease priorities. Infect. Dis. Poverty 2020, 9, 1–9. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; de Souza, M.V.N. Current leishmaniasis drug discovery. RSC Med. Chem. 2022, 13, 1029–1043. [Google Scholar] [CrossRef]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 22–25. [Google Scholar] [CrossRef]
- Desjeux, P. Leishmaniasis: Current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 305–318. [Google Scholar] [CrossRef]
- Pal, M.; Gutama, K.P.; Steinmetz, C.H.; Dave, P. Leishmaniasis: An Emerging and Re-emerging Disease of Global Public Health Concern. Am. J. Infect. Dis. 2022, 10, 22–25. [Google Scholar] [CrossRef]
- Shah, V.V.; Patel, V.M.; Vyas, P. Human African Trypanosomiasis–A rare case report from India. Indian J. Med. Microbiol. 2022, 40, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Simarro, P.P.; Cecchi, G.; Paone, M.; Franco, J.R.; Diarra, A.; Ruiz, J.A.; Fèvre, E.M.; Courtin, F.; Mattioli, R.C.; Jannin, J.G. The Atlas of human African trypanosomiasis: A contribution to global mapping of neglected tropical diseases. Int. J. Health Geogr. 2010, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chimelli, L.; Scaravilli, F. Trypanosomiasis. Brain Pathol. 1997, 7, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.P.; Parise, M.E.; Dotson, E.M.; Bialek, S.R. What do we know about Chagas disease in the United States? Am. J. Trop. Med. Hyg. 2016, 95, 1225. [Google Scholar] [CrossRef]
- Beteck, R.M.; Smit, F.J.; Haynes, R.K.; N’Da, D.D. Recent progress in the development of anti-malarial quinolones. Malar. J. 2014, 13, 1–10. [Google Scholar] [CrossRef]
- Ouji, M.; Augereau, J.-M.; Paloque, L.; Benoit-Vical, F. Plasmodium falciparum resistance to artemisinin-based combination therapies: A sword of Damocles in the path toward malaria elimination. Parasite 2018, 25, 24. [Google Scholar] [CrossRef]
- Thu, A.M.; Phyo, A.P.; Landier, J.; Parker, D.M.; Nosten, F.H. Combating multidrug-resistant Plasmodium falciparum malaria. FEBS J. 2017, 284, 2569–2578. [Google Scholar] [CrossRef]
- Duru, V.; Khim, N.; Leang, R.; Kim, S.; Domergue, A.; Kloeung, N.; Ke, S.; Chy, S.; Eam, R.; Khean, C. Plasmodium falciparum dihydroartemisinin-piperaquine failures in Cambodia are associated with mutant K13 parasites presenting high survival rates in novel piperaquine in vitro assays: Retrospective and prospective investigations. BMC Med. 2015, 13, 305. [Google Scholar] [CrossRef]
- Leang, R.; Taylor, W.R.; Bouth, D.M.; Song, L.; Tarning, J.; Char, M.C.; Kim, S.; Witkowski, B.; Duru, V.; Domergue, A. Evidence of Plasmodium falciparum malaria multidrug resistance to artemisinin and piperaquine in western Cambodia: Dihydroartemisinin-piperaquine open-label multicenter clinical assessment. Antimicrob. Agents Chemother. 2015, 59, 4719–4726. [Google Scholar] [CrossRef]
- AlKadi, H.O. Antimalarial drug toxicity: A review. Chemotherapy 2007, 53, 385–391. [Google Scholar] [CrossRef]
- da Silva, V.B.R.; Campos, B.R.K.L.; de Oliveira, J.F.; Decout, J.-L.; de Lima, M.d.C.A. Medicinal chemistry of antischistosomal drugs: Praziquantel and oxamniquine. Bioorg. Med. Chem. 2017, 25, 3259–3277. [Google Scholar] [CrossRef]
- Lespine, A.; Ménez, C.; Bourguinat, C.; Prichard, R.K. P-glycoproteins and other multidrug resistance transporters in the pharmacology of anthelmintics: Prospects for reversing transport-dependent anthelmintic resistance. Int. J. Parasitol. Drugs Drug Resist. 2012, 2, 58–75. [Google Scholar] [CrossRef]
- Bermudez, J.; Davies, C.; Simonazzi, A.; Real, J.P.; Palma, S. Current drug therapy and pharmaceutical challenges for Chagas disease. Acta Trop. 2016, 156, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, L.; Almutairi, M.M.; Hotez, P.J.; Pollet, J. Enlisting the mRNA vaccine platform to combat parasitic infections. Vaccines 2019, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.-Y.; Li, H.-J.; Li, Q.-Y.; Wu, Y.-C. Application of marine natural products in drug research. Bioorg. Med. Chem. 2021, 35, 116058. [Google Scholar] [CrossRef] [PubMed]
- Danovaro, R.; Corinaldesi, C.; Dell’Anno, A.; Snelgrove, P.V. The deep-sea under global change. Curr. Biol. 2017, 27, R461–R465. [Google Scholar] [CrossRef]
- Thomas, T.R.A.; Kavlekar, D.P.; LokaBharathi, P.A. Marine drugs from sponge-microbe association—A review. Mar. Drugs 2010, 8, 1417–1468. [Google Scholar] [CrossRef] [PubMed]
- Nweze, J.A.; Mbaoji, F.N.; Li, Y.-M.; Yang, L.-Y.; Huang, S.-S.; Chigor, V.N.; Eze, E.A.; Pan, L.-X.; Zhang, T.; Yang, D.-F. Potentials of marine natural products against malaria, leishmaniasis, and trypanosomiasis parasites: A review of recent articles. Infect. Dis. Poverty 2021, 10, 1–19. [Google Scholar] [CrossRef]
- Lenz, K.D.; Klosterman, K.E.; Mukundan, H.; Kubicek-Sutherland, J.Z. Macrolides: From toxins to therapeutics. Toxins 2021, 13, 347. [Google Scholar] [CrossRef]
- Rushdi, M.I.; Abdel-Rahman, I.A.; Attia, E.Z.; Abdelraheem, W.M.; Saber, H.; Madkour, H.A.; Amin, E.; Hassan, H.M.; Abdelmohsen, U.R. A review on the diversity, chemical and pharmacological potential of the green algae genus Caulerpa. S. Afr. J. Bot. 2020, 132, 226–241. [Google Scholar] [CrossRef]
- Nweze, J.A.; Mbaoji, F.N.; Huang, G.; Li, Y.; Yang, L.; Zhang, Y.; Huang, S.; Pan, L.; Yang, D. Antibiotics development and the potentials of marine-derived compounds to stem the tide of multidrug-resistant pathogenic bacteria, fungi, and protozoa. Mar. Drugs 2020, 18, 145. [Google Scholar] [CrossRef]
- Murillo, T.; Schneider, D.; Fichtel, C.; Daniel, R. Dietary shifts and social interactions drive temporal fluctuations of the gut microbiome from wild redfronted lemurs. ISME Commun. 2022, 2, 1–11. [Google Scholar] [CrossRef]
- Ioset, J.-R.; Brun, R.; Wenzler, T.; Kaiser, M.; Yardley, V. Drug Screening for Kinetoplastid Diseases: A Training Manual for Screening in Neglected Diseases. DNDi 2009, 1–74. [Google Scholar]
- Oluwabusola, E.T.; Tabudravu, J.N.; Al Maqbali, K.S.; Annang, F.; Pérez-Moreno, G.; Reyes, F.; Jaspars, M. Antiparasitic activity of bromotyrosine alkaloids and new analogues isolated from the Fijian marine sponge Aplysinella rhax. Chem. Biodivers. 2020, 17, e2000335. [Google Scholar] [CrossRef] [PubMed]
- Imperatore, C.; Gimmelli, R.; Persico, M.; Casertano, M.; Guidi, A.; Saccoccia, F.; Ruberti, G.; Luciano, P.; Aiello, A.; Parapini, S. Investigating the antiparasitic potential of the marine sesquiterpene avarone, its reduced form avarol, and the novel semisynthetic thiazinoquinone analogue thiazoavarone. Mar. Drugs 2020, 18, 112. [Google Scholar] [CrossRef]
- Campos, P.-E.; Pichon, E.; Moriou, C.; Clerc, P.; Trepos, R.; Frederich, M.; De Voogd, N.; Hellio, C.; Gauvin-Bialecki, A.; Al-Mourabit, A. New antimalarial and antimicrobial tryptamine derivatives from the marine sponge Fascaplysinopsis reticulata. Mar. Drugs 2019, 17, 167. [Google Scholar] [CrossRef]
- Ju, E.; Latif, A.; Kong, C.-S.; Seo, Y.; Lee, Y.-J.; Dalal, S.R.; Cassera, M.B.; Kingston, D.G. Antimalarial activity of the isolates from the marine sponge Hyrtios erectus against the chloroquine-resistant Dd2 strain of Plasmodium falciparum. Z. Für Nat. C 2018, 73, 397–400. [Google Scholar] [CrossRef]
- Shady, N.H.; Fouad, M.A.; Ahmed, S.; Pimentel-Elardo, S.M.; Nodwell, J.R.; Kamel, M.S.; Abdelmohsen, U.R. A new antitrypanosomal alkaloid from the Red Sea marine sponge Hyrtios sp. J. Antibiot. 2018, 71, 1036–1039. [Google Scholar] [CrossRef]
- Chianese, G.; Silber, J.; Luciano, P.; Merten, C.; Erpenbeck, D.; Topaloglu, B.L.; Kaiser, M.; Tasdemir, D. Antiprotozoal linear furanosesterterpenoids from the marine sponge Ircinia oros. J. Nat. Prod. 2017, 80, 2566–2571. [Google Scholar] [CrossRef]
- Majer, T.; Bhattarai, K.; Straetener, J.; Pohlmann, J.; Cahill, P.; Zimmermann, M.O.; Hübner, M.P.; Kaiser, M.; Svenson, J.; Schindler, M. Discovery of Ircinianin Lactones B and C—Two New Cyclic Sesterterpenes from the Marine Sponge Ircinia wistarii. Mar. Drugs 2022, 20, 532. [Google Scholar] [CrossRef]
- Kurimoto, S.-i.; Ohno, T.; Hokari, R.; Ishiyama, A.; Iwatsuki, M.; Ōmura, S.; Kobayashi, J.I.; Kubota, T. Ceratinadins E and F, new bromotyrosine alkaloids from an Okinawan marine sponge Pseudoceratina sp. Mar. Drugs 2018, 16, 463. [Google Scholar] [CrossRef]
- Campos, P.-E.; Wolfender, J.-L.; Queiroz, E.F.; Marcourt, L.; Al-Mourabit, A.; Frederich, M.; Bordignon, A.; De Voogd, N.; Illien, B.; Gauvin-Bialecki, A. Unguiculin A and ptilomycalins E–H, antimalarial guanidine alkaloids from the marine sponge Monanchora unguiculata. J. Nat. Prod. 2017, 80, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Sala, S.; Fromont, J.; Gomez, O.; Vuong, D.; Lacey, E.; Flematti, G.R. Albanitriles A–G: Antiprotozoal Polyacetylene Nitriles from a Mycale Marine Sponge. J. Nat. Prod. 2019, 82, 3450–3455. [Google Scholar] [CrossRef] [PubMed]
- Parra, L.L.; Bertonha, A.F.; Severo, I.R.; Aguiar, A.C.; de Souza, G.E.; Oliva, G.; Guido, R.V.; Grazzia, N.; Costa, T.R.; Miguel, D.C. Isolation, derivative synthesis, and structure–activity relationships of antiparasitic bromopyrrole alkaloids from the marine sponge Tedania brasiliensis. J. Nat. Prod. 2018, 81, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Murtihapsari, M.; Salam, S.; Kurnia, D.; Darwati, D.; Kadarusman, K.; Abdullah, F.F.; Herlina, T.; Husna, M.H.; Awang, K.; Shiono, Y. A new antiplasmodial sterol from Indonesian marine sponge, Xestospongia sp. Nat. Prod. Res. 2021, 35, 937–944. [Google Scholar] [CrossRef]
- Fröhlich, T.; Çapcı Karagöz, A.; Reiter, C.; Tsogoeva, S.B. Artemisinin-derived dimers: Potent antimalarial and anticancer agents. J. Med. Chem. 2016, 59, 7360–7388. [Google Scholar] [CrossRef] [PubMed]
- Limon, A.-C.D.; Patabendige, H.M.; Azhari, A.; Sun, X.; Kyle, D.E.; Wilson, N.G.; Baker, B.J. Chemistry and Bioactivity of the Deep-Water Antarctic Octocoral Alcyonium sp. Mar. Drugs 2022, 20, 576. [Google Scholar] [CrossRef]
- Wright, A.E.; Collins, J.E.; Roberts, B.; Roberts, J.C.; Winder, P.L.; Reed, J.K.; Diaz, M.C.; Pomponi, S.A.; Chakrabarti, D. Antiplasmodial compounds from deep-water marine invertebrates. Mar. Drugs 2021, 19, 179. [Google Scholar] [CrossRef]
- Lima, M.L.; Romanelli, M.M.; Borborema, S.E.; Johns, D.M.; Migotto, A.E.; Lago, J.H.G.; Tempone, A.G. Antitrypanosomal activity of isololiolide isolated from the marine hydroid Macrorhynchia philippina (Cnidaria, Hydrozoa). Bioorg. Chem. 2019, 89, 103002. [Google Scholar] [CrossRef]
- Thomas, S.A.; Von Salm, J.L.; Clark, S.; Ferlita, S.; Nemani, P.; Azhari, A.; Rice, C.A.; Wilson, N.G.; Kyle, D.E.; Baker, B.J. Keikipukalides, furanocembrane diterpenes from the Antarctic deep sea octocoral Plumarella delicatissima. J. Nat. Prod. 2018, 81, 117–123. [Google Scholar] [CrossRef]
- Pham, G.N.; Kang, D.Y.; Kim, M.J.; Han, S.J.; Lee, J.H.; Na, M. Isolation of sesquiterpenoids and steroids from the soft coral sinularia brassica and determination of their absolute configuration. Mar. Drugs 2021, 19, 523. [Google Scholar] [CrossRef]
- Qin, G.-F.; Tang, X.-L.; Sun, Y.-T.; Luo, X.-C.; Zhang, J.; Van Ofwegen, L.; Sung, P.-J.; Li, P.-L.; Li, G.-Q. Terpenoids from the soft coral Sinularia sp. collected in Yongxing Island. Mar. Drugs 2018, 16, 127. [Google Scholar] [CrossRef] [PubMed]
- Kleks, G.; Holland, D.C.; Kennedy, E.K.; Avery, V.M.; Carroll, A.R. Antiplasmodial alkaloids from the Australian bryozoan Amathia lamourouxi. J. Nat. Prod. 2020, 83, 3435–3444. [Google Scholar] [CrossRef] [PubMed]
- Kleks, G.; Duffy, S.; Lucantoni, L.; Avery, V.M.; Carroll, A.R. Orthoscuticellines A–E, β-Carboline Alkaloids from the Bryozoan Orthoscuticella ventricosa Collected in Australia. J. Nat. Prod. 2020, 83, 422–428. [Google Scholar] [CrossRef]
- Cartuche, L.; Sifaoui, I.; Cruz, D.; Reyes-Batlle, M.; López-Arencibia, A.; Javier Fernández, J.; Díaz-Marrero, A.R.; Piñero, J.E.; Lorenzo-Morales, J. Staurosporine from Streptomyces sanyensis activates programmed cell death in Acanthamoeba via the mitochondrial pathway and presents low in vitro cytotoxicity levels in a macrophage cell line. Sci. Rep. 2019, 9, 11651. [Google Scholar] [CrossRef] [PubMed]
- Martens, M.C.; Liu, Y.; Sanford, A.G.; Wallick, A.I.; Warner, R.C.; Li, R.; Davis, P.H. Analogs of Marinopyrrole A Show Enhancement to Observed In Vitro Potency against Acute Toxoplasma gondii Infection. Antimicrob. Agents Chemother. 2022, 66, e00794-21. [Google Scholar] [CrossRef]
- Martínez-Luis, S.; Cherigo, L.; Spadafora, C.; Gutiérrez, M. Antiparasitic Compounds from the Panamanian Marine Bacterium Pseudomonas aeruginosa. Nat. Prod. Commun. 2019, 14, 1934578X1901400109. [Google Scholar] [CrossRef]
- Iwasaki, A.; Ohtomo, K.; Kurisawa, N.; Shiota, I.; Rahmawati, Y.; Jeelani, G.; Nozaki, T.; Suenaga, K. Isolation, structure determination, and total synthesis of hoshinoamide c, an antiparasitic lipopeptide from the marine cyanobacterium Caldora penicillata. J. Nat. Prod. 2020, 84, 126–135. [Google Scholar] [CrossRef]
- Iwasaki, A.; Teranuma, K.; Kurisawa, N.; Rahmawati, Y.; Jeelani, G.; Nozaki, T.; Gerwick, W.H.; Suenaga, K. First Total Synthesis and Structure–Activity Relationship of Iheyamide A, an Antitrypanosomal Linear Peptide Isolated from a Dapis sp. Marine Cyanobacterium. J. Nat. Prod. 2021, 84, 2587–2593. [Google Scholar] [CrossRef]
- Kurisawa, N.; Iwasaki, A.; Jeelani, G.; Nozaki, T.; Suenaga, K. Iheyamides A–C, antitrypanosomal linear peptides isolated from a marine Dapis sp. cyanobacterium. J. Nat. Prod. 2020, 83, 1684–1690. [Google Scholar] [CrossRef]
- Takahashi, H.; Iwasaki, A.; Kurisawa, N.; Suzuki, R.; Jeelani, G.; Matsubara, T.; Sato, T.; Nozaki, T.; Suenaga, K. Motobamide, an antitrypanosomal cyclic peptide from a Leptolyngbya sp. marine cyanobacterium. J. Nat. Prod. 2021, 84, 1649–1655. [Google Scholar] [CrossRef]
- Keller, L.; Siqueira-Neto, J.L.; Souza, J.M.; Eribez, K.; LaMonte, G.M.; Smith, J.E.; Gerwick, W.H. Palstimolide A: A complex polyhydroxy macrolide with antiparasitic activity. Molecules 2020, 25, 1604. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, K.; Iwasaki, A.; Sumimoto, S.; Matsubara, T.; Sato, T.; Nozaki, T.; Saito-Nakano, Y.; Suenaga, K. Ikoamide, an antimalarial lipopeptide from an Okeania sp. marine cyanobacterium. J. Nat. Prod. 2020, 83, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Iwasaki, A.; Sezawa, D.; Fujimura, H.; Nozaki, T.; Saito-Nakano, Y.; Suenaga, K.; Teruya, T. Isolation and total synthesis of mabuniamide, a lipopeptide from an Okeania sp. marine cyanobacterium. J. Nat. Prod. 2019, 82, 2907–2915. [Google Scholar] [CrossRef]
- Kurisawa, N.; Otomo, K.; Iwasaki, A.; Jeelani, G.; Nozaki, T.; Suenaga, K. Isolation and Total Synthesis of Kinenzoline, an Antitrypanosomal Linear Depsipeptide Isolated from a Marine Salileptolyngbya sp. Cyanobacterium. J. Org. Chem. 2021, 86, 12528–12536. [Google Scholar] [CrossRef] [PubMed]
- Almaliti, J.; Malloy, K.L.; Glukhov, E.; Spadafora, C.; Gutiérrez, M.; Gerwick, W.H. Dudawalamides A–D, antiparasitic cyclic depsipeptides from the marine cyanobacterium Moorea producens. J. Nat. Prod. 2017, 80, 1827–1836. [Google Scholar] [CrossRef]
- Bunbamrung, N.; Intaraudom, C.; Dramae, A.; Komwijit, S.; Laorob, T.; Khamsaeng, S.; Pittayakhajonwut, P. Antimicrobial, antimalarial and anticholinesterase substances from the marine-derived fungus Aspergillus terreus BCC51799. Tetrahedron 2020, 76, 131496. [Google Scholar] [CrossRef]
- Xu, W.-F.; Wu, N.-N.; Wu, Y.-W.; Qi, Y.-X.; Wei, M.-Y.; Pineda, L.M.; Ng, M.G.; Spadafora, C.; Zheng, J.-Y.; Lu, L. Structure modification, antialgal, antiplasmodial, and toxic evaluations of a series of new marine-derived 14-membered resorcylic acid lactone derivatives. Mar. Life Sci. Technol. 2022, 4, 88–97. [Google Scholar] [CrossRef]
- Coronado, L.; Zhang, X.-Q.; Dorta, D.; Escala, N.; Pineda, L.M.; Ng, M.G.; Del Olmo, E.; Wang, C.-Y.; Gu, Y.-C.; Shao, C.-L. Semisynthesis, antiplasmodial activity, and mechanism of action studies of isocoumarin derivatives. J. Nat. Prod. 2021, 84, 1434–1441. [Google Scholar] [CrossRef]
- Braun, G.H.; Ramos, H.P.; Candido, A.C.; Pedroso, R.C.; Siqueira, K.A.; Soares, M.A.; Dias, G.M.; Magalhães, L.G.; Ambrósio, S.R.; Januário, A.H. Evaluation of antileishmanial activity of harzialactone a isolated from the marine-derived fungus Paecilomyces sp. Nat. Prod. Res. 2021, 35, 1644–1647. [Google Scholar] [CrossRef]
- Morales-Landa, J.L.; Lazcano-Pérez, F.; Cedillo-Rivera, R.; Sánchez-Rodríguez, J. Ultrastructure and molecular toxicological effects of the Coronate Scyphomedusa Linuche unguiculata Venom on Giardia duodenalis. Biologia 2021, 76, 1033–1039. [Google Scholar] [CrossRef]
- Shamikh, Y.I.; El Shamy, A.A.; Gaber, Y.; Abdelmohsen, U.R.; Madkour, H.A.; Horn, H.; Hassan, H.M.; Elmaidomy, A.H.; Alkhalifah, D.H.M.; Hozzein, W.N. Actinomycetes from the Red Sea sponge Coscinoderma mathewsi: Isolation, diversity, and potential for bioactive compounds discovery. Microorganisms 2020, 8, 783. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, P.J.; Roberts, B.F.; Carbonell, A.; Roberts, J.; Wright, A.E.; Chakrabarti, D. Marine microbiome as a source of antimalarials. Trop. Med. Infect. Dis. 2019, 4, 103. [Google Scholar] [CrossRef] [PubMed]
- Quintero, M.; Blandón, L.M.; Vidal, O.M.; Guzman, J.D.; Gómez-Marín, J.E.; Patiño, A.D.; Molina, D.A.; Puerto-Castro, G.M.; Gómez-León, J. In vitro biological activity of extracts from marine bacteria cultures against Toxoplasma gondii and Mycobacterium tuberculosis. J. Appl. Microbiol. 2022, 132, 2705–2720. [Google Scholar] [CrossRef] [PubMed]
- Alkhalifah, D.H.M. Sponge-associated sp. RM66 metabolome induction with N-acetylglucosamine: Antibacterial, antifungal and anti-trypanosomal activities. Saudi J. Biol. Sci. 2021, 28, 4691–4698. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.D.; Vitorino, I.; de la Cruz, M.; Díaz, C.; Cautain, B.; Annang, F.; Pérez-Moreno, G.; Gonzalez, I.; Tormo, J.R.; Martin, J. Diketopiperazines and other bioactive compounds from bacterial symbionts of marine sponges. Antonie Van Leeuwenhoek 2020, 113, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Ghania, A.; Nabila, B.-B.; Larbi, B.; Elisabeth, M.; Philippe, G.; Mariem, B.; Khadidja, K.-K.; Wacila, B.-R.; Fawzia, A.-B. Antimicrobial and antiparasitic activities of three algae from the northwest coast of Algeria. Nat. Prod. Res. 2019, 33, 742–745. [Google Scholar] [CrossRef]
- Chiboub, O.; Ktari, L.; Sifaoui, I.; López-Arencibia, A.; Reyes-Batlle, M.; Mejri, M.; Valladares, B.; Abderrabba, M.; Piñero, J.E.; Lorenzo-Morales, J. In vitro amoebicidal and antioxidant activities of some Tunisian seaweeds. Exp. Parasitol. 2017, 183, 76–80. [Google Scholar] [CrossRef]
- Stein, E.M.; Tajú, S.G.; Miyasato, P.A.; de Freitas, R.P.; Tallarico, L.d.F.; Dos Santos, G.S.; Luiz, G.L.; Rofatto, H.K.; da Silva, F.N.; Colepicolo, P. The prospective use of Brazilian marine macroalgae in schistosomiasis control. Mar. Drugs 2021, 19, 234. [Google Scholar] [CrossRef]
- Saini, A.; Kumar, S.; Raj, R.; Chowdhary, S.; Gendrot, M.; Mosnier, J.; Fonta, I.; Pradines, B.; Kumar, V. Synthesis and antiplasmodial evaluation of 1H-1, 2, 3-triazole grafted 4-aminoquinoline-benzoxaborole hybrids and benzoxaborole analogues. Bioorg. Chem. 2021, 109, 104733. [Google Scholar] [CrossRef]
- Wilson, D.W.; Langer, C.; Goodman, C.D.; McFadden, G.I.; Beeson, J.G. Defining the timing of action of antimalarial drugs against Plasmodium falciparum. Antimicrob. Agents Chemother. 2013, 57, 1455–1467. [Google Scholar] [CrossRef] [PubMed]
- Dalisay, D.S.; Williams, D.E.; Wang, X.L.; Centko, R.; Chen, J.; Andersen, R.J. Marine sediment-derived Streptomyces bacteria from British Columbia, Canada are a promising microbiota resource for the discovery of antimicrobial natural products. PLoS ONE 2013, 8, e77078. [Google Scholar] [CrossRef]
- Subramani, R.; Aalbersberg, W. Marine actinomycetes: An ongoing source of novel bioactive metabolites. Microbiol. Res. 2012, 167, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E. Resistance mechanisms in Pseudomonas aeruginosa and other nonfermentative gram-negative bacteria. Clin. Infect. Dis. 1998, 27 (Suppl. 1), S93–S99. [Google Scholar] [CrossRef] [PubMed]
- Haste, N.M.; Hughes, C.C.; Tran, D.N.; Fenical, W.; Jensen, P.R.; Nizet, V.; Hensler, M.E. Pharmacological properties of the marine natural product marinopyrrole A against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2011, 55, 3305–3312. [Google Scholar] [CrossRef]
- Khan, N.A. Pathogenesis of Acanthamoeba infections. Microb. Pathog. 2003, 34, 277–285. [Google Scholar] [CrossRef]
- Lorenzo-Morales, J.; Martín-Navarro, C.M.; López-Arencibia, A.; Arnalich-Montiel, F.; Piñero, J.E.; Valladares, B. Acanthamoeba keratitis: An emerging disease gathering importance worldwide? Trends Parasitol. 2013, 29, 181–187. [Google Scholar] [CrossRef]
- Antonsson, A.; Persson, J.L. Induction of apoptosis by staurosporine involves the inhibition of expression of the major cell cycle proteins at the G2/M checkpoint accompanied by alterations in Erk and Akt kinase activities. Anticancer Res. 2009, 29, 2893–2898. [Google Scholar]
- Wang, B.; Waters, A.L.; Sims, J.W.; Fullmer, A.; Ellison, S.; Hamann, M.T. Complex marine natural products as potential epigenetic and production regulators of antibiotics from a marine Pseudomonas aeruginosa. Microb. Ecol. 2013, 65, 1068–1075. [Google Scholar] [CrossRef]
- Aly, A.H.; Debbab, A.; Proksch, P. Fungal endophytes: Unique plant inhabitants with great promises. Appl. Microbiol. Biotechnol. 2011, 90, 1829–1845. [Google Scholar] [CrossRef]
- Hyde, K.; Soytong, K. The fungal endophyte dilemma. Fungal Divers 2008, 33, e173. [Google Scholar]
- Bhadury, P.; Mohammad, B.T.; Wright, P.C. The current status of natural products from marine fungi and their potential as anti-infective agents. J. Ind. Microbiol. Biotechnol. 2006, 33, 325. [Google Scholar] [CrossRef] [PubMed]
- Debbab, A.; Aly, A.H.; Proksch, P. Bioactive secondary metabolites from endophytes and associated marine derived fungi. Fungal Divers. 2011, 49, 1–12. [Google Scholar] [CrossRef]
- Singh, R.P.; Kumari, P.; Reddy, C. Antimicrobial compounds from seaweeds-associated bacteria and fungi. Appl. Microbiol. Biotechnol. 2015, 99, 1571–1586. [Google Scholar] [CrossRef] [PubMed]
- Amagata, T.; Usami, Y.; Minoura, K.; Ito, T.; NUMATA, A. Cytotoxic substances produced by a fungal strain from a sponge: Physico-chemical properties and structures. J. Antibiot. 1998, 51, 33–40. [Google Scholar] [CrossRef]
- Rivas, L.; Rojas, V. Cyanobacterial peptides as a tour de force in the chemical space of antiparasitic agents. Arch. Biochem. Biophys. 2019, 664, 24–39. [Google Scholar] [CrossRef]
- Demay, J.; Bernard, C.; Reinhardt, A.; Marie, B. Natural products from cyanobacteria: Focus on beneficial activities. Mar. Drugs 2019, 17, 320. [Google Scholar] [CrossRef]
- Niedermeyer, T.H.J. Anti-infective natural products from cyanobacteria. Planta Med. 2015, 81, 1309–1325. [Google Scholar] [CrossRef]
- Horton, D.A.; Bourne, G.T.; Smythe, M.L. Exploring privileged structures: The combinatorial synthesis of cyclic peptides. J. Comput. -Aided Mol. Des. 2002, 16, 415–431. [Google Scholar] [CrossRef]
- Sawadogo, W.R.; Boly, R.; Cerella, C.; Teiten, M.H.; Dicato, M.; Diederich, M. A survey of marine natural compounds and their derivatives with anti-cancer activity reported in 2012. Molecules 2015, 20, 7097–7142. [Google Scholar] [CrossRef]
- Walvekar, S.; Anwar, A.; Anwar, A.; Lai, N.J.Y.; Yow, Y.-Y.; Khalid, M.; Siddiqui, R.; Khan, N.A. Conjugation with Silver Nanoparticles Enhances Anti-Acanthamoebic Activity of Kappaphycus alvarezii. J. Parasitol. 2021, 107, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Hamann, M.T. Marine pharmacology in 2001–2002: Marine compounds with anthelmintic, antibacterial, anticoagulant, antidiabetic, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2005, 140, 265–286. [Google Scholar]
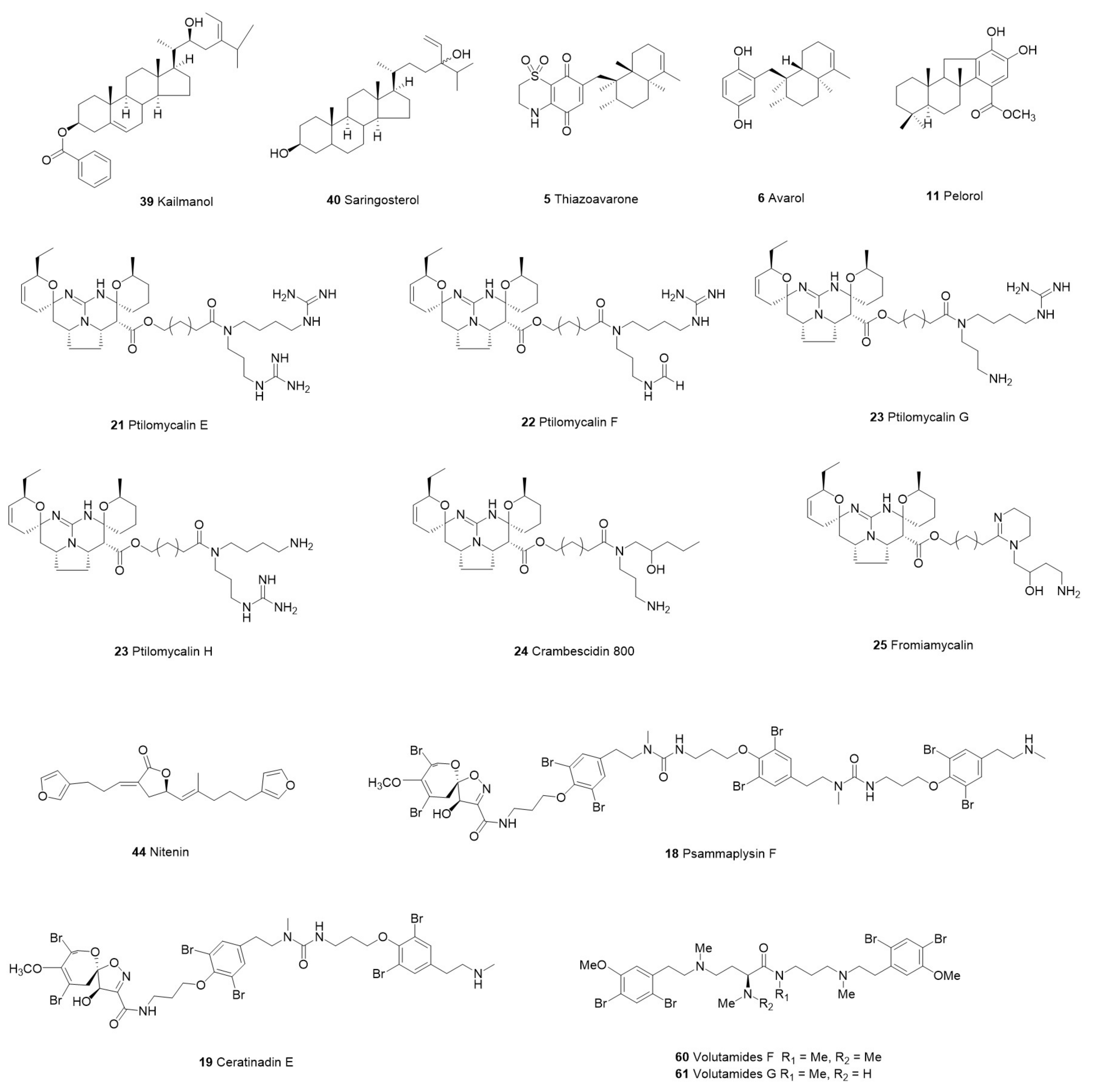
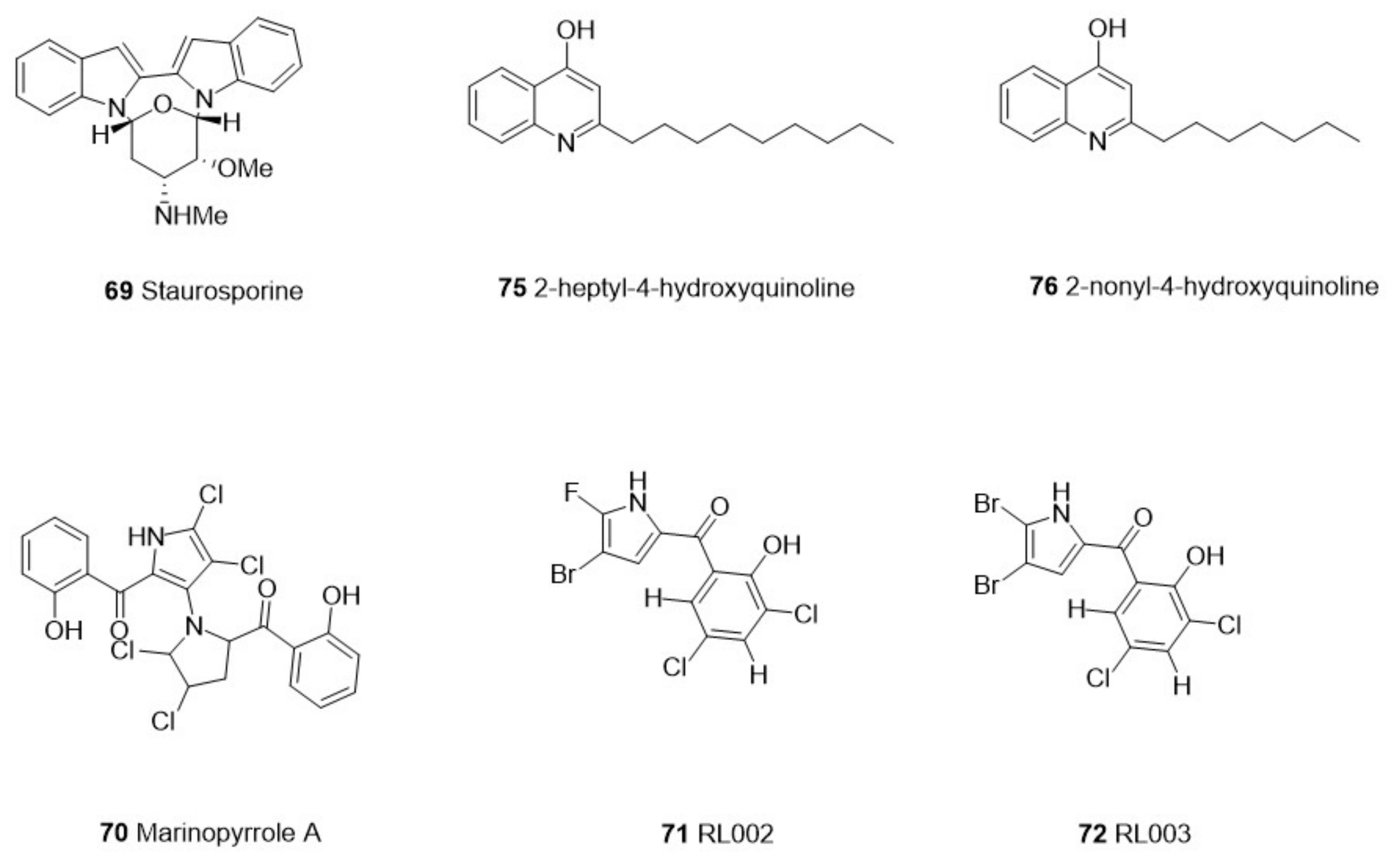
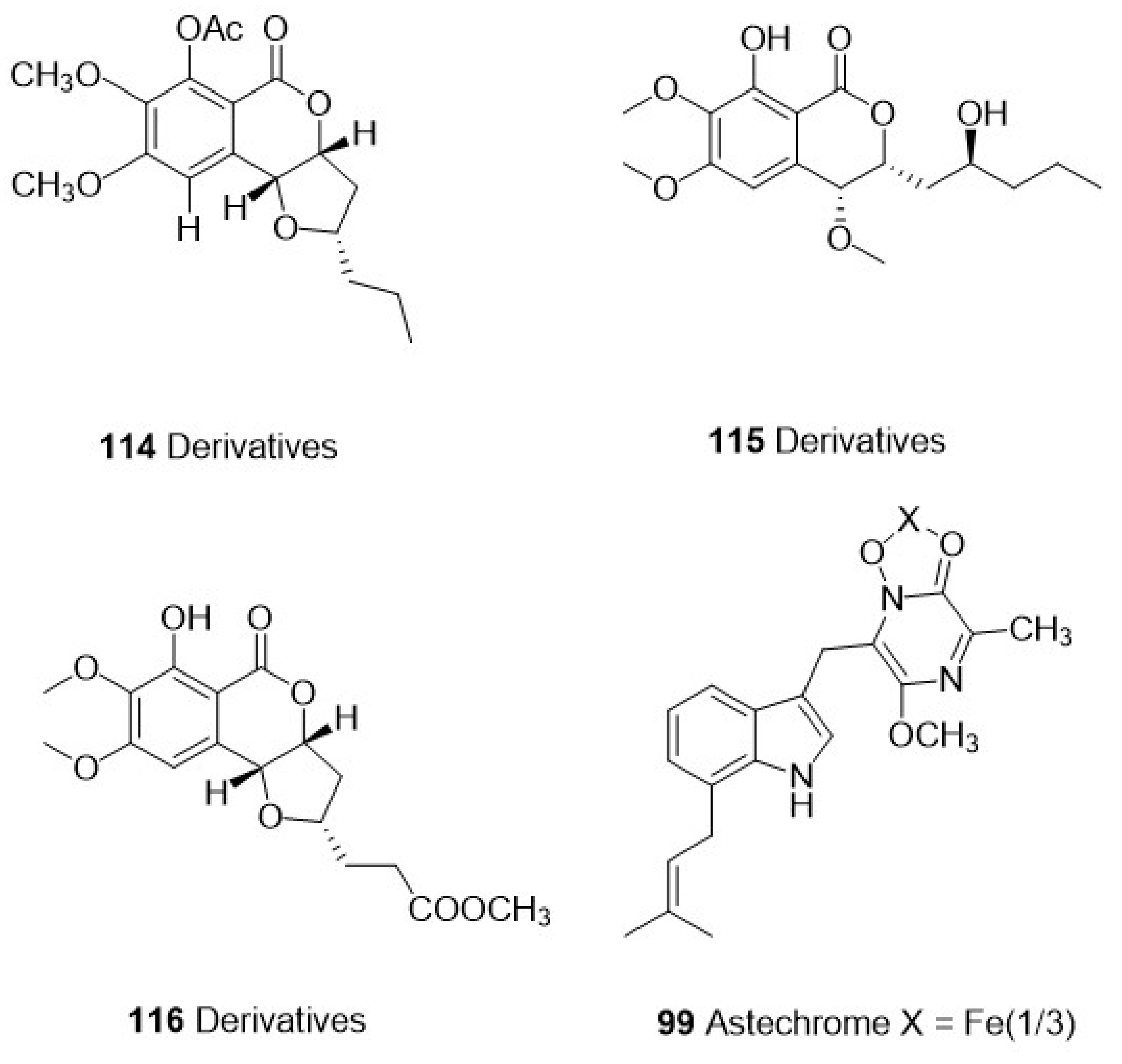
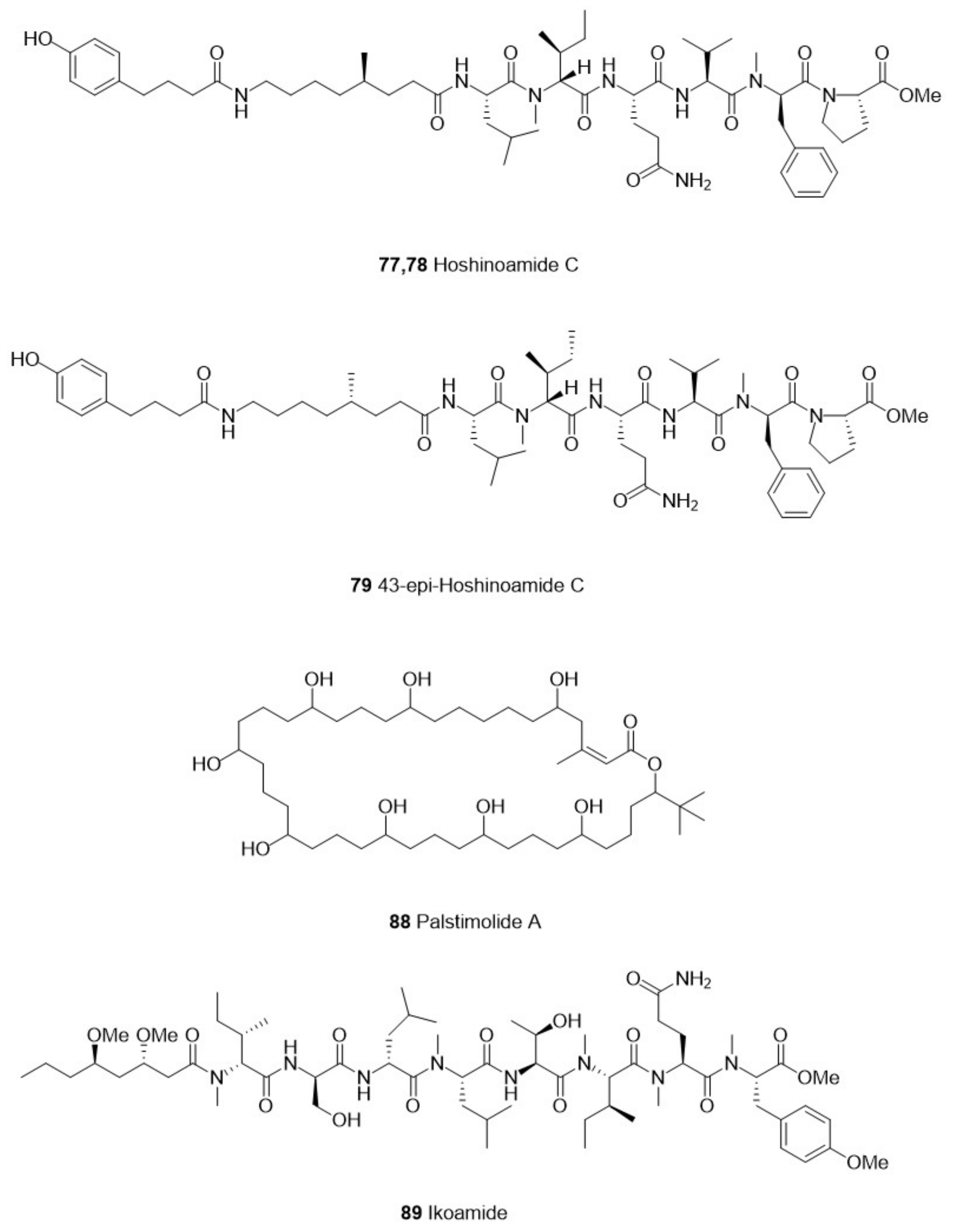
| Category | Species | Compounds | Chemistry | Target Parasite | Stage/Strain | IC50 | Cytotoxicity | Site | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of Cells | IC50 | ||||||||||
| Invertebrate | sponges | Aplysinella rhax | 1 Psammaplin A | Bromotyrosine Alkaloids | T. cruzi | C2C4 | 30 μM | NT | NT | Fiji Islands | [36] |
| P. falciparum | 3D7 | 60 μM | |||||||||
| 2 Psammaplin D | T. cruzi | C2C4 | 43 μM | ||||||||
| P. falciparum | 3D7 | 67 μM | |||||||||
| 3 Bisaprasin | T. cruzi | C2C4 | 19 μM | ||||||||
| P. falciparum | 3D7 | 29 μM | |||||||||
| Benznidazole * | - | T. cruzi | C2C4 | 2.6 μM | - | - | - | ||||
| Chloroquine * | - | P. falciparum | 3D7 | 0.017 μM | |||||||
| Dysidea avara | 4 Avarone | Sesquiterpene Quinone Avarone | P. falciparum | D10 | 2.74 μM | Human microvascular endothelial cells, HMEC-1 | 62.19 μM | Bay of Izmir, Turkey | [37] | ||
| W2 | 2.09 μM | ||||||||||
| 3D7 elo1-pfs16-CBG99 | 15.53 μM | ||||||||||
| L. infantum | promastigote | 28.21 μM | Human acute monocytic leukemia cells, THP-1 | >100 μM | |||||||
| L. tropica | promastigote | 20.28 μM | |||||||||
| L. infantum | amastigotes | 7.64 μM | |||||||||
| S. mansoni | schistosomula | 42.77 μM | |||||||||
| 5 Thiazoavarone | P. falciparum | D10 | 0.38 μM | Human microvascular endothelial cells, HMEC-1 | 3.31 μM | ||||||
| W2 | 0.21 μM | ||||||||||
| 3D7 elo1-pfs16-CBG99 | 15.01 μM | ||||||||||
| L. infantum | promastigote | 8.78 μM | Human acute monocytic leukemia cells, THP-1 | 7.41 μM | |||||||
| L. tropica | promastigote | 9.52 μM | |||||||||
| L. infantum | amastigotes | 4.99 μM | |||||||||
| S. mansoni | schistosomula | 5.90 μM | |||||||||
| 6 Avarol | P. falciparum | D10 | 0.96 μM | Human microvascular endothelial cells, HMEC-1 | 36.85 μM | ||||||
| W2 | 1.10 μM | ||||||||||
| 3D7 elo1-pfs16-CBG99 | 9.30 μM | ||||||||||
| L. infantum | promastigote | 7.42 μM | Human acute monocytic leukemia cells, THP-1 | 31.75 μM | |||||||
| L. tropica | promastigote | 7.08 μM | |||||||||
| L. infantum | amastigotes | 3.19 μM | |||||||||
| S. mansoni | schistosomula | 33.97 μM | |||||||||
| Chloroquine * | - | P. falciparum | D10 | 0.04 μM | - | - | - | ||||
| W2 | 0.54 μM | ||||||||||
| Methylene blue * | - | 3D7 elo1-pfs16-CBG99 | 0.155 μM | ||||||||
| Amphotericin B * | - | L. infantum | promastigote | 0.2 μM | |||||||
| L. tropica | promastigote | 0.17 μM | |||||||||
| L. infantum | amastigotes | 0.189 μM | |||||||||
| Fascaplysinopsis reticulata | 7 8-oxo-tryptamine | Tryptophan-Derived Alkaloids | P. falciparum | 3D7 | 8.8 µg/mL | NT | NT | Mayotte | [38] | ||
| 8 The mixture of the known (E) and (Z)-6-bromo-2′-demethyl-3′-N-methylaplysinopsin | 8.0 µg/mL | ||||||||||
| Artemisinin * | - | 0.006 μg/mL | - | - | - | ||||||
| Hyrtios erectus | 9 Smenotronic acid | Sesquiterpenoids | P. falciparum | Dd2 | 3.51 μM | NT | NT | Sesquiterpenoids | [39] | ||
| 10 Ilimaquinone | 2.11 μM | ||||||||||
| 11 Pelorol | 0.80 μM | ||||||||||
| Hyrtios sp. | 12 Hyrtiodoline A | Alkaloid | T. brucei brucei | - | 48 h: 15.26 μM | J774.1 macrophages | >200 μM | Red Sea at Sharm el-Sheikh, Egypt | [40] | ||
| 72 h: 7.48 μM | |||||||||||
| Ircinia oros | 13 Ircinin-1 | Linear Furanosesterterpenoids | T. b. rhodesiense | - | 97 μM | L6 rat myoblast cells | 150 μM | Gökçeada, Northern Aegean Sea, Turkey | [41] | ||
| T. cruzi | 120 μM | ||||||||||
| L. donovani | 31 μM | ||||||||||
| P. falciparum | 58 μM | ||||||||||
| 14 Ircinin-2 | T. b. rhodesiense | 65 μM | 140 μM | ||||||||
| T. cruzi | 110 μM | ||||||||||
| L. donovani | 28 μM | ||||||||||
| P. falciparum | 56 μM | ||||||||||
| 15 Ircinialactam E | T. b. rhodesiense | 130 μM | >200 μM | ||||||||
| P. falciparum | 95 μM | ||||||||||
| 16 Ircinialactam F | T. b. rhodesiense | 130 μM | >200 μM | ||||||||
| L. donovani | 95 μM | ||||||||||
| Melarsoprol * | - | T. b. rhodesiense | - | 0.015 μM | - | - | - | ||||
| Benznidazole * | T. cruzi | 3.07 μM | |||||||||
| Miltefosine * | L. donovani | 0.51 μM | |||||||||
| Chloroquine * | P. falciparum | 0.009 μM | |||||||||
| Podophyllotoxin * | - | - | - | L6 rat myoblast cells | 0.010 μM | ||||||
| Ircinia wistarii | 17 Ircinianin | Sesterterpenes | P. falciparum | NF54 | 25.4 μM | HeLa | >64 μg/mL | Wistari Reef, Great Barrier Reef, Australia | [42] | ||
| T. brucei rhodesiense | STIB900 | 82.8 μM | |||||||||
| T. cruzi | C2C4 | 190.9 μM | L6 | 59.5 μg/mL | |||||||
| L. donovani | MHOM/ET/67/L82 | 16.6 μM | |||||||||
| Chloroquine * | - | P. falciparum | NF54 | 0.006 μM | - | - | - | ||||
| Melarsoprol * | T. brucei rhodesiense | STIB900 | 0.020 μM | ||||||||
| Benznidazole * | T. cruzi | C2C4 | 3.36 μM | ||||||||
| Miltefosine * | L. donovani | MHOM/ET/67/L82 | 0.486 μM | ||||||||
| Pseudoceratina sp. | 18 Psammaplysin F | Bromotyrosine Alkaloid | P. falciparum | K1 | 3.77 µg/mL | MRC-5 | 12.65 µg/mL | Okinawa, Japan | [43] | ||
| FCR3 | 2.45 µg/mL | ||||||||||
| 19 Ceratinadin E | K1 | 1.03 µg/mL | 15.99 µg/mL | ||||||||
| FCR3 | 0.77 µg/mL | ||||||||||
| Chloroquine * | - | K1 | 0.34 µg/mL | >25.80 µg/mL | - | ||||||
| FCR3 | 0.035 µg/mL | ||||||||||
| Artemisinin * | K1 | 0.010 µg/mL | >14.12 µg/mL | ||||||||
| FCR3 | 0.0088 µg/mL | ||||||||||
| Monanchora unguiculata | 20 Unguiculin A | Acyclic Guanidine Alkaloid | P. falciparum | 3D7 | 12.89 μM | KB Cells | 7.66 μM | Mitsio Islands, Madagascar | [44] | ||
| 21 Ptilomycalin E | Pentacyclic Alkaloids | 0.35 μM | 0.85 μM | ||||||||
| 22 Ptilomycalin F | 0.23 μM | 1.61 μM | |||||||||
| 23 Ptilomycalins G+H | 0.46 μM | 0.92 μM | |||||||||
| 24 Crambescidin 800 | Acyclic Guanidine Alkaloid | 0.52 μM | 1.31 μM | ||||||||
| 25 Fromiamycalin | 0.24 μM | 1.17 μM | |||||||||
| Artemisinin * | - | 0.004 μM | -- | - | - | ||||||
| Mycale sp. SS5 | 26 Albanitrile A | Nitrile-Bearing Polyacetylenes | Giardia duodenalis | 713 | 12 μM | Mammalian myeloma cell line NS-1 | 50 μM | Near Albany | [45] | ||
| Normal nontumor NFF cells | 100 μM | ||||||||||
| 27 Albanitrile B | 25 μM | Mammalian myeloma cell line NS-1 | 50 μM | ||||||||
| Normal nontumor NFF cells | 100 μM | ||||||||||
| 28 Albanitrile C | 90 μM | Mammalian myeloma cell line NS-1 | 180 μM | ||||||||
| Normal nontumor NFF cells | 90 μM | ||||||||||
| Metronidazole * | 2.9 μM | - | - | - | |||||||
| Sparsomycin * | - | - | - | - | Mammalian myeloma cell line NS-1 | 0.55 μM | |||||
| Normal nontumor NFF cells | 1.7 μM | ||||||||||
| Tedania brasiliensis | 29 Pseudoceratidine | Bromopyrrole Alkaloids | P. falciparum | 3D7 | EC50 = 1 μM | Bone marrow-derived macrophages | NT | Cabo Frio, Rio de Janeiro state, Brazil | [46] | ||
| 1.1 μM | |||||||||||
| K1 | 1.1 μM | ||||||||||
| 30 Pseudoceratidine derivative | P. falciparum | 3D7 | EC50 = 6 μM | ||||||||
| 31 Pseudoceratidine derivative | EC50 = 4 μM | ||||||||||
| 32 Pseudoceratidine derivative | L. infantum | promastigotes | EC50 = 24 μM | 52 μM | |||||||
| L. amazonensis | promastigotes | EC50 = 19 μM | |||||||||
| T. cruzi | epimastigotes | EC50 = 7 μM | |||||||||
| 33 Pseudoceratidine derivative | L. infantum | promastigotes | EC50 = 19 μM | >100 μM | |||||||
| L. amazonensis | promastigotes | EC50 = 7 μM | |||||||||
| P. falciparum | 3D7 | EC50 = 19 μM | |||||||||
| 34 Pseudoceratidine derivative | P. falciparum | 3D7 | EC50 = 44 μM | NT | |||||||
| 35 Pseudoceratidine derivative | L. infantum | promastigotes | EC50 = 2 μM | 66 μM | |||||||
| L. amazonensis | promastigotes | EC50 = 3 μM | |||||||||
| T. cruzi | epimastigotes | EC50 = 24 μM | |||||||||
| 36 Pseudoceratidine derivative | P. falciparum | 3D7 | EC50 = 7 μM | NT | |||||||
| 37 Pseudoceratidine derivative | L. infantum | promastigotes | EC50 = 20 μM | >100 μM | |||||||
| L. amazonensis | promastigotes | EC50 = 76 μM | |||||||||
| 38 Pseudoceratidine derivative | L. infantum | promastigotes | EC50 = 23 μM | 82 μM | |||||||
| L. amazonensis | promastigotes | EC50 = 18 μM | |||||||||
| P. falciparum | 3D7 | EC50 = 3 μM | NT | ||||||||
| Chloroquine * | - | P. falciparum | 3D7 | 0.013 μM | - | - | - | ||||
| K1 | 0.167 μM | ||||||||||
| Pyrimethamine * | - | P. falciparum | 3D7 | 0.03 μM | - | - | - | ||||
| K1 | 3.9 μM | ||||||||||
| Cycloguanil * | - | P. falciparum | 3D7 | 0.010 μM | - | - | - | ||||
| K1 | 0.54 μM | ||||||||||
| Artesunate * | - | P. falciparum | 3D7 | 0.004 μM | - | - | - | ||||
| K1 | 0.003 μM | ||||||||||
| Xestospongia sp. | 39 Kaimanol | Sterol | P. falciparum | 3D7 | 0.359 μM | NT | NT | Indonesia | [47] | ||
| 40 Saringosterol | 0.00025 μM | ||||||||||
| Artemisinin * | - | 5.207 × 10−3 nM | - | - | - | [48] | |||||
| Cnidaria | Alcyonium sp. | 41 Alcyopterosin V | Illudalane Sesquiterpenes | L. donovani | - | 7.0 μM | J774.A1 macrophages | 110 μM | Scotia Arc of Antarctica | [49] | |
| Host cell lines HEK293T | 220 μM | ||||||||||
| Host cell lines HepG2 | 288 μM | ||||||||||
| 42 Alcyopterosin E | 3.1 μM | J774.A1 macrophages | 62 μM | ||||||||
| Host cell lines HEK293T | 570 μM | ||||||||||
| Host cell lines HepG2 | 331 μM | ||||||||||
| Miltefosine * | - | 6.2 μM | - | - | - | ||||||
| Bebryce grandis | 43 Bebrycin A | Diterpene | P. falciparum | Dd2 | EC50 = 1.08 μM | HepG2 human hepatocyte carcinoma cell line | EC50 =21.8 μM | Southeast coast of Curacao, East of Fuikbaai | [50] | ||
| 44 Nitenin | C21 Degraded Terpene | EC50 = 0.29 μM | EC50 =18.3 μM | ||||||||
| Macrorhynchia philippina | 45 Isololiolide | Carotenoid Isololiolide | T. cruzi | trypomastigotes | 31.9 μM | BMM cells | >200 μM | São Sebastião Channel, Brazil | [51] | ||
| amastigotes | 40.4 μM | ||||||||||
| Benznidazole * | - | trypomastigotes | 16.2 μM | >200 μM | - | ||||||
| amastigotes | 5.3 μM | ||||||||||
| Plumarella delicatissima | 46 Keikipukalide A | Furanocembranoid Diterpenes | L. donovani | amastigotes | > 28 μM | Human lung carcinoma, cells, A549 cytotoxicity | >50 μM | Stanley, Falkland Islands (Islas Malvinas), in the Southern Ocean | [52] | ||
| 47 Keikipukalide B | 8.5 μM | >50 μM | |||||||||
| 48 Keikipukalide C | 8.8 μM | >50 μM | |||||||||
| 49 Keikipukalide D | 12 μM | >50 μM | |||||||||
| 50 Keikipukalide E | 8.8 μM | >50 μM | |||||||||
| 51 Pukalide aldehyde | 1.9 μM | >50 μM | |||||||||
| 52 Norditerpenoid ineleganolide | 4.4 μM | >50 μM | |||||||||
| Miltefosine * | - | 6.2 μM | - | - | - | ||||||
| Sinularia brassica | 53 Chlorinated steroid | Steroid | L. donovaniamastigote | amastigote | Inhibition of a growth of L. donovani at 50 μM = 58.7% | THP-1 cells at 50 μM | 88.8% | Van Phong bay, Khanh Hoa province, Vietnam and Institute of Oceanography, Nha Trang, Vietnam | [53] | ||
| 54 Pinnaterpene C | Dibromoditerpene | Inhibition of a growth of L. donovani at 50 μM = 74.3% | 106.2% | ||||||||
| 55 24-methylenecholestane-3β-5α,6β-triol-6-monoacetate | Steroid | Inhibition of a growth of L. donovani at 50μM = 54.7% | 96.1% | ||||||||
| 56 Cholestane-3β-5α,6β-triol-6-monoacetate | Inhibition of growth of L. donovani at 50μM = 39.0% | 92.7% | |||||||||
| Sinularia sp. | 57 Sinuketal | Sesquiterpenoids | P. falciparum | 3D7 | 80 μM | Jurkat | 24.9 μM | Yongxing Island (16°50′ N, 112°20′ E) of Xisha Islands in the South China Sea | [54] | ||
| MDA-MB-231 | 32.3 μM | ||||||||||
| U2OS | 41.7 μM | ||||||||||
| Dihydroartemisinine * | - | 10 nM | - | - | - | ||||||
| Bryozoa | Amathia lamourouxi | 58 Convolutamines K | Brominated Alkaloids | P. falciparum | 3D7 | 1.7 μM | Human embryonic kidney cell line, HEK293 | 17.01 μM | Rock pools of Woolgoolga, New South Wales, Australia | [55] | |
| 59 Convolutamines L | 3D7 | 11 μM | IA at 40 μM | ||||||||
| 60 Volutamides F | 3D7 | 0.61 μM | IA at 40 μM | ||||||||
| Dd2 | 0.75 μM | ||||||||||
| 61 Volutamides G | 3D7 | 0.57 μM | 11 μM | ||||||||
| Dd2 | 0.85 μM | ||||||||||
| 62 Volutamides H | 3D7 | 1.6 μM | IA at 40 μM | ||||||||
| Dd2 | 1.9 μM | ||||||||||
| Chloroquine * | - | 3D7 | 0.025 μM | 67% at 4 μM | - | ||||||
| Dd2 | 0.18 μM | ||||||||||
| Dihydroartemisinin * | - | 3D7 | 0.0020 μM | IA at 0.1 μM | - | ||||||
| Dd2 | 0.0020 μM | ||||||||||
| Puromycin * | - | 3D7 | 0.11 μM | 0.81 μM | - | ||||||
| Dd2 | 0.068 μM | ||||||||||
| Orthoscuticella ventricosa | 63 Orthoscuticellines A | Alkaloids | P. falciparum | 3D7 | 10 μM | Human embryonic kidney cell line, HEK293 | 10 μM | Northern NSW, Australia | [56] | ||
| 64 Orthoscuticellines B | > 40 μM | >40 μM | |||||||||
| 65 Orthoscuticellines D | 14 μM | >40 μM | |||||||||
| 66 Orthoscuticellines E | 12 μM | >40 μM | |||||||||
| 67 1-ethyl-4-methylsulfone-β-carboline | 21 μM | >40 μM | |||||||||
| 68 1-ethyl-β-carboline | 18 μM | >40 μM | |||||||||
| Chloroquine * | - | 0.007 μM | >40 μM | ||||||||
| Artesunate * | - | 0.0003 μM | - | - | - | ||||||
| Microorganisms | Actinomy-cetes | Streptomyces sp. PBLC04 | 69 Staurosporine | Alkaloid | Acanthamoeba castellanii | Trophozoites | 0.265 µg/mL | Murine macrophage J774.A1 cell line | 4.076 μM | Jambelí mangrove, Ecuador | [57] |
| Cysts | 0.771 µg/mL | ||||||||||
| Streptomyces sp. | 70 Marinopyrrole A | Alkaloids | T. gondii | Tachyzoites/Type I RH | 0.31 μM | Human foreskin fibroblast (HFF) | >50 μM | Marinopyrrole A was obtained from Sigma-Aldrich | [58] | ||
| Human hepatocarcinoma (HepG2) | 5.3 μM | ||||||||||
| 71 RL002 | 0.17 μM | Human foreskin fibroblast (HFF) | >50 μM | ||||||||
| Human hepatocarcinoma (HepG2) | 29.0 μM | ||||||||||
| 72 RL003 | 0.09 μM | Human foreskin fibroblast (HFF) | >50 μM | ||||||||
| Human hepatocarcinoma (HepG2) | 49.7 μM | ||||||||||
| 73 RL125 | 0.16 μM | Human foreskin fibroblast (HFF) | >50 μM | ||||||||
| Human hepatocarcinoma (HepG2) | 46.5 μM | ||||||||||
| Pyrimethamine * | - | 0.61 μM | - | - | - | ||||||
| Proteobacteria | Pseudomonas aeruginosa | 74 3-heptyl-3-hydroxy-1,2,3,4-tetrahydroquinoline-2.4-dione | Hydroxyquinoline | P. falciparum | Indochina W2 | 3.47 µg/mL | NT | NT | Pacific of Panama | [59] | |
| 75 2-heptyl-4-hydroxyquinoline | P. falciparum | Indochina W2 | 2.57 µg/mL | ||||||||
| T. cruzi | C4 | 3.66 µg/mL | |||||||||
| 76 2-nonyl-4-hydroxyquinoline | P. falciparum | Indochina W2 | 2.79 µg/mL | ||||||||
| T. cruzi | C4 | 3.99 µg/mL | |||||||||
| Chloroquine * | - | P. falciparum | Indochina W2 | 0.03 µg/mL | - | - | - | ||||
| Nifurtimox * | - | T. cruzi | C4 | 1.6 µg/mL | - | - | - | ||||
| Cyanophyta | Caldora penicillata | 77 Hoshinoamide C (natural) | Lipopeptide | P. falciparum | 3D7 | 0.96 μM | Human cancer cells, HeLa and HL60 | No cytotoxicity at 10 μM | Ikei Island, Okinawa, Japan | [60] | |
| T. brucei rhodesiense | IL-1501 | 2.9 μM | |||||||||
| 78 Hoshinoamide C(synthetic) | P. falciparum | 3D7 | 3.2 μM | ||||||||
| T. brucei rhodesiense | IL-1501 | 3.7 μM | |||||||||
| 79 43-epi-hoshinoamide C(synthetic) | P. falciparum | 3D7 | 0.87 μM | ||||||||
| T. brucei rhodesiense | IL-1501 | 4.4 μM | |||||||||
| Atovaquone * | - | P. falciparum | 3D7 | 0.00096 μM | - | - | - | ||||
| Pentamidine * | - | T. brucei rhodesiense | IL-1501 | 0.001 μM | - | - | - | ||||
| Dapis sp. | 80 Iheyanone | Linear Peptides | T. brucei rhodesiense | IL-1501 | 35 μM | WI-38 cells | >50 μM | Noho Island, Okinawa, Japan | [61] | ||
| 81 Peptides | 33 μM | >50 μM | |||||||||
| 82 Peptides | 24 μM | >50 μM | |||||||||
| 83 Peptides | 15 μM | >50 μM | |||||||||
| 84 Peptides | 17 μM | >50 μM | |||||||||
| 85 Peptides | 6.2 μM | >50 μM | |||||||||
| Pentamidine * | - | T. brucei rhodesiense | IL-1501 | 0.05 μM | - | -- | - | ||||
| Dapis sp. | 86 Iheyamides A | Linear Peptides | T. b. rhodesiense | IL-1501 | 1.5 μM | Normal human fibroblasts, WI-38 cells | 18 μM | Noho Island, Okinawa, Japan | [62] | ||
| T. b. brucei | 221 | 1.5 μM | |||||||||
| T. b. rhodesiense | IL-1501 | > 20 μM | >20 μM | ||||||||
| T. b. brucei | 221 | > 20 μM | |||||||||
| T. b. rhodesiense | IL-1501 | > 20 μM | >20 μM | ||||||||
| T. b. brucei | 221 | > 20 μM | |||||||||
| Pentamide * | - | T. b. rhodesiense | IL-1501 | 0.005 μM | - | - | - | ||||
| T. b. brucei | 221 | 0.001 μM | |||||||||
| Leptolyngbya sp. | 87 Motobamide | Cyclic Peptide | T. b. rhodesiense | IL-1501 | 2.3 μM | WI-38 cells | 55 μM | Bise, Okinawa Island, Okinawa Prefecture, Japan | [63] | ||
| HeLa or HL60 cells | IA at 10 μM | ||||||||||
| Leptolyngbya sp. | 88 Palstimolide A | Polyhydroxy Macrolide | P. falciparum | Dd2 | 0.1725 μM | HepG2 human liver cell line | 5.04 μM | Palmyra Atoll | [64] | ||
| L. donovani | promastigotes | 4.67 μM | B10R murine macrophages (L. donovani host cell toxicity) | >10 μM | |||||||
| Okeania sp. | 89 Ikoamide | Lipopeptide | P. falciparum | 3D7 | 0.14 μM | HeLa cells or HL60 cells | No cytotoxicity at 10 μM | Iko-pier, Kuroshima Island, Okinawa, Japan | [65] | ||
| Chloroquine * | - | 6.9 nM | - | - | - | ||||||
| doxorubicin | - | - | - | - | HeLa cells | 0.24 μM | - | ||||
| HL60 cells | 46 nM | - | |||||||||
| Okeania sp. | 90 Mabuniamide | Lipopeptide | P. falciparum | 3D7 | 1.4 μM | L6 myotubes | No cytotoxicity at 10–40 μM | The coast of Odo, Okinawa, Japan | [66] | ||
| 91 Stereoisomer 2 | 1.4 μM | ||||||||||
| Chloroquine * | - | 7.6 nM | - | - | - | ||||||
| Salileptolyngbya sp. | 92 Kinenzoline (natural) | Linear Depsipeptide | T. b. rhodesiense | IL-1501 | 5.0 μM | WI-38 cells | >20 μM | Kinenhama beach, Kagoshima, Japan | [67] | ||
| 93 Kinenzoline (synthetic) | 4.5 μM | >100 μM | |||||||||
| Pentamide * | - | 0.001 μM | - | - | - | ||||||
| Adriamycin * | - | - | - | - | WI-38 cells | 0.73 μM | - | ||||
| Moorea producens | 94 Dudawalamide A | Cyclic Depsipeptides | P. falciparum | W2 | 3.6 μM | H-460 human lung cancer cell line | Little to no cytotoxicity | Papua New Guinea | [68] | ||
| T. cruzi | Transgenic β-galactosidase-expressing strain | 12% GI (Percentage growth inhibition) at 10 μg/mL | |||||||||
| L. donovani | WR2810 | > 10 μM | |||||||||
| 95 Dudawalamide B | P. falciparum | W2 | 8.0 μM | ||||||||
| T. cruzi | Transgenic β-galactosidase-expressing strain | 7% GI at 10 μg/mL | |||||||||
| L. donovani | WR2810 | > 10 μM | |||||||||
| 96 Dudawalamide C | P. falciparum | W2 | 10 μM | ||||||||
| 97 Dudawalamide D | P. falciparum | W2 | 3.5 μM | ||||||||
| T. cruzi | Transgenic β-galactosidase-expressing strain | 60% GI at 10 μg/mL | |||||||||
| L. donovani | WR2810 | 2.6 μM | |||||||||
| Ascomycetes | Aspergillus terreus BCC51799 | 98 Astepyrazinoxide | Alkaloid | P. falciparum | K-1 | 24.82 μM | MCF-7 | 34.70 μM | The marine fungus was isolated from a decayed wood sample at Hat Bang Pu, Khao Sam Roi Yot National Park, Prachuap Khiri Khan Province | [69] | |
| NCI–H187 | 5.98 μM | ||||||||||
| Vero | 15.61 μM | ||||||||||
| 99 Astechrome | 0.94 μM | MCF-7 | IA | ||||||||
| NCI–H187 | IA | ||||||||||
| Vero | 7.9 μM | ||||||||||
| Dihydroartemisinin * | - | 2.12 × 10−3 μM | - | - | - | ||||||
| Mefloquine * | - | 0.422 μM | - | - | - | ||||||
| Ellipticine * | - | - | - | - | NCI–H187 | 9.87 μM | - | ||||
| Vero | 5.32 μM | ||||||||||
| Doxorubicin * | - | - | - | - | MCF-7 | 10.97 μM | - | ||||
| NCI–H187 | 0.16 μM | ||||||||||
| Tamoxifen * | - | - | - | - | MCF-7 | 32.95 μM | - | ||||
| Cochliobolus lunatus TA26-46 | 100 Derivatives | 14-Membered Resorcylic Acid Lactone Derivatives | P. falciparum | HB3 | 12.59 μmol/L | HUVEC | NT | Marine-derived | [70] | ||
| 101 Derivatives | 12.39 μmol/L | NT | |||||||||
| 102 Derivatives | 11.55 μmol/L | NT | |||||||||
| 103 Derivatives | 8.06 μmol/L | >100 μmol/L | |||||||||
| 104 Derivatives | 6.69 μmol/L | >100 μmol/L | |||||||||
| 105 Derivatives | 7.82 μmol/L | >100 μmol/L | |||||||||
| 106 Derivatives | 9.72 μmol/L | >100 μmol/L | |||||||||
| 107 Derivatives | 7.82 μmol/L | >100 μmol/L | |||||||||
| 108 Derivatives | 7.25 μmol/L | >100 μmol/L | |||||||||
| 109 Acyl derivatives | 9.18 μmol/L | NT | |||||||||
| 110 Acyl derivatives | 6.91 μmol/L | >100 μmol/L | |||||||||
| 111 Acyl derivatives | 3.54 μmol/L | >100 μmol/L | |||||||||
| Chloroquine * | - | 32.9 nmol/L | - | - | - | ||||||
| Exserohilum sp. | 112 Isocoumarins | Polyketide | P. falciparum | HB3 | 1.13 μM | Vero cells | 87.5 μM | Zoanthid Palythoa haddoni | [71] | ||
| 113 Isocoumarins | 11.7 μM | 124.2 μM | |||||||||
| 114 Derivatives | 0.77 μM | 258.0 μM | |||||||||
| 115 Derivatives | 0.38 μM | 106.3 μM | |||||||||
| 116 Derivatives | 2.58 μM | 262.5 μM | |||||||||
| Paecilomyces sp. 7A22 | 117 Harzialactone A | Polyketone | L. amazonensis | promastigotes | 5.25 μg/mL | Peritoneal macrophages | 35.21 μg/mL | Ascidian Aplidiopsis sp. collected from São Sebastião Channel in Brazil | [72] | ||
| amastigotes | 18.18 μg/mL | ||||||||||
| Amphotericin B * | - | L. amazonensis | promastigotes | 0.119 μg/mL | 22.41 μg/mL | - | |||||
| amastigotes | 0.095 μg/mL | ||||||||||
| Category | Species | Extract Type | Target Parasite | Stage/Strain | IC50 | Site | References |
|---|---|---|---|---|---|---|---|
| Cnidaria | Linuche unguiculata | Distilled water | Giardia duodenalis | Trophozoites, IMSS 0989:1 strain | 63 µg/mL | Puerto Morelos Reef Lagoon, Mexico | [73] |
| Actinomycetes | Nocardia sp. UA 23 | ISP2 medium | Trypanosoma brucei | TC 221 | MIC, 72 h = 7.2 µg/mL | Coscinoderma mathewsi was collected from Ahia Reefs | [74] |
| Micromonospora sp. W305 | Resin, MeOH | Antiplasmodial Activities | Dd2 | 0.42 µg/mL | The microbial population associated with deep-water invertebrates | [75] | |
| Nocardiopsis sp. V671 | ASE, MeOH | Antiplasmodial Activities | Dd2 | 0.88 µg/mL | The microbial population associated with deep-water invertebrates | [75] | |
| Streptomyces tendae V324 | Resin, MeOH/CH2Cl2 | Antiplasmodial Activities | Dd2 | 0.35 µg/mL | The microbial population associated with deep-water invertebrates | [75] | |
| Streptomyces sp. INV ACT2 | Ethyl acetate | T. gondii | GFP-RH tachyzoites | Inhibition ≥ 80% at 120 μg/mL | Caño Aguas Negras | [76] | |
| Streptomyces sp. RM66 | On ISP2, solid media with GlcNAc | Trypanosoma brucei | TC 221 | MIC, 72 h = 4.7 µg/mL | Hurghada (Egypt) | [77] | |
| Streptomyces sp. V881 | Resin, CH2Cl2 | Antiplasmodial Activities | Dd2 | 0.062 µg/mL | The microbial population associated with deep-water invertebrates | [75] | |
| Streptomyces sp. E677 | Resin, MeOH/CH2Cl2 | Antiplasmodial Activities | Dd2 | 0.037 µg/mL | The microbial population associated with deep-water invertebrates | [75] | |
| Unidentified actinomycete V663 | ASE, heptane | Antiplasmodial Activities | Dd2 | 0.89 µg/mL | The microbial population associated with deep-water invertebrates | [75] | |
| Bacteroides | Alcanivorax sp. V174 (G-) | Resin, MeOH/CH2Cl2 | Antiplasmodial Activities | Dd2 | 0.969 µg/mL | The microbial population associated with deep-water invertebrates | [75] |
| Alcanivorax sp. V193 (G-) | Resin, MeOH/CH2Cl2 | Antiplasmodial Activities | Dd2 | 1.079 µg/mL | The microbial population associated with deep-water invertebrates | [75] | |
| Endozoicomonas numazuensis H402 (G-) | Resin, MeOH/CH2Cl2 | Antiplasmodial Activities | Dd2 | 0.978 µg/mL | The microbial population associated with deep-water invertebrates | [75] | |
| Marinobacter sp. V184 (G-) | Resin, MeOH/CH2Cl2 | Antiplasmodial Activities | Dd2 | 1.008 µg/mL | The microbial population associated with deep-water invertebrates | [75] | |
| Marinobacter sp. V201 (G-) | Resin, MeOH/CH2Cl2 | Antiplasmodial Activities | Dd2 | 1.091 µg/mL | The microbial population associated with deep-water invertebrates | [75] | |
| Marinobacter sp. V208 (G-) | Resin, MeOH/CH2Cl2 | Antiplasmodial Activities | Dd2 | 1.091 µg/mL | The microbial population associated with deep-water invertebrates | [75] | |
| Firmicutes | Bacillus sp. INV FIR35 | Ethyl acetate | T. gondii | GFP-RH tachyzoites | Inhibition ≥ 80% at 48 μg/mL | Punta Betín | [76] |
| Bacillus sp. INV FIR48 | Ethyl acetate | T. gondii | GFP-RH tachyzoites | Inhibition ≥ 80% at 120 μg/mL | Caño Grande | [76] | |
| Fictibacillus sp. INV FIR149 | Ethyl acetate | T. gondii | GFP-RH tachyzoites | Inhibition ≥ 80% at 1080 μg/mL | Caño Grande | [76] | |
| Paenibacillus sp. #91_7 (IN-CRY) | Waters™ Oasis® HLB extraction plates, with the sorbent Oasis® HLB, was equilibrated using methanol and HPLC grade water | T. cruzi | Tulahuen C4 | 97% | Isolated from marine sponges of the Erylus genus, collected in Portuguese waters | [78] | |
| Penicillium citrinum V170 | Resin, MeOH/CH2Cl2 | Antiplasmodial Activities | Dd2 | 1.069 µg/mL | The microbial population associated with deep-water invertebrates | [75] | |
| Penicillium sp. N161 | Resin, MeOH/CH2Cl2 | Antiplasmodial Activities | Dd2 | 0.266 µg/mL | The microbial population associated with deep-water invertebrates | [75] | |
| Penicillium sp. Z691 | Resin, CH2Cl2 | Antiplasmodial Activities | Dd2 | 0.049 µg/mL | The microbial population associated with deep-water invertebrates | [75] | |
| Talaromyces rotundus S920 | Resin, MeOH/CH2Cl2 | Antiplasmodial Activities | Dd2 | 0.677 µg/mL | The microbial population associated with deep-water invertebrates | [75] | |
| Tritirachium sp. V199 | Resin, MeOH/CH2Cl2 | Antiplasmodial Activities | Dd2 | 0.339 µg/mL | The microbial population associated with deep-water invertebrates | [75] | |
| ɣ-Proteobacteria | Enterococcus faecalis #118_3 (IN-CRY) | EPA vials: Sepabeads® SP207ss resin, HPLC-grade water and acetone; medium IN-CRY | T. cruzi | Tulahuen C4 | Percentage of growth inhibition = 81% | Isolated from marine sponges of the Erylus genus, collected in Portuguese waters | [78] |
| Enterococcus faecalis #118_3 (IN-CRY) | Duetz extraction: Waters™ Oasis® HLB extraction plates, with the sorbent Oasis® HLB, was equilibrated using methanol and HPLC grade water; medium IN-CRY | T. cruzi | Tulahuen C4 | Percentage of growth inhibition = 102% | Isolated from marine sponges of the Erylus genus, collected in Portuguese waters | [78] | |
| Enterococcus faecalis #118_4 (IN-CRY) | Duetz extraction: Waters™ Oasis® HLB extraction plates, with the sorbent Oasis® HLB, was equilibrated using methanol and HPLC grade water; medium IN-CRY | T. cruzi | Tulahuen C4 | Percentage of growth inhibition = 103% | Isolated from marine sponges of the Erylus genus, collected in Portuguese waters | [78] | |
| Pseudoalteromonas sp. INV PRT33 | Ethyl acetate | T. gondii | GFP-RH tachyzoites | Inhibition ≥ 80% at 48 μg/mL | Caño Grande | [76] | |
| Phaeophyta | Cladostephus hirsutus | Ethyl acetate | T. brucei brucei | - | 27.2 μg/mL | North-west coast of Algeria | [79] |
| Cystoseira sedoides | Hexane | Acanthamoeba castellanii | Trophozoite/Neff | 1009 μg/mL | Tunisian coasts, Tabarka | [80] | |
| Ethyl acetate | 860 μg/mL | ||||||
| Methanol | 836 μg/mL | ||||||
| Dictyota ciliolata | Hexane | Schistosoma mansoni | Death Ratio = 100% | Espírito Santo State, Southeastern Brazil | [81] | ||
| Chloroform | Death Ratio = 100% | ||||||
| Supercritical fluid | Death Ratio = 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Zhang, Q.; Zhang, Q.; Cui, X.; Zhu, L. Promising Antiparasitic Natural and Synthetic Products from Marine Invertebrates and Microorganisms. Mar. Drugs 2023, 21, 84. https://doi.org/10.3390/md21020084
Zhang M, Zhang Q, Zhang Q, Cui X, Zhu L. Promising Antiparasitic Natural and Synthetic Products from Marine Invertebrates and Microorganisms. Marine Drugs. 2023; 21(2):84. https://doi.org/10.3390/md21020084
Chicago/Turabian StyleZhang, Mingyue, Qinrong Zhang, Qunde Zhang, Xinyuan Cui, and Lifeng Zhu. 2023. "Promising Antiparasitic Natural and Synthetic Products from Marine Invertebrates and Microorganisms" Marine Drugs 21, no. 2: 84. https://doi.org/10.3390/md21020084
APA StyleZhang, M., Zhang, Q., Zhang, Q., Cui, X., & Zhu, L. (2023). Promising Antiparasitic Natural and Synthetic Products from Marine Invertebrates and Microorganisms. Marine Drugs, 21(2), 84. https://doi.org/10.3390/md21020084







