Multiple Effects of Echinochrome A on Selected Ion Channels Implicated in Skin Physiology
Abstract
1. Introduction
2. Results
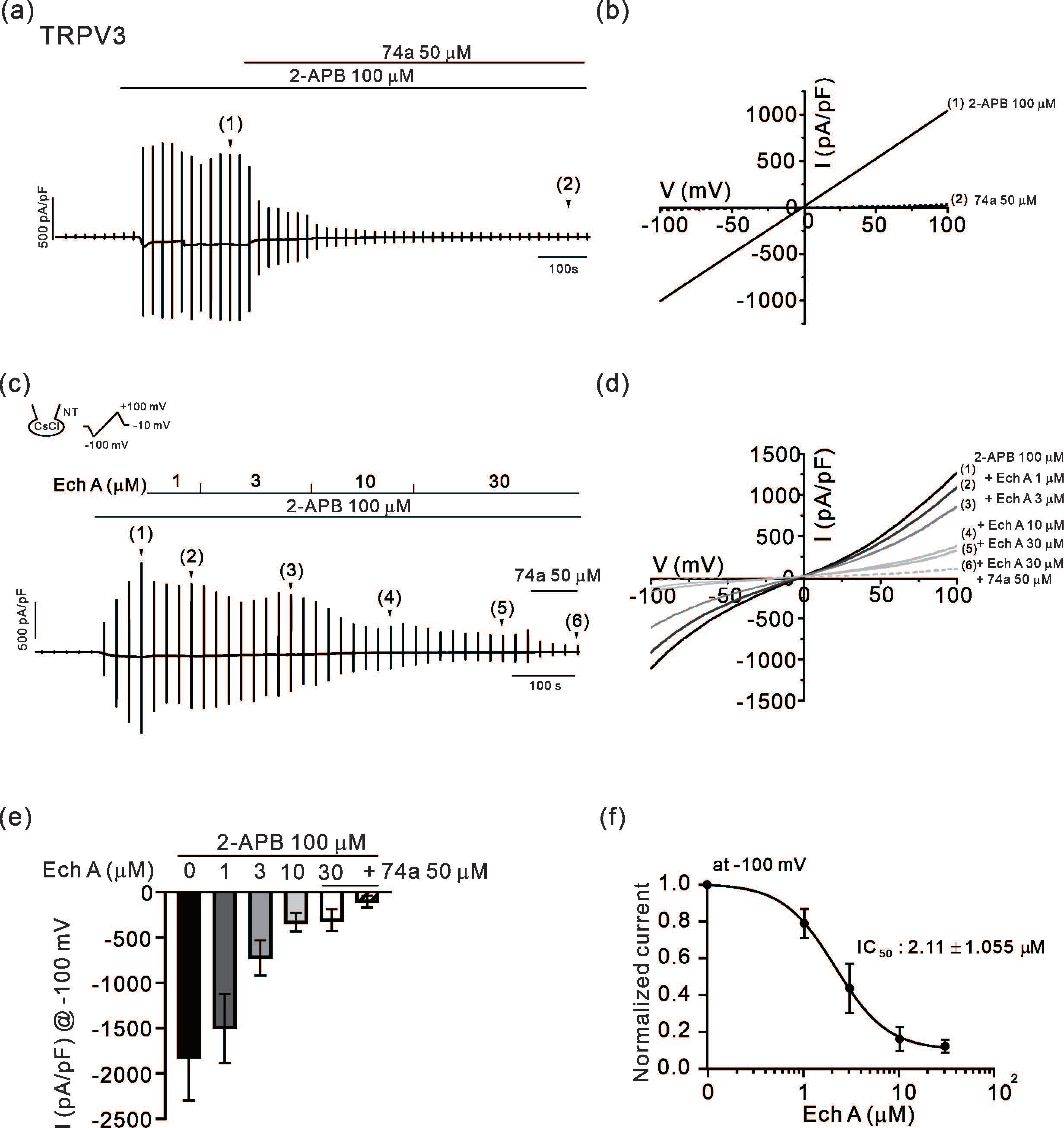
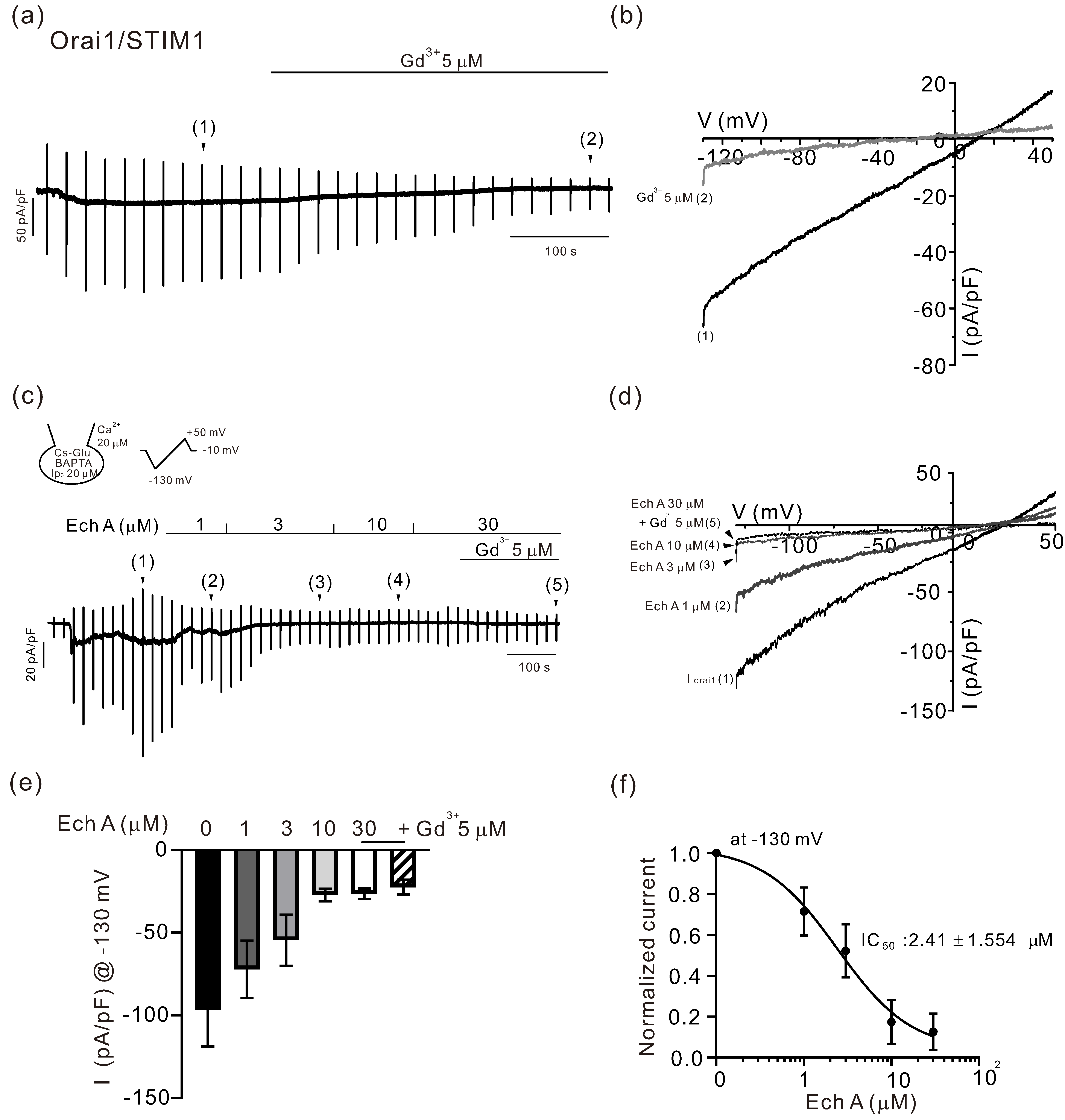
2.1. Inhibitory Effects of Ech A on TRPV3 and Orai1
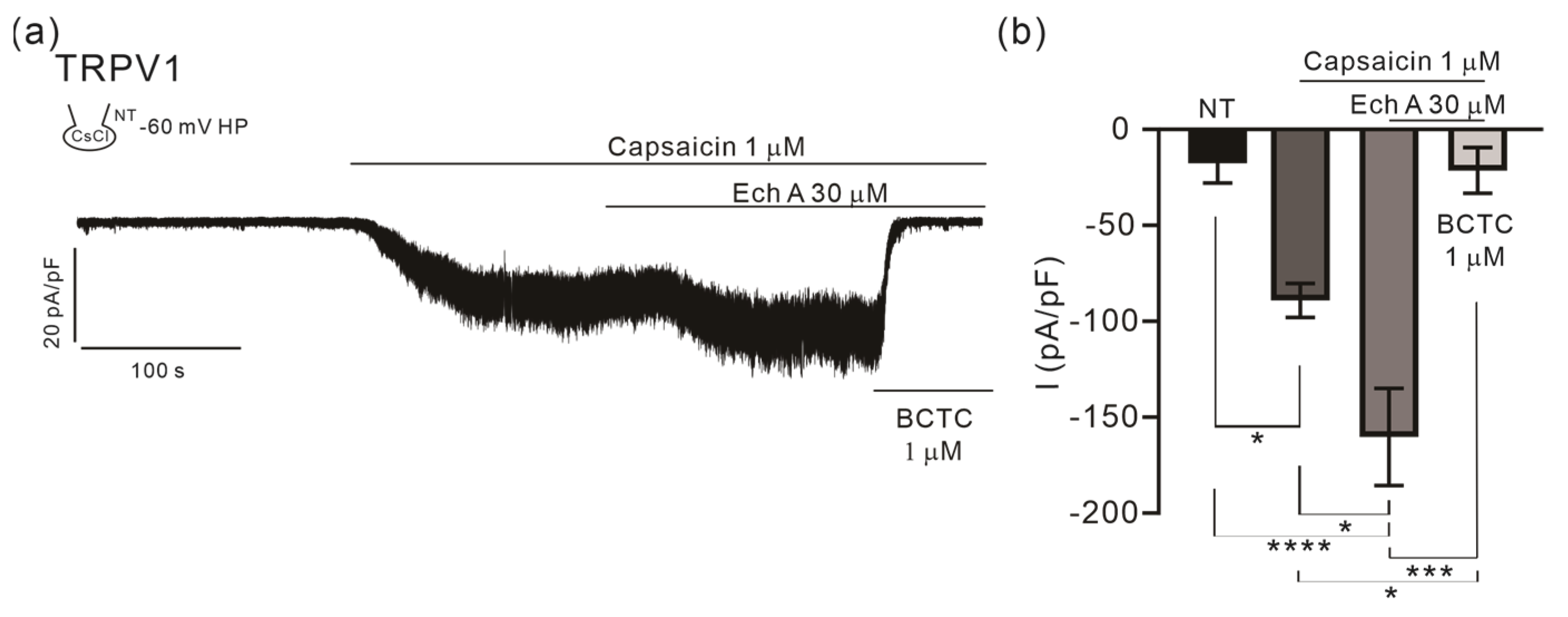
2.2. Facilitating Effect of Ech A on TRPV1
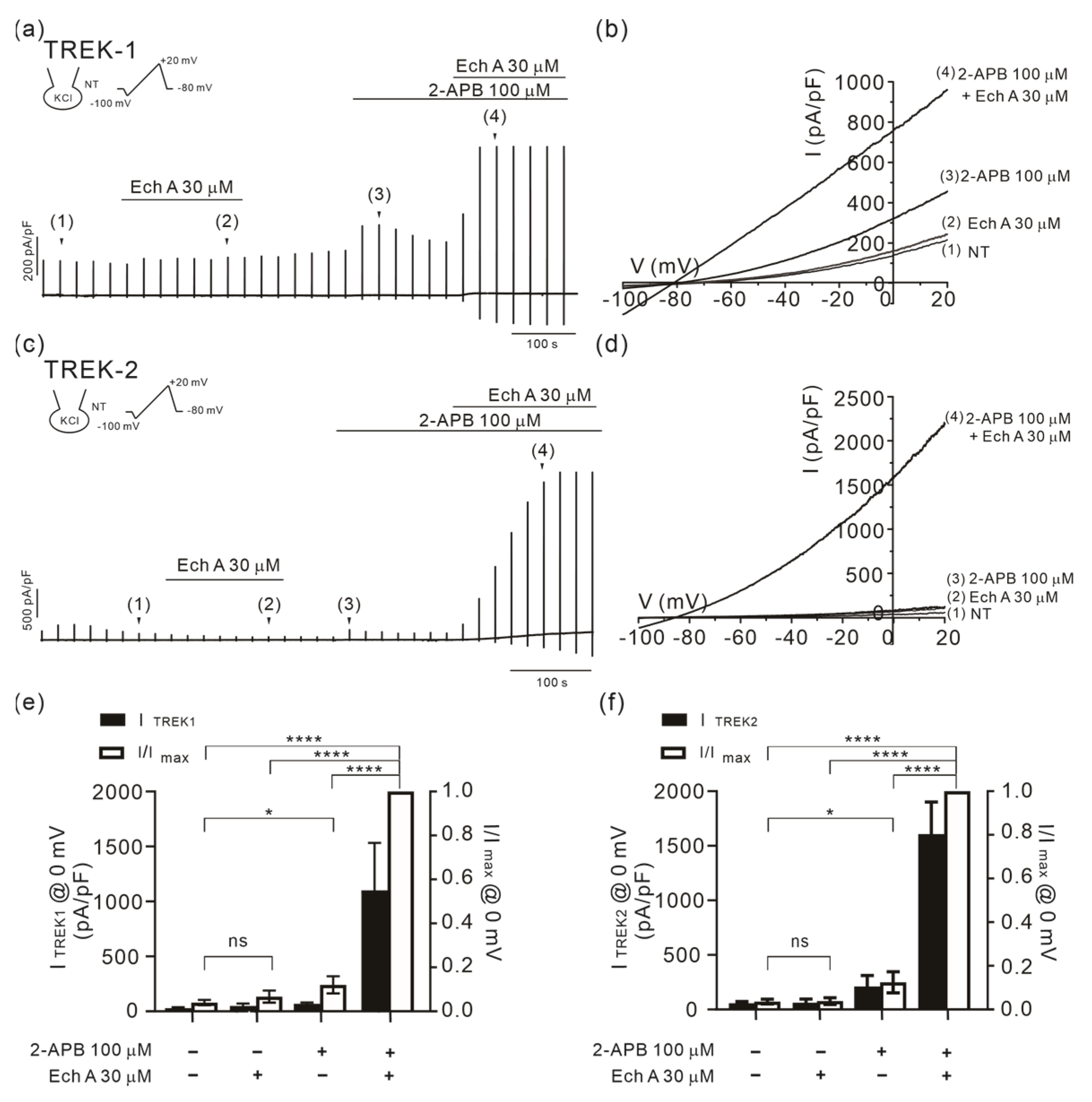
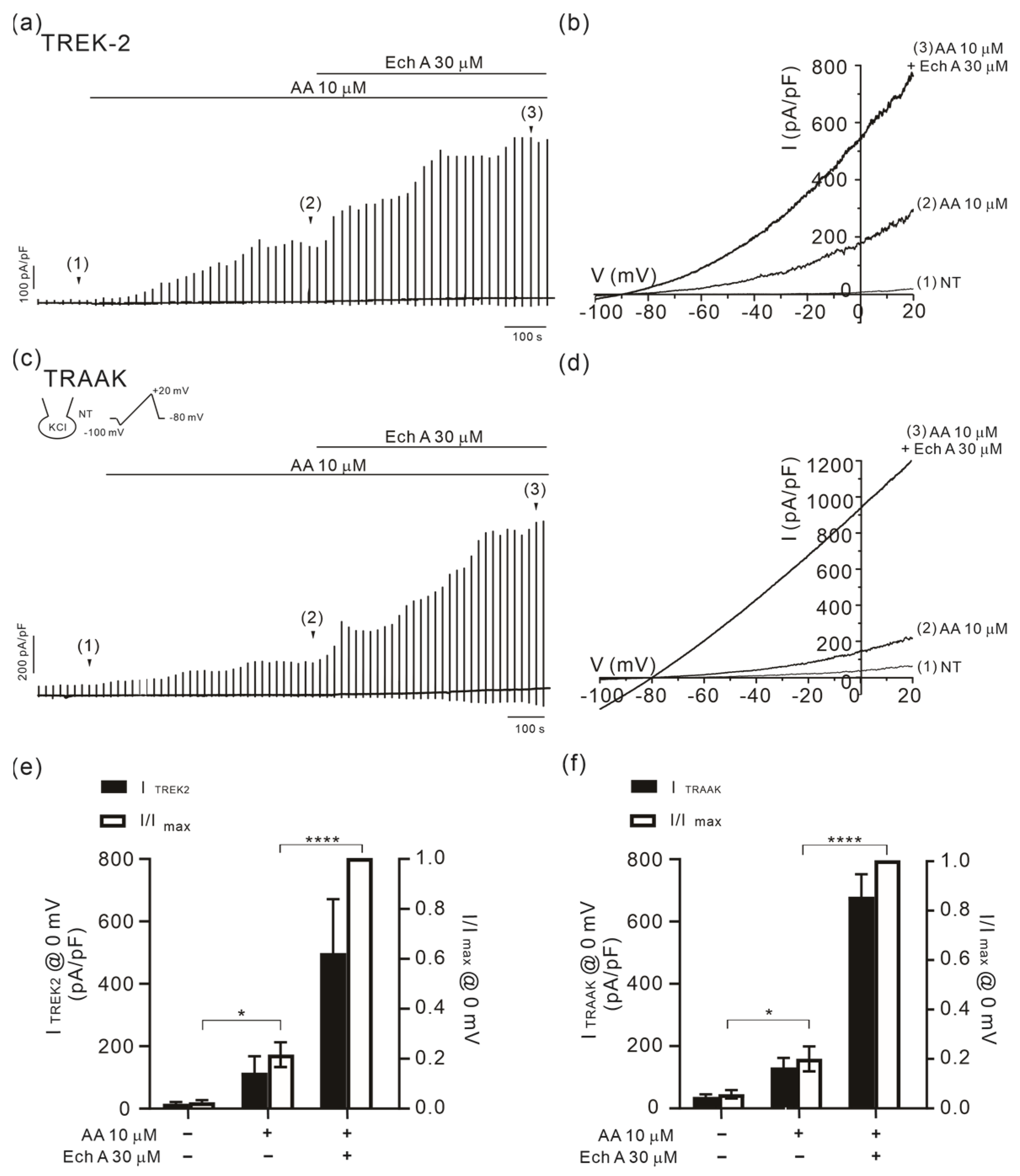
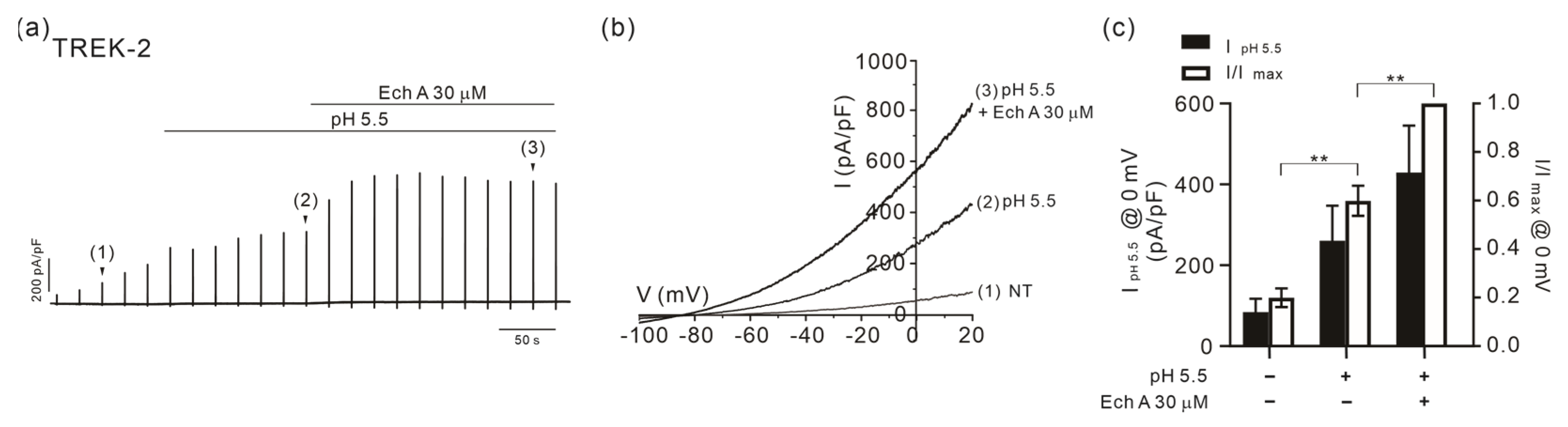
2.3. Facilitation of TREK/TRAAK Activities by Ech A in the Presence of Agonists
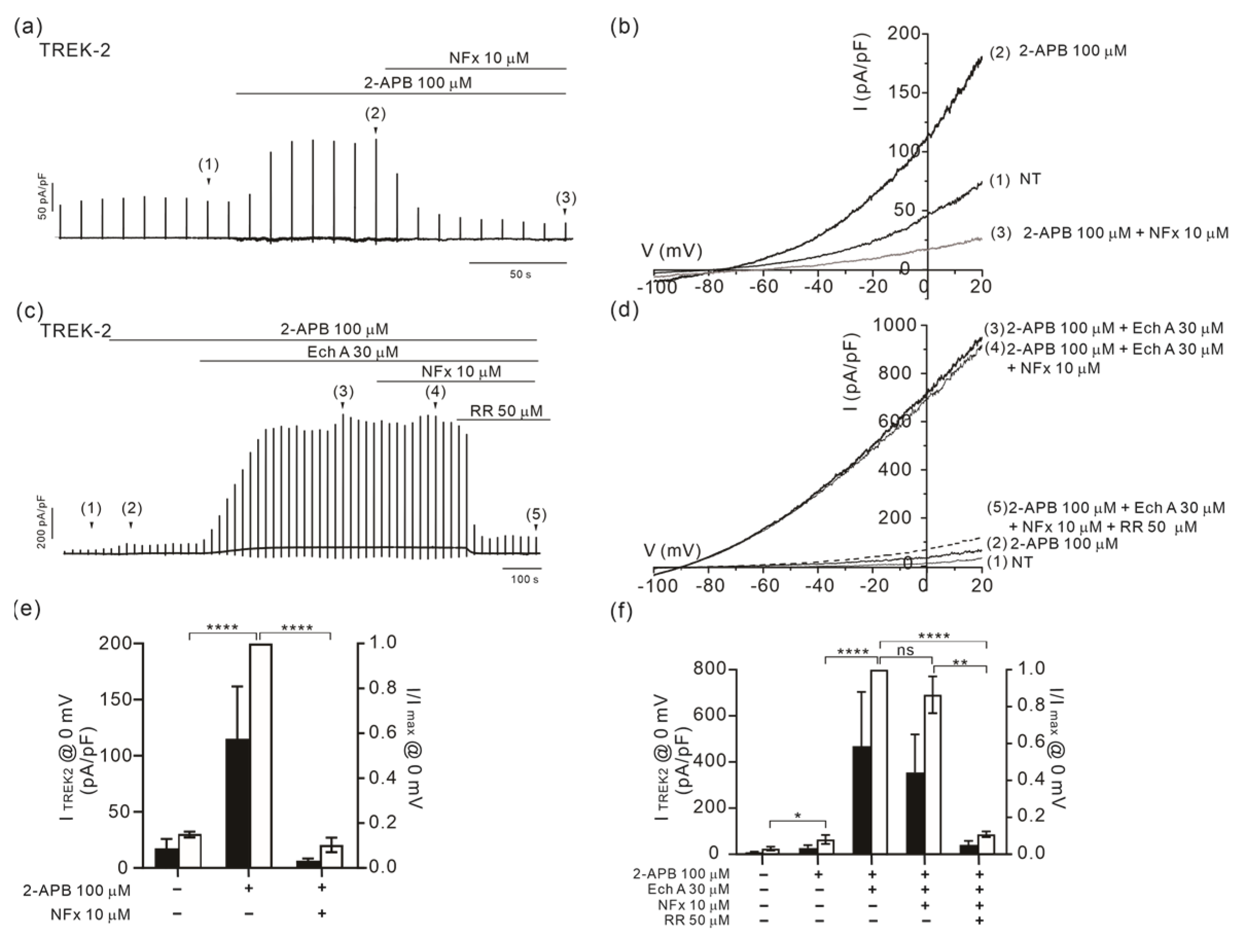
2.4. Altered Sensitivity of TREK-2 to Norfluoxetine by Ech A
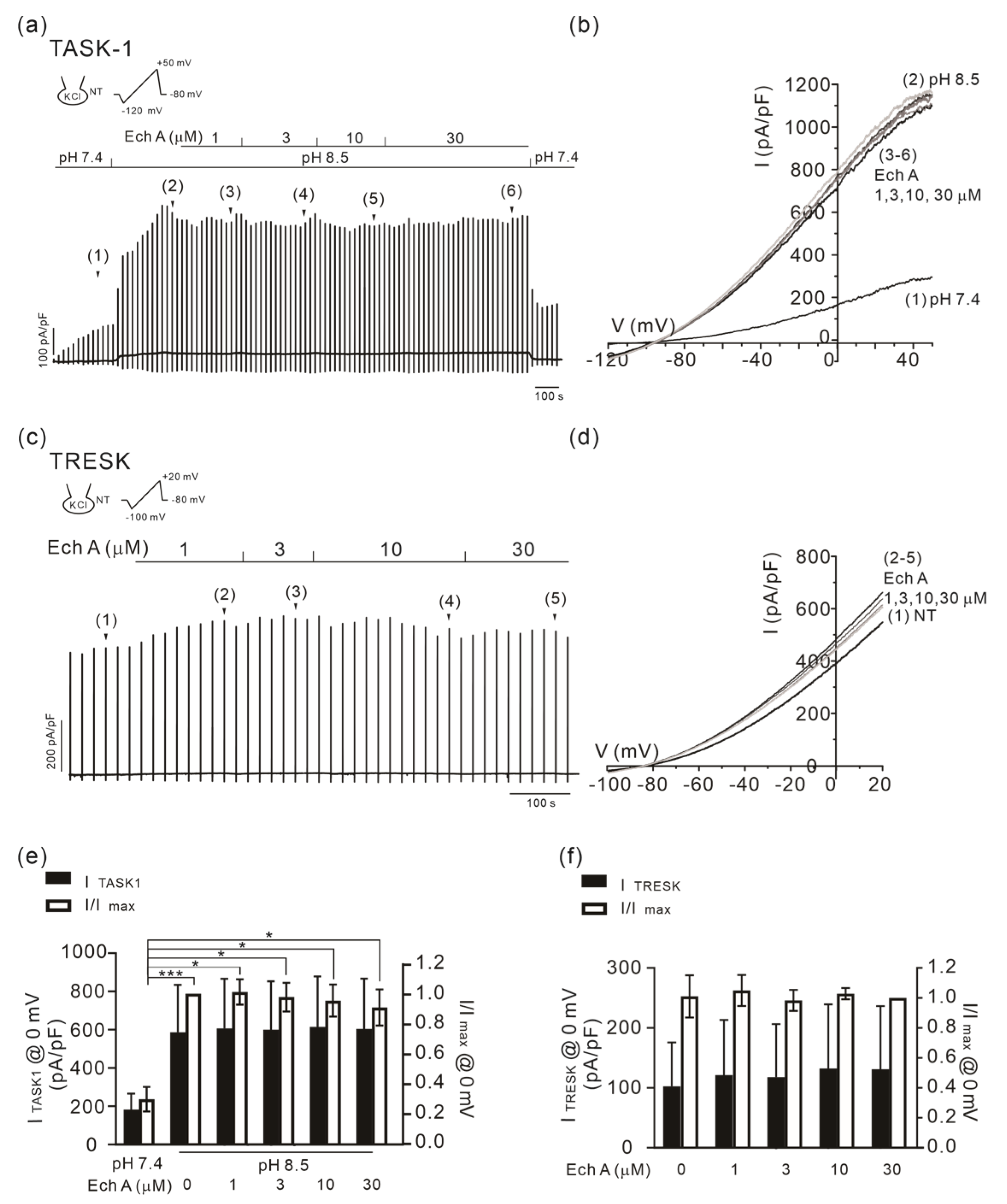
2.5. No Changes in TASK-1 and TRESK Activity by Ech A
3. Discussion
3.1. Inhibitory Effect of Ech A on TRPV3
3.2. Inhibitory Effect of Ech A on Orai1
3.3. Strong Facilitation of TREK/TRAAK Channels by Ech A
3.4. State-Dependent Effects of Ech A on TREK-2
3.5. Facilitation of TRPV1 by Ech A
4. Materials and Methods
4.1. Cell Culture
4.2. Chemicals
4.3. Electrophysiological Recording
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Wang, K. Exploiting the diversity of ion channels: Modulation of ion channels for therapeutic indications. Handb. Exp. Pharmacol. 2019, 260, 187–205. [Google Scholar] [PubMed]
- Imbrici, P.; Nicolotti, O.; Leonetti, F.; Conte, D.; Liantonio, A. Ion channels in drug discovery and safety pharmacology. Methods Mol. Biol. 2018, 1800, 313–326. [Google Scholar] [PubMed]
- Bajaj, S.; Ong, S.T.; Chandy, K.G. Contributions of natural products to ion channel pharmacology. Nat. Prod. Rep. 2020, 37, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Kanitakis, J. Anatomy, histology and immunohistochemistry of normal human skin. Eur. J. Dermatol. 2002, 12, 390–399. [Google Scholar] [PubMed]
- Peier, A.M.; Reeve, A.J.; Andersson, D.A.; Moqrich, A.; Earley, T.J.; Hergarden, A.C.; Story, G.M.; Colley, S.; Hogenesch, J.B.; McIntyre, P.; et al. A heat-sensitive TRP channel expressed in keratinocytes. Science 2002, 296, 2046–2049. [Google Scholar] [CrossRef]
- Wang, G.; Wang, K. The Ca2+-permeable cation transient receptor potential trpv3 channel: An emerging pivotal target for itch and skin diseases. Mol. Pharmacol. 2017, 92, 193–200. [Google Scholar] [CrossRef]
- Blaydon, D.C.; Kelsell, D.P. Defective channels lead to an impaired skin barrier. J. Cell. Sci. 2014, 127, 4343–4350. [Google Scholar] [CrossRef]
- Steinhoff, M.; Bíró, T. A TR(I)P to pruritus research: Role of TRPV3 in inflammation and itch. J. Investig. Dermatol. 2009, 129, 531–535, Erratum in J. Investig. Dermatol. 2010, 130, 908. [Google Scholar] [CrossRef]
- Mauro, T.; Dixon, D.B.; Komuves, L.; Hanley, K.; Pappone, P.A. Keratinocyte K+ channels mediate Ca2+-induced differentiation. J. Investig. Dermatol. 1997, 108, 864–870. [Google Scholar] [CrossRef]
- Kang, D.; Kim, S.H.; Hwang, E.M.; Kwon, O.S.; Yang, H.Y.; Kim, E.S.; Choi, T.H.; Park, J.Y.; Hong, S.G.; Han, J. Expression of thermosensitive two-pore domain K+ channels in human keratinocytes cell line HaCaT cells. Exp. Dermatol. 2007, 16, 1016–1022. [Google Scholar] [CrossRef]
- Smith, G.D.; Gunthorpe, M.J.; Kelsell, R.E.; Hayes, P.D.; Reilly, P.; Facer, P.; Wright, J.E.; Jerman, J.C.; Walhin, J.P.; Ooi, L.; et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 2002, 418, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ramsey, I.S.; Kotecha, S.A.; Moran, M.M.; Chong, J.A.; Lawson, D.; Ge, P.; Lilly, J.; Silos-Santiago, I.; Xie, Y.; et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 2002, 418, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Jin, J.; Hu, L.; Shen, D.; Dong, X.P.; Samie, M.A.; Knoff, J.; Eisinger, B.; Liu, M.L.; Huang, S.M.; et al. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell 2010, 141, 331–343. [Google Scholar] [CrossRef]
- Moqrich, A.; Hwang, S.W.; Earley, T.J.; Petrus, M.J.; Murray, A.N.; Spencer, K.S.; Andahazy, M.; Story, G.M.; Patapoutian, A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 2005, 307, 1468–1472. [Google Scholar] [CrossRef]
- Lin, Z.; Chen, Q.; Lee, M.; Cao, X.; Zhang, J.; Ma, D.; Chen, L.; Hu, X.; Wang, H.; Wang, X.; et al. Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am. J. Hum. Genet. 2012, 90, 558–564. [Google Scholar] [CrossRef]
- He, Y.; Zeng, K.; Zhang, X.; Chen, Q.; Wu, J.; Li, H.; Zhou, Y.; Glusman, G.; Roach, J.; Etheridge, A.; et al. A gain-of-function mutation in TRPV3 causes focal palmoplantar keratoderma in a Chinese family. J. Investig. Dermatol. 2015, 135, 907–909. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Chae, J.; Yoo, H.Y.; Kim, J.I.; Kim, S.J. The novel high-frequency variant of TRPV3 p.A628T in East Asians showing faster sensitization in response to chemical agonists. Pflugers. Arch. 2019, 471, 1273–1289. [Google Scholar] [CrossRef] [PubMed]
- Takei, K.; Denda, S.; Nagayama, M.; Denda, M. Role of STIM1-Orai1 system in intra-cellular calcium elevation induced by ATP in cultured human keratinocytes. Exp. Dermatol. 2016, 25, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Vandenberghe, M.; Raphaël, M.; Lehen’kyi, V.; Gordienko, D.; Hastie, R.; Oddos, T.; Rao, A.; Hogan, P.G.; Skryma, R.; Prevarskaya, N. ORAI1 calcium channel orchestrates skin homeostasis. Proc. Natl. Acad. Sci. USA 2013, 110, E4839–E4848. [Google Scholar] [CrossRef]
- Wulff, H.; Beeton, C.; Chandy, K.G. Potassium channels as therapeutic targets for autoimmune disorders. Curr. Opin. Drug. Discov. Devel. 2003, 6, 640–647. [Google Scholar]
- Varga, Z.; Hajdu, P.; Panyi, G. Ion channels in T lymphocytes: An update on facts, mechanisms and therapeutic targeting in autoimmune diseases. Immunol. Lett. 2010, 130, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.L.; Woods, C.G. Painful and painless channelopathies. Lancet. Neurol. 2014, 13, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Das, V. An introduction to pain pathways and pain “targets”. Prog. Mol. Biol. Transl. Sci. 2015, 131, 1–30. [Google Scholar]
- Gada, K.; Plant, L.D. Two-pore domain potassium channels: Emerging targets for novel analgesic drugs: IUPHAR Review 26. Br. J. Pharmacol. 2019, 176, 256–266. [Google Scholar] [CrossRef]
- Kang, D.; Kim, D. TREK-2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am. J. Physiol. Cell. Physiol. 2006, 291, C138–C146. [Google Scholar] [CrossRef]
- Mathie, A.; Veale, E.L.; Golluscio, A.; Holden, R.G.; Walsh, Y. Pharmacological Approaches to Studying Potassium Channels. Handb. Exp. Pharmacol. 2021, 267, 83–111. [Google Scholar] [PubMed]
- Choi, S.W.; Woo, J.; Park, K.S.; Ko, J.; Jeon, Y.K.; Choi, S.W.; Yoo, H.Y.; Kho, I.; Kim, T.J.; Kim, S.J. Higher expression of KCNK10 (TREK-2) K+ channels and their functional upregulation by lipopolysaccharide treatment in mouse peritoneal B1a cells. Pflugers. Arch. 2021, 473, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Nam, J.H.; Pang, B.; Shin, D.H.; Kim, J.S.; Chun, Y.S.; Park, J.W.; Bang, H.; Kim, W.K.; Earm, Y.E.; et al. Identification of the large-conductance background K+ channel in mouse B cells as TREK-2. Am. J. Physiol. Cell. Physiol. 2009, 297, C188–C197. [Google Scholar] [CrossRef]
- Vaeth, M.; Kahlfuss, S.; Feske, S. CRAC channels and calcium signaling in T cell-mediated immunity. Trends Immunol. 2020, 41, 878–901. [Google Scholar] [CrossRef]
- Feske, S. Calcium signalling in lymphocyte activation and disease. Nat. Rev. Immunol. 2007, 7, 690–702. [Google Scholar] [CrossRef]
- Yan, S.; Chen, W.; Zhang, Y.; Li, J.; Chen, X. Calcium release-activated calcium modulator 1 as a therapeutic target in allergic skin diseases. Life Sci. 2019, 228, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Majtan, J.; Bucekova, M.; Jesenak, M. Natural Products and Skin Diseases. Molecules 2021, 26, 4489. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, K.W. Molecular Targets of Phytochemicals for Skin Inflammation. Curr. Pharm. Des. 2018, 24, 1533–1550. [Google Scholar] [CrossRef] [PubMed]
- Egorov, E.A.; Alekhina, V.A.; Volobueva, T.M.; Fedoreev, S.A.; Mishchenko, N.P.; Kol’tsova, E.A. Histochrome, a new antioxidant in the treatment of ocular diseases. Vestn. Oftalmol. 1999, 115, 34–35. [Google Scholar]
- Mishchenko, N.; Fedoreev, S.; Bagirova, V. Histochrome: A new original domestic drug. Pharm. Chem. J. 2003, 37, 48–52. [Google Scholar] [CrossRef]
- Arthukov, A.A.; Zelepuga, E.A.; Bogdanovich, L.N.; Lupach, N.M.; Novikov, V.L.; Rutckova, T.A.; Kozlovskaya, E.P. Marine Polyhydroxynaphthoquinone, Echinochrome A: Prevention of Atherosclerotic Inflammation and Probable Molecular Targets. J. Clin. Med. 2020, 9, 1494. [Google Scholar] [CrossRef]
- Jeong, S.H.; Kim, H.K.; Song, I.S.; Lee, S.J.; Ko, K.S.; Rhee, B.D.; Kim, N.; Mishchenko, N.P.; Fedoryev, S.A.; Stonik, V.A.; et al. Echinochrome A protects mitochondrial function in cardiomyocytes against cardiotoxic drugs. Mar. Drugs 2014, 12, 2922–2936. [Google Scholar] [CrossRef]
- Kim, H.K.; Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A.; Han, J. Multifaceted clinical effects of Echinochrome. Mar. Drugs 2021, 19, 412. [Google Scholar] [CrossRef]
- Oh, S.J.; Seo, Y.; Ahn, J.S.; Shin, Y.Y.; Yang, J.W.; Kim, H.K.; Han, J.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; et al. Echinochrome A reduces colitis in mice and induces in vitro generation of regulatory immune cells. Mar. Drugs 2019, 17, 622. [Google Scholar] [CrossRef]
- Sayed, D.A.; Soliman, A.M.; Fahmy, S.R. Echinochrome pigment as novel therapeutic agent against experimentally-induced gastric ulcer in rats. Biomed. Pharmacother. 2018, 107, 90–95. [Google Scholar] [CrossRef]
- Park, G.T.; Yoon, J.W.; Yoo, S.B.; Song, Y.C.; Song, P.; Kim, H.K.; Han, J.; Bae, S.J.; Ha, K.T.; Mishchenko, N.P.; et al. Echinochrome A treatment alleviates fibrosis and inflammation in bleomycin-induced scleroderma. Mar. Drugs 2021, 19, 237. [Google Scholar] [CrossRef] [PubMed]
- Seol, J.E.; Ahn, S.W.; Seol, B.; Yun, H.R.; Park, N.; Kim, H.K.; Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; et al. Echinochrome A protects against ultraviolet b-induced photoaging by lowering collagen degradation and inflammatory cell infiltration in hairless mice. Mar. Drugs 2021, 19, 550. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.R.; Ahn, S.W.; Seol, B.; Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Han, J.; Ko, K.S.; Rhee, B.D.; et al. Echinochrome A treatment alleviates atopic dermatitis-like skin lesions in NC/Nga mice via IL-4 and IL-13 suppression. Mar. Drugs 2021, 19, 622. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, L.; Beltrán, M.; Aguado, A.; Gisselmann, G.; Hatt, H. 2-Aminoethoxydiphenyl borate activates the mechanically gated human KCNK channels KCNK 2 (TREK-1), KCNK 4 (TRAAK), and KCNK 10 (TREK-2). Front. Pharmacol. 2013, 4, 63. [Google Scholar] [CrossRef] [PubMed]
- Sandoz, G.; Douguet, D.; Chatelain, F.; Lazdunski, M.; Lesage, F. Extracellular acidification exerts opposite actions on TREK1 and TREK2 potassium channels via a single conserved histidine residue. Proc. Natl. Acad. Sci. USA 2009, 106, 14628–14633. [Google Scholar] [CrossRef]
- Kennard, L.E.; Chumbley, J.R.; Ranatunga, K.M.; Armstrong, S.J.; Veale, E.L.; Mathie, A. Inhibition of the human two-pore domain potassium channel, TREK-1, by fluoxetine and its metabolite norfluoxetine. Br. J. Pharmacol. 2005, 144, 821–829. [Google Scholar] [CrossRef]
- Proks, P.; Schewe, M.; Conrad, L.J.; Rao, S.; Rathje, K.; Rödström, K.E.J.; Carpenter, E.P.; Baukrowitz, T.; Tucker, S.J. Norfluoxetine inhibits TREK-2 K2P channels by multiple mechanisms including state-independent effects on the selectivity filter gate. J. Gen. Physiol. 2021, 153, e202012812. [Google Scholar] [CrossRef]
- Pope, L.; Minor, D.L., Jr. The Polysite Pharmacology of TREK K2P Channels. Adv. Exp. Med. Biol. 2021, 1349, 51–65. [Google Scholar]
- Han, Y.; Luo, A.; Kamau, P.M.; Takomthong, P.; Hu, J.; Boonyarat, C.; Luo, L.; Lai, R. A plant-derived TRPV3 inhibitor suppresses pain and itch. Br. J. Pharmacol. 2021, 178, 1669–1683. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, J.; Wei, X.; Hu, J.; Ping, C.; Gao, Y.; Xie, C.; Wang, P.; Cao, P.; Cao, Z.; et al. Therapeutic inhibition of keratinocyte TRPV3 sensory channel by local anesthetic dyclonine. eLife 2021, 10, e68128. [Google Scholar] [CrossRef]
- Hoth, M.; Penner, R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature 1992, 355, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Seo, E.Y.; Pang, B.; Nam, J.H.; Kim, H.S.; Kim, W.K.; Kim, S.J. Inhibition of Ca2+-release-activated Ca2+ channel (CRAC) and K+ channels by curcumin in Jurkat-T cells. J. Pharmacol. Sci. 2011, 115, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.U.; Weon, K.Y.; Nam, D.Y.; Nam, J.H.; Kim, W.K. Skin protective effect of guava leaves against UV-induced melanogenesis via inhibition of ORAI1 channel and tyrosinase activity. Exp. Dermatol. 2016, 25, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Şterbuleac, D. Molecular determinants of chemical modulation of two-pore domain potassium channels. Chem. Biol. Drug Des. 2019, 94, 1596–1614. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Jeon, Y.K.; Zhang, Y.H.; Nam, J.H.; Shin, D.H.; Kim, S.J. Triple arginine residues in the proximal C-terminus of TREK K+channels are critical for biphasic regulation by phosphatidylinositol 4,5-bisphosphate. Am. J. Physiol. Cell. Physiol. 2019, 316, C312–C324. [Google Scholar] [CrossRef] [PubMed]
- Djillani, A.; Mazella, J.; Heurteaux, C.; Borsotto, M. Role of TREK-1 in Health and Disease, Focus on the Central Nervous System. Front. Pharmacol. 2019, 10, 379. [Google Scholar] [CrossRef]
- Alloui, A.; Zimmermann, K.; Mamet, J.; Duprat, F.; Noël, J.; Chemin, J.; Guy, N.; Blondeau, N.; Voilley, N.; Rubat-Coudert, C.; et al. TREK-1, a K+ channel involved in polymodal pain perception. EMBO J. 2006, 25, 2368–2376. [Google Scholar] [CrossRef]
- Acosta, C.; Djouhri, L.; Watkins, R.; Berry, C.; Bromage, K.; Lawson, S.N. TREK2 expressed selectively in IB4-binding C-fiber nociceptors hyperpolarizes their membrane potentials and limits spontaneous pain. J. Neurosci. 2014, 34, 1494–1509. [Google Scholar] [CrossRef]
- Noël, J.; Zimmermann, K.; Busserolles, J.; Deval, E.; Alloui, A.; Diochot, S.; Guy, N.; Borsotto, M.; Reeh, P.; Eschalier, A.; et al. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 2009, 28, 1308–1318. [Google Scholar] [CrossRef]
- McCoull, D.; Ococks, E.; Large, J.M.; Tickle, D.C.; Mathie, A.; Jerman, J.; Wright, P.D. A “Target Class” Screen to Identify Activators of Two-Pore Domain Potassium (K2P) Channels. SLAS Discov. 2021, 26, 428–438. [Google Scholar] [CrossRef]
- Pope, L.; Lolicato, M.; Minor, D.L., Jr. Polynuclear ruthenium amines inhibit K2P channels via a “Finger in the Dam” mechanism. Cell. Chem. Biol. 2020, 27, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Lolicato, M.; Arrigoni, C.; Mori, T.; Sekioka, Y.; Bryant, C.; Clark, K.A.; Minor, D.L., Jr. K2P2.1 (TREK-1)-activator complexes reveal a cryptic selectivity filter binding site. Nature 2017, 547, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.Y.; Pike, A.C.; Mackenzie, A.; McClenaghan, C.; Aryal, P.; Dong, L.; Quigley, A.; Grieben, M.; Goubin, S.; Mukhopadhyay, S.; et al. K2P channel gating mechanisms revealed by structures of TREK-2 and a complex with Prozac. Science 2015, 347, 1256–1259. [Google Scholar] [CrossRef] [PubMed]
- Schewe, M.; Sun, H.; Mert, Ü.; Mackenzie, A.; Pike, A.C.W.; Schulz, F.; Constantin, C.; Vowinkel, K.S.; Conrad, L.J.; Kiper, A.K.; et al. A pharmacological master key mechanism that unlocks the selectivity filter gate in K+channels. Science 2019, 363, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Ingólfsson, H.I.; Thakur, P.; Herold, K.F.; Hobart, E.A.; Ramsey, N.B.; Periole, X.; de Jong, D.H.; Zwama, M.; Yilmaz, D.; Hall, K.; et al. Phytochemicals Perturb Membranes and Promiscuously Alter Protein Function. ACS Chem. Biol. 2014, 9, 8. [Google Scholar] [CrossRef]
- Benarroch, E.E. Ion channels in nociceptors: Recent developments. Neurology 2015, 84, 1153–1164. [Google Scholar] [CrossRef]
- Mishchenko, N.P.; Vasileva, E.A.; Gerasimenko, A.V.; Grigorchuk, V.P.; Dmit-renok, P.S.; Fedoreyev, S.A. Isolation and Structure Determination of Echinochrome A Oxidative Degradation Products. Molecules 2020, 25, 4778. [Google Scholar] [CrossRef]
- Sadek, S.A.; Hassanein, S.S.; Mohamed, A.S.; Soliman, A.M.; Fahmy, S.R. Echinochrome pigment extracted from sea urchin suppress the bacterial activity, inflammation, nociception, and oxidative stress resulted in the inhibition of renal injury in septic rats. J. Food Biochem. 2022, 46, e13729. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.E.; Chung, E.D.S.; Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Kim, H.K.; Nam, J.H.; Kim, S.J. Multiple Effects of Echinochrome A on Selected Ion Channels Implicated in Skin Physiology. Mar. Drugs 2023, 21, 78. https://doi.org/10.3390/md21020078
Kim SE, Chung EDS, Vasileva EA, Mishchenko NP, Fedoreyev SA, Stonik VA, Kim HK, Nam JH, Kim SJ. Multiple Effects of Echinochrome A on Selected Ion Channels Implicated in Skin Physiology. Marine Drugs. 2023; 21(2):78. https://doi.org/10.3390/md21020078
Chicago/Turabian StyleKim, Sung Eun, Elina Da Sol Chung, Elena A. Vasileva, Natalia P. Mishchenko, Sergey A. Fedoreyev, Valentin A. Stonik, Hyoung Kyu Kim, Joo Hyun Nam, and Sung Joon Kim. 2023. "Multiple Effects of Echinochrome A on Selected Ion Channels Implicated in Skin Physiology" Marine Drugs 21, no. 2: 78. https://doi.org/10.3390/md21020078
APA StyleKim, S. E., Chung, E. D. S., Vasileva, E. A., Mishchenko, N. P., Fedoreyev, S. A., Stonik, V. A., Kim, H. K., Nam, J. H., & Kim, S. J. (2023). Multiple Effects of Echinochrome A on Selected Ion Channels Implicated in Skin Physiology. Marine Drugs, 21(2), 78. https://doi.org/10.3390/md21020078










