The Phospholipid Molecular Species Profile of Apostichopus japonicus Tissues Modifies through Exposure to n-3 Polyunsaturated Fatty Acid-Deficient Diet
Abstract
1. Introduction
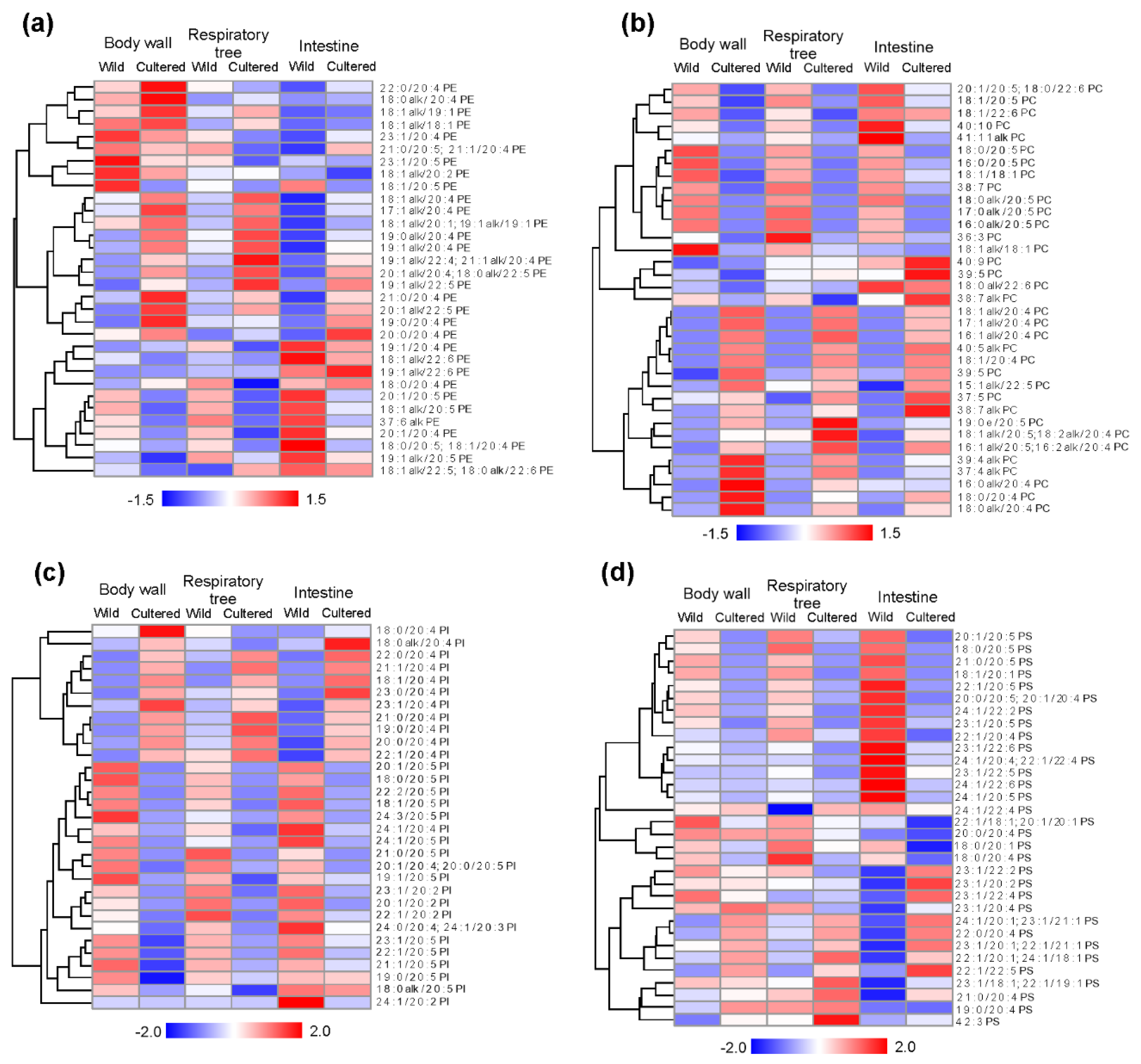
2. Results
3. Discussion
3.1. Phospholipid Molecular Species Profile in Tissues of A. japonicus
3.2. Differences in Lipid Profile Composition between Wild and Cultured Sea Cucumbers
4. Materials and Methods
4.1. Sample Collection
4.2. The Lipid Analysis
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, Y.C.; Zhang, L.B.; Liu, S.L.; Ru, X.S.; Xing, L.L.; Cao, X.B.; Zhang, T.; Yang, H.S. The effect of salinity on the growth, energy budget and physiological performance of green, white and purple color morphs of sea cucumber, Apostichopus japonicus. Aquaculture 2015, 437, 297–303. [Google Scholar] [CrossRef]
- Purcell, S.W.; Conand, C.; Uthicke, S.; Byrne, M. Ecological roles of exploited sea cucumbers. In Oceanography and Marine Biology: An Annual Review; Taylor & Francis Inc: London, UK, 2016; Volume 54, pp. 367–386. [Google Scholar]
- Zhang, X.M.; Li, X.B.; Zhang, S.S.; He, Q.X.; Hou, H.R.; Dang, L.; Guo, J.L.; Chen, Y.F.; Yu, T.; Peng, D.J.; et al. Lc-Ms/Ms Identification of Novel Saponins from the Viscera of Sea Cucumber Apostichopus Japonicus. Chem. Nat. Compd. 2018, 54, 721–725. [Google Scholar] [CrossRef]
- Khotimchenko, Y. Pharmacological Potential of Sea Cucumbers. Int. J. Mol. Sci. 2018, 19, 1342. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Lu, F.; Xiao, C.; Yang, L.; Chen, J.; Zhou, K.; Wen, D.D.; Li, Z.; Wu, M.Y.; Jiang, J.M.; et al. beta-Eliminative depolymerization of the fucosylated chondroitin sulfate and anticoagulant activities of resulting fragments. Carbohydr. Polym. 2015, 127, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Yano, A.; Abe, A.; Aizawa, F.; Yamada, H.; Minami, K.; Matsui, M.; Kishi, M. The Effect of Eating Sea Cucumber Jelly on Candida Load in the Oral Cavity of Elderly Individuals in a Nursing Home. Mar. Drugs 2013, 11, 4993–5007. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Z.L.; Zhang, M.S.; Meng, X.M.; Xia, X.K.; Yuan, W.P.; Xue, F.; Liu, C.H. Antioxidant and antihyperlipidemic activities of polysaccharides from sea cucumber Apostichopus japonicus. Carbohydr. Polym. 2012, 90, 1664–1670. [Google Scholar] [CrossRef]
- Zheng, R.; Li, X.M.; Cao, B.B.; Zuo, T.; Wu, J.; Wang, J.F.; Xue, C.H.; Tang, Q.J. Dietary Apostichopus japonicus enhances the respiratory and intestinal mucosal immunity in immunosuppressive mice. Biosci. Biotechnol. Biochem. 2015, 79, 253–259. [Google Scholar] [CrossRef]
- Sargent, J.R.; Tocher, D.R.; Bell, J.G. 4—The Lipids. In Fish Nutrition, 3rd ed.; Halver, J.E., Hardy, R.W., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 181–257. [Google Scholar] [CrossRef]
- Imbs, A.B.; Svetashev, V.I.; Rodkina, S.A. Differences in lipid class and fatty acid composition between wild and cultured sea cucumbers, Apostichopus japonicus, explain modification and deposition of lipids. Aquac. Res. 2021, 53, 810–819. [Google Scholar] [CrossRef]
- Xu, J.; Duan, J.J.; Xue, C.H.; Feng, T.Y.; Dong, P.; Sugawara, T.; Hirata, T. Analysis and Comparison of Glucocerebroside Species from Three Edible Sea Cucumbers Using Liquid Chromatography-Ion Trap-Time-of-Flight Mass Spectrometry. J. Agric. Food Chem. 2011, 59, 12246–12253. [Google Scholar] [CrossRef]
- Kostetsky, E.Y.; Sanina, N.M.; Velansky, P.V. The thermotropic behavior and major molecular species composition of the phospholipids of echinoderms. Russ. J. Mar. Biol. 2014, 40, 131–139. [Google Scholar] [CrossRef]
- Liao, M.-L.; Ren, T.; He, L.; Han, Y.; Jiang, Z. Optimum dietary proportion of soybean meal with fish meal, and its effects on growth, digestibility, and digestive enzyme activity of juvenile sea cucumber Apostichopus japonicus. Fish. Sci. 2015, 81, 915–922. [Google Scholar] [CrossRef]
- Wen, B.; Sun, Y.-J.; Gao, Q.-F.; Dong, S.-L.; Chen, Z.-Z.; Jian Zhong, G. Effects of dietary macroalgae meal and lipid source on growth performance and body wall fatty acid composition of sea cucumber Apostichopus japonicus. Aquac. Res. 2017, 49, 776–785. [Google Scholar] [CrossRef]
- Yu, H.; Gao, Q.-F.; Dong, S.-L.; Wen, B. Changes in fatty acid profiles of sea cucumber Apostichopus japonicus (Selenka) induced by terrestrial plants in diets. Aquaculture 2015, 442, 119–124. [Google Scholar] [CrossRef]
- Yuan, X.; Yang, H.; Zhou, Y.; Mao, Y.; Zhang, T.; Liu, Y. The influence of diets containing dried bivalve feces and/or powdered algae on growth and energy distribution in sea cucumber Apostichopus japonicus (Selenka) (Echinodermata: Holothuroidea). Aquaculture 2006, 256, 457–467. [Google Scholar] [CrossRef]
- Zhao, L.t.; Feng, Z.f.; Lu, N.; Yang, S.h.; Zhu, W. Effects of dietary n-3 PUFA supplements on composition of n-3 PUFA and expression of fatty acid elongase 5 (AJELOVL5) in sea cucumber, Apostichopus japonicus. Aquac. Res. 2018, 50, 209–218. [Google Scholar] [CrossRef]
- Zadorozhnyj, P.A.; Pivnenko, T.N.; Kovalev, N.N. Development of Fatty Acid Biomarkers for the Identification of Wild and Aquacultured Sea Cucumber (Apostichopus japonicus). J. Ocean Univ. China 2016, 15, 177–183. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, W.; Ding, B.; Zhang, Y.; Huang, X.; Liu, X.; Zuo, R.; Chang, Y.; Ding, J. Comparative lipidomics profiling of the sea urchin, Strongylocentrotus intermedius. Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 40, 100900. [Google Scholar] [CrossRef]
- Imbs, A.B.; Ermolenko, E.V.; Grigorchuk, V.P.; Sikorskaya, T.V.; Velansky, P.V. Current Progress in Lipidomics of Marine Invertebrates. Mar Drugs 2021, 19, 660. [Google Scholar] [CrossRef]
- Wang, X.C.; Cong, P.X.; Chen, Q.S.; Li, Z.J.; Xu, J.; Xue, C.H. Characterizing the phospholipid composition of six edible sea cucumbers by NPLC-Triple TOF-MS/MS. J. Food Compos. Anal. 2020, 94, 103626. [Google Scholar] [CrossRef]
- Xu, Q.Z.; Xu, Q.; Zhang, X.L.; Peng, Q.C.; Yang, H.S. Fatty acid component in sea cucumber Apostichopus japonicus from different tissues and habitats. J. Mar. Biol. Assoc. UK 2016, 96, 197–204. [Google Scholar] [CrossRef]
- Diaz, M.; Dopido, R.; Gomez, T.; Rodriguez, C. Membrane lipid microenvironment modulates thermodynamic properties of the Na+-K+-ATPase in branchial and intestinal epithelia in euryhaline fish in vivo. Front. Physiol. 2016, 7, 589. [Google Scholar] [CrossRef] [PubMed]
- Castellano, G.C.; Souza, M.M.; Freire, C.A. Volume regulation of intestinal cells of echinoderms: Putative role of ion transporters (Na+/K+-ATPase and NKCC). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 201, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Dolmatov, I.; Frolova, L.; Zakharova, E.; Ginanova, T. Development of respiratory trees in the holothurian Apostichopus japonicus (Aspidochirotida: Holothuroidea). Cell Tissue Res. 2011, 346, 327–338. [Google Scholar] [CrossRef]
- Martínez-Milián, G.; Olvera-Novoa, M.A.; Toledo-Cuevas, E.M. Novel findings in sea cucumber’s digestive capacities: Enzymatic activities in the respiratory tree, implications for aquaculture. J. World Aquac. Soc. 2021, 52, 1259–1272. [Google Scholar] [CrossRef]
- De Craene, J.-O.; Bertazzi, D.; Bär, S.; Friant, S. Phosphoinositides, Major Actors in Membrane Trafficking and Lipid Signaling Pathways. Int. J. Mol. Sci. 2017, 18, 634. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Kunisaki, N.; Urano, N.; Kimura, S. Collagen as the Major Edible Component of Sea Cucumber (Stichopus japonicus). J. Food Sci. 2006, 67, 1319–1322. [Google Scholar] [CrossRef]
- Zacarias-Soto, M.; Tec-Tec, P.; Olvera-Novoa, M. Effect of diet on growth and body biochemical composition of juvenile four-sided sea cucumber Isostichopus badionotus (Selenka, 1867). Aquac. Res. 2017, 49, 939–946. [Google Scholar] [CrossRef]
- Seo, J.Y.; Lee, S.M. Optimum dietary protein and lipid levels for growth of juvenile sea cucumber Apostichopus japonicus. Aquac. Nutr. 2010, 17, e56–e61. [Google Scholar] [CrossRef]
- Tocher, D.; Bendiksen, E.; Campbell, P.; Bell, J.G.B. The role of phospholipids in nutrition and metabolism of teleost fish. Aquaculture 2008, 280, 21–34. [Google Scholar] [CrossRef]
- Lands, B.; Bibus, D.; Stark, K.D. Dynamic interactions of n-3 and n-6 fatty acid nutrients. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 15–21. [Google Scholar] [CrossRef]
- Stanley-Samuelson, D. Comparative eicosanoid physiology in invertebrate animals. Am. J. Physiol. 1991, 260, R849–R853. [Google Scholar] [CrossRef] [PubMed]
- Toda, A.; Yokomizo, T.; Shimizu, T. Leukotriene B4 receptors. Prostaglandins Other Lipid Mediat. 2002, 68–69, 575–585. [Google Scholar] [CrossRef]
- Pope, E.C.; Taylor, G.W.; Rowley, A.F. Biosynthesis and functions of eicosanoids generated by the coelomocytes of the starfish, Asterias rubens. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 147, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, D.; Mouchlis, V.D.; Dennis, E.A. Each phospholipase A2 type exhibits distinct selectivity toward sn-1 ester, alkyl ether, and vinyl ether phospholipids. Biochim. Et. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2022, 1867, 159067. [Google Scholar] [CrossRef] [PubMed]
- Tocher, D.R. Metabolism and Functions of Lipids and Fatty Acids in Teleost Fish. Rev. Fish. Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G.A. A simple methods for the isolation and pyrification of total lipid extraction from animal tissue. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Sikorskaya, T.V.; Ermolenko, E.V.; Efimova, K.V. Lipids of Indo-Pacific gorgonian corals are modified under the influence of microbial associations. Coral Reefs 2022, 41, 277–291. [Google Scholar] [CrossRef]
- Sikorskaya, T.V.; Imbs, A.B. Study of Total Lipidome of the Sinularia siaesensis Soft Coral. Russ. J. Bioorganic Chem. 2018, 44, 712–723. [Google Scholar] [CrossRef]
- Liput, K.P.; Lepczyński, A.; Ogłuszka, M.; Nawrocka, A.; Poławska, E.; Grzesiak, A.; Ślaska, B.; Pareek, C.S.; Czarnik, U.; Pierzchała, M. Effects of Dietary n–3 and n–6 Polyunsaturated Fatty Acids in Inflammation and Cancerogenesis. Int. J. Mol. Sci. 2021, 22, 6965. [Google Scholar] [CrossRef]
- Bouwens, M.; van de Rest, O.; Dellschaft, N.; Bromhaar, M.G.; De Groot, L.C.P.G.M.; Geleijnse, J.M.; Müller, M.; Afman, L.A. Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells. Am. J. Clin. Nutr. 2009, 90, 415–424. [Google Scholar] [CrossRef]
- Itariu, B.K.; Zeyda, M.; Hochbrugger, E.E.; Neuhofer, A.; Prager, G.; Schindler, K.; Bohdjalian, A.; Mascher, D.; Vangala, S.; Schranz, M.; et al. Long-chain n−3 PUFAs reduce adipose tissue and systemic inflammation in severely obese nondiabetic patients: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 96, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Ahmmed, M.; Ahmmed, F.; Tian, H.; Carne, A.; Bekhit, A. Marine omega-3 (n-3) phospholipids: A comprehensive review of their properties, sources, bioavailability, and relation to brain health. Compr. Rev. Food Sci. Food Saf. 2019, 19, 64–123. [Google Scholar] [CrossRef] [PubMed]
- Schverer, M.; O’Mahony, S.M.; O’Riordan, K.J.; Donoso, F.; Roy, B.L.; Stanton, C.; Dinan, T.G.; Schellekens, H.; Cryan, J.F. Dietary phospholipids: Role in cognitive processes across the lifespan. Neurosci. Biobehav. Rev. 2020, 111, 183–193. [Google Scholar] [CrossRef] [PubMed]

| PL Groups | Wild | Cultured | ||||
|---|---|---|---|---|---|---|
| Body Wall | RT | Intestine | Body Wall | RT | Intestine | |
| Phosphatidylethanolamines (% from total PE) | ||||||
| C20:5 PE | 30.62 ± 1.26 a | 31.39 ± 1.61 a | 42.30 ± 1.74 a | 11.02 ± 0.67 b | 10.41 ± 0.39 b | 22.64 ± 4.19 b |
| C20:4 PE | 43.85 ± 1.54 a | 44.75 ± 3.22 a | 27.15 ± 0.93 c | 62.45 ± 0.54 b | 57.40 ± 2.78 b | 48.12 ± 4.00 c |
| Alkyl/acyl PE | 83.76 ± 2.10 a | 82.30 ± 1.65 a | 81.30 ± 2.29 a | 85.46 ± 0.18 a | 92.80 ± 1.05 b | 84.46 ± 1.54 a |
| C22:6 PE | 10.02 ± 1.21 a | 8.11 ± 1.10 a | 16.32 ± 1.11 b | 8.26 ± 0.58 a | 9.68 ± 1.91 a | 13.38 ± 0.86 c |
| Phosphatidylcholines (% from total PC) | ||||||
| C20:5 PC | 46.82 ± 0.90 a | 44.36 ± 0.82 a | 46.89 ± 1.01 a | 8.76 ± 0.97 b | 13.23 ± 1.81 c | 16.61 ± 1.15 d |
| C20:4 PC | 2.86 ± 0.19 a | 2.81 ± 0.45 a | 2.56 ± 0.20 a | 47.35 ± 0.88 b | 42.31 ± 1.31 c | 31.60 ± 2.11 d |
| Alkyl/acyl PC | 42.62 ± 2.68 a | 44.42 ± 3.33 a | 41.63 ± 1.24 a | 70.76 ± 0.94 b | 66.66 ± 1.91 b | 55.12 ± 0.77 c |
| C22:6 PC | 12.49 ± 0.41 a | 11.61 ± 1.46 a | 15.78 ± 0.42 b | 7.15 ± 0.60 c | 6.37 ± 1.16 c | 13.22 ± 1.17 d |
| Phosphatidylinositols (% from total PI) | ||||||
| C20:5 PI | 36.16 ± 1.75 a | 28.21 ± 0.51 b | 35.57 ± 0.90 a | 6.41 ± 0.90 c | 9.20 ± 0.87 c | 13.87 ± 2.03 d |
| C20:4 PI | 40.25 ± 2.75 a | 51.30 ± 2.54 b | 31.93 ± 1.23 c | 86.49 ± 1.00 d | 82.23 ± 3.03 d | 76.21 ± 2.71 e |
| odd-chain PI | 31.34 ± 2.26 a | 37.36 ± 1.41 b | 24.39 ± 1.98 c | 54.36 ± 1.89 d | 56.24 ± 3.97 d | 47.98 ± 2.08 e |
| very long chain PI | 34.25 ± 2.07 a | 29.96 ± 1.22 a | 43.61 ± 3.08 b | 35.45 ± 0.60 a | 25.30 ± 2.42 c | 31.44 ± 1.71 a |
| Phosphatidylserines (% from total PS) | ||||||
| C20:5 PS | 10.99 ± 1.11 a | 13.66 ± 1.45 b | 27.19 ± 0.69 c | 2.73 ± 0.28 d | 3.30 ± 0.45 d | 3.56 ± 0.90 d |
| C20:4 PS | 20.75 ± 2.09 | 23.60 ± 2.59 a | 16.93 ± 1.94 b | 22.33 ± 0.45 | 21.56 ± 3.94 | 18.90 ± 4.02 |
| odd-chain PS | 49.62 ± 1.86 a | 46.41 ± 1.04 b | 32.67 ± 0.99 c | 50.42 ± 0.94 a | 48.78 ± 1.01 b | 54.26 ± 1.62 d |
| very long chain PS | 77.04 ± 1.52 a | 75.42 ± 2.52 a | 79.59 ± 2.51 a | 83.27 ± 1.46 b | 80.75 ± 1.75 b | 87.32 ± 3.27 b |
| C22:6 PS | 1.02 ± 0.11 | 1.11 ± 0.26 | 6.40 ± 0.76 a | 0.41 ± 0.02 | 0.10 ± 0.06 | 0.67 ± 0.24 b |
| Monounsaturated PS | 37.88 ± 1.72 a | 34.95 ± 2.07 a | 20.22 ± 2.56 c | 49.61 ± 0.75 d | 49.99 ± 3.11 d | 51.88 ± 3.99 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ermolenko, E.V.; Sikorskaya, T.V.; Grigorchuk, V.P. The Phospholipid Molecular Species Profile of Apostichopus japonicus Tissues Modifies through Exposure to n-3 Polyunsaturated Fatty Acid-Deficient Diet. Mar. Drugs 2022, 20, 578. https://doi.org/10.3390/md20090578
Ermolenko EV, Sikorskaya TV, Grigorchuk VP. The Phospholipid Molecular Species Profile of Apostichopus japonicus Tissues Modifies through Exposure to n-3 Polyunsaturated Fatty Acid-Deficient Diet. Marine Drugs. 2022; 20(9):578. https://doi.org/10.3390/md20090578
Chicago/Turabian StyleErmolenko, Ekaterina V., Tatyana V. Sikorskaya, and Valeria P. Grigorchuk. 2022. "The Phospholipid Molecular Species Profile of Apostichopus japonicus Tissues Modifies through Exposure to n-3 Polyunsaturated Fatty Acid-Deficient Diet" Marine Drugs 20, no. 9: 578. https://doi.org/10.3390/md20090578
APA StyleErmolenko, E. V., Sikorskaya, T. V., & Grigorchuk, V. P. (2022). The Phospholipid Molecular Species Profile of Apostichopus japonicus Tissues Modifies through Exposure to n-3 Polyunsaturated Fatty Acid-Deficient Diet. Marine Drugs, 20(9), 578. https://doi.org/10.3390/md20090578







