Antarctic Marine Algae Extracts as a Potential Natural Resource to Protect Epithelial Barrier Integrity
Abstract
:1. Introduction
2. Results
2.1. GC–MS Profiles
2.2. Radical-Scavenging Activity
2.3. Cell viability Protection
2.4. Intracellular ROS Scavenging
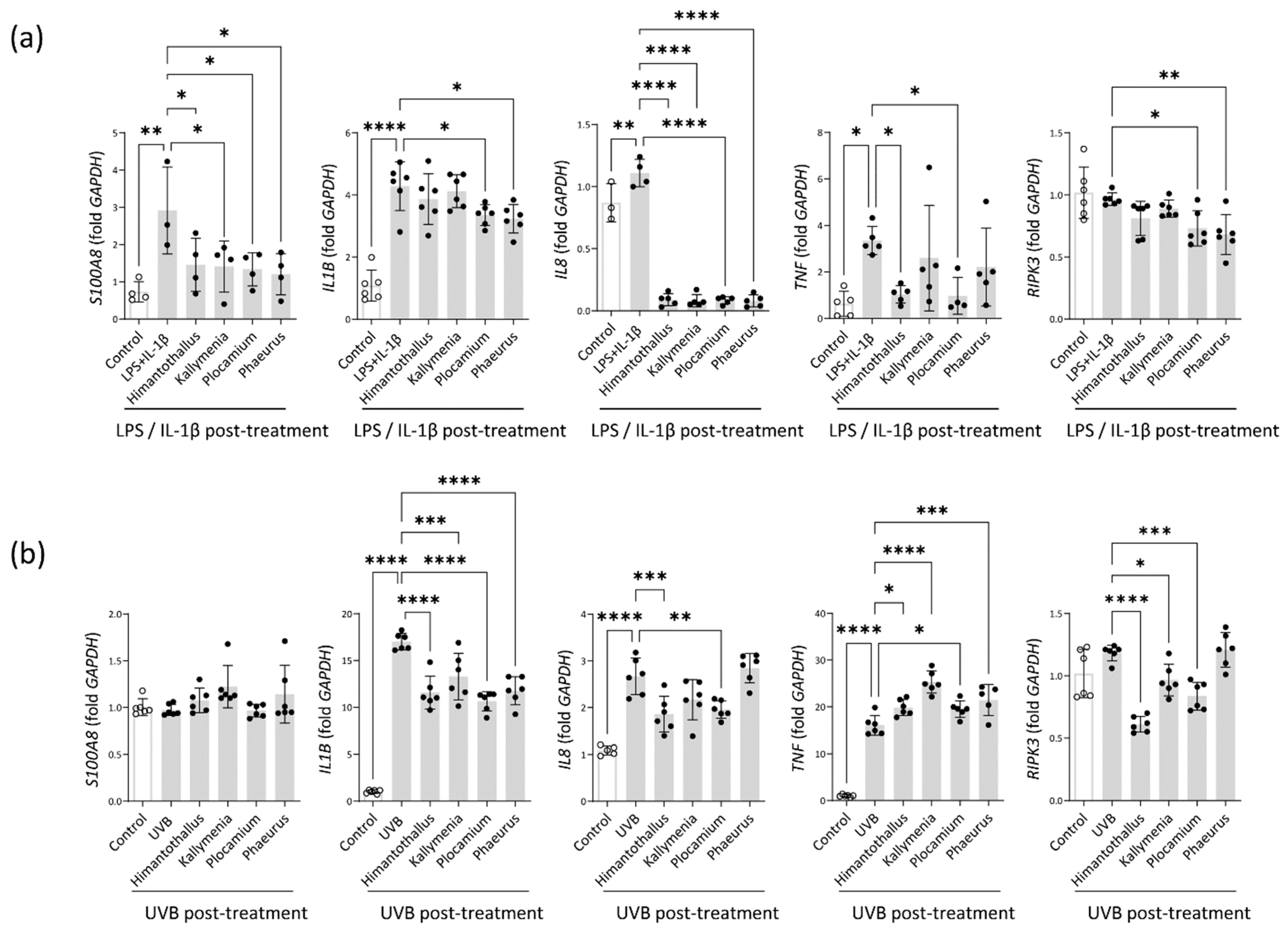
2.5. Anti-Inflammatory Activity
2.6. Epithelial Barrier Protection
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Sample Preparation
4.3. GC–MS Analysis
4.4. Cell Culture
4.5. Radical-Scavenging Assay
4.6. Cell Viability Assay
4.7. Cell-Based ROS Scavenging Assay
4.8. Real-Time Polymerase Chain Reacion (PCR) Analysis
4.9. TEER Assay
4.10. FITC-Dextran Permeability Assay
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.T.; Paus, R. The gut-skin axis in health and disease: A paradigm with therapeutic implications. Bioessays 2016, 38, 1167–1176. [Google Scholar] [CrossRef]
- Shaykhiev, R.; Bals, R. Interactions between epithelial cells and leukocytes in immunity and tissue homeostasis. J. Leukoc. Biol. 2007, 82, 1–15. [Google Scholar] [CrossRef]
- Artis, D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat. Rev. Immunol. 2008, 8, 411–420. [Google Scholar] [CrossRef]
- Goto, Y.; Kiyono, H. Epithelial barrier: An interface for the cross-communication between gut flora and immune system. Immunol. Rev. 2012, 245, 147–163. [Google Scholar] [CrossRef]
- Hikima, T.; Tojo, K.; Maibach, H.I. Skin metabolism in transdermal therapeutic systems. Ski. Pharmacol. Physiol. 2005, 18, 153–159. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Zhang, C.; Lv, J.; Qin, X.; Peng, Z.; Lin, H. Novel Antioxidant Peptides from Crassostrea Hongkongensis Improve Photo-Oxidation in UV-Induced HaCaT Cells. Mar. Drugs 2022, 20, 100. [Google Scholar] [CrossRef]
- Um, H.N.; Baek, J.O.; Park, S.; Lee, E.H.; Jang, J.; Park, W.J.; Roh, J.Y.; Jung, Y. Small intestinal immune-environmental changes induced by oral tolerance inhibit experimental atopic dermatitis. Cell Death Dis. 2021, 12, 243. [Google Scholar] [CrossRef]
- Zhu, T.H.; Zhu, T.R.; Tran, K.A.; Sivamani, R.K.; Shi, V.Y. Epithelial barrier dysfunctions in atopic dermatitis: A skin-gut-lung model linking microbiome alteration and immune dysregulation. Br. J. Dermatol. 2018, 179, 570–581. [Google Scholar] [CrossRef]
- Schleimer, R.P.; Berdnikovs, S. Etiology of epithelial barrier dysfunction in patients with type 2 inflammatory diseases. J. Allergy Clin. Immunol. 2017, 139, 1752–1761. [Google Scholar] [CrossRef] [Green Version]
- Spalinger, M.R.; Sayoc-Becerra, A.; Santos, A.N.; Shawki, A.; Canale, V.; Krishnan, M.; Niechcial, A.; Obialo, N.; Scharl, M.; Li, J.; et al. PTPN2 Regulates Interactions Between Macrophages and Intestinal Epithelial Cells to Promote Intestinal Barrier Function. Gastroenterology 2020, 159, 1763–1777.e14. [Google Scholar] [CrossRef]
- Krishnan, M.; Penrose, H.M.; Shah, N.N.; Marchelletta, R.R.; McCole, D.F. VSL#3 Probiotic Stimulates T-cell Protein Tyrosine Phosphatase-mediated Recovery of IFN-gamma-induced Intestinal Epithelial Barrier Defects. Inflamm. Bowel Dis. 2016, 22, 2811–2823. [Google Scholar]
- Tripathi, V.C.; Satish, S.; Horam, S.; Raj, S.; Lal, A.; Arockiaraj, J.; Pasupuleti, M.; Dikshit, D.K. Natural products from polar organisms: Structural diversity, bioactivities and potential pharmaceutical applications. Polar Sci. 2018, 18, 147–166. [Google Scholar] [CrossRef]
- Amirkia, V.; Heinrich, M. Natural products and drug discovery: A survey of stakeholders in industry and academia. Front. Pharmacol. 2015, 6, 237. [Google Scholar] [CrossRef]
- Gribble, G.W. Biological Activity of Recently Discovered Halogenated Marine Natural Products. Mar. Drugs 2015, 13, 4044–4136. [Google Scholar] [CrossRef]
- Ryan, K.G.; McMinn, A.; Hegseth, E.N.; Davy, S.K. The Effects of Ultraviolet-B Radiation on Antarctic Sea-Ice Algae. J. Phycol. 2012, 48, 74–84. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Pacheco, D.; Gonçalves, A.M.M.; Pereira, L. A Comprehensive Review of the Nutraceutical and Therapeutic Applications of Red Seaweeds (Rhodophyta). Life 2020, 10, 19. [Google Scholar] [CrossRef]
- Martins, R.M.; Nedel, F.; Guimaraes, V.B.S.; da Silva, A.F.; Colepicolo, P.; de Pereira, C.M.P.; Lund, R.G. Macroalgae Extracts From Antarctica Have Antimicrobial and Anticancer Potential. Front. Microbiol. 2018, 9, 412. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Sali, V.K.; Mani, S.; Vasanthi, H.R. Neophytadiene from Turbinaria ornata Suppresses LPS-Induced Inflammatory Response in RAW 264.7 Macrophages and Sprague Dawley Rats. Inflammation 2020, 43, 937–950. [Google Scholar] [CrossRef]
- Bhardwaj, K.; Sharma, R.; Cruz-Martins, N.; Valko, M.; Upadhyay, N.K.; Kuča, K.; Bhardwaj, P. Studies of Phytochemicals, Antioxidant, and Antibacterial Activities of Pinus gerardiana and Pinus roxburghii Seed Extracts. Biomed. Res. Int. 2022, 2022, 5938610. [Google Scholar] [CrossRef]
- Ahirwar, A.; Kesharwani, K.; Deka, R.; Muthukumar, S.; Khan, M.J.; Rai, A.; Vinayak, V.; Varjani, S.; Joshi, K.B.; Morjaria, S. Microalgal drugs: A promising therapeutic reserve for the future. J. Biotechnol. 2022, 349, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Zabel, M.; Nackenoff, A.; Kirsch, W.M.; Harrison, F.E.; Perry, G.; Schrag, M. Markers of oxidative damage to lipids, nucleic acids and proteins and antioxidant enzymes activities in Alzheimer’s disease brain: A meta-analysis in human pathological specimens. Free Radic. Biol. Med. 2018, 115, 351–360. [Google Scholar] [PubMed]
- Sanders, S.E.; Madara, J.L.; McGuirk, D.K.; Gelman, D.S.; Colgan, S.P. Assessment of inflammatory events in epithelial permeability: A rapid screening method using fluorescein dextrans. Epithelial. Cell Biol. 1995, 4, 25–34. [Google Scholar]
- Al-Sadi, R.M.; Ma, T.Y. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J. Immunol. 2007, 178, 4641–4649. [Google Scholar] [CrossRef]
- Moriwaki, K.; Chan, F.K. The Inflammatory Signal Adaptor RIPK3: Functions Beyond Necroptosis. Int. Rev. Cell Mol. Biol. 2017, 328, 253–275. [Google Scholar] [PubMed]
- Ransy, C.; Vaz, C.; Lombès, A.; Bouillaud, F. Use of H2O2 to Cause Oxidative Stress, the Catalase Issue. Int. J. Mol. Sci. 2020, 21, 9149. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.W.; Choi, J.Y.; Zeng, H.H.; Lee, Y.S.; Kim, H.S.; Yoon, S.H.; Chung, M.H. Leukemic cell line, KG-1 has a functional loss of hOGG1 enzyme due to a point mutation and 8-hydroxydeoxyguanosine can kill KG-1. Oncogene 2000, 19, 4476–4479. [Google Scholar] [CrossRef]
- Virág, L.; Szabó, E.; Gergely, P.; Szabó, C. Peroxynitrite-induced cytotoxicity: Mechanism and opportunities for intervention. Toxicol. Lett. 2003, 140–141, 113–124. [Google Scholar] [CrossRef]
- Whiteman, M.; Ketsawatsakul, U.; Halliwell, B. A reassessment of the peroxynitrite scavenging activity of uric acid. Ann. N. Y. Acad. Sci. 2002, 962, 242–259. [Google Scholar] [CrossRef]
- Kajino-Sakamoto, R.; Omori, E.; Nighot, P.K.; Blikslager, A.T.; Matsumoto, K.; Ninomiya-Tsuji, J. TGF-beta-activated kinase 1 signaling maintains intestinal integrity by preventing accumulation of reactive oxygen species in the intestinal epithelium. J. Immunol. 2010, 185, 4729–4737. [Google Scholar] [CrossRef]
- Trouba, K.J.; Hamadeh, H.K.; Amin, R.P.; Germolec, D.R. Oxidative stress and its role in skin disease. Antioxid. Redox Signal. 2002, 4, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Ko, S.H.; Ye, S.K.; Chung, M.H. 8-Oxo-2’-deoxyguanosine ameliorates UVB-induced skin damage in hairless mice by scavenging reactive oxygen species and inhibiting MMP expression. J. Dermatol. Sci. 2013, 70, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inflammatory property of n-hexadecanoic acid: Structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef]
- Fritsche, K.L. The science of fatty acids and inflammation. Adv. Nutr. 2015, 6, 293S–301S. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R.; Traber, M.G. Vitamin E: Function and metabolism. FASEB J. 1999, 13, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.M.; Ahmad, S.F.; Nadeem, A.; Attia, M.S.M.; Ansari, M.A.; As Sobeai, H.M.; Al-Mazroua, H.A.; Alasmari, A.F.; Bakheet, S.A. 3-Aminobenzamide alleviates elevated DNA damage and DNA methylation in a BTBR T(+)Itpr3(tf)/J mouse model of autism by enhancing repair gene expression. Pharmacol. Biochem. Behav. 2020, 199, 173057. [Google Scholar] [CrossRef]
- Plaza, M.; Santoyo, S.; Jaime, L.; Garcia-Blairsy Reina, G.; Herrero, M.; Senorans, F.J.; Ibanez, E. Screening for bioactive compounds from algae. J. Pharm. Biomed. Anal. 2010, 51, 450–455. [Google Scholar] [CrossRef]
- Rodríguez-Luna, A.; Ávila-Román, J.; González-Rodríguez, M.L.; Cózar, M.J.; Rabasco, A.M.; Motilva, V.; Talero, E. Fucoxanthin-Containing Cream Prevents Epidermal Hyperplasia and UVB-Induced Skin Erythema in Mice. Mar. Drugs 2018, 16, 378. [Google Scholar] [CrossRef]
- Coates, M.; Lee, M.J.; Norton, D.; MacLeod, A.S. The Skin and Intestinal Microbiota and Their Specific Innate Immune Systems. Front. Immunol. 2019, 10, 2950. [Google Scholar] [CrossRef]
- Ananthapadmanabhan, K.P.; Mukherjee, S.; Chandar, P. Stratum corneum fatty acids: Their critical role in preserving barrier integrity during cleansing. Int. J. Cosmet. Sci. 2013, 35, 337–345. [Google Scholar] [CrossRef]
- Wei, X.; Yang, Z.; Rey, F.E.; Ridaura, V.K.; Davidson, N.O.; Gordon, J.I.; Semenkovich, C.F. Fatty acid synthase modulates intestinal barrier function through palmitoylation of mucin 2. Cell Host Microbe 2012, 11, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, C.; Harrison, O.J.; Schmitt, V.; Pelletier, M.; Spencer, S.P.; Urban, J.F., Jr.; Ploch, M.; Ramalingam, T.R.; Siegel, R.M.; Belkaid, Y. Critical role of fatty acid metabolism in ILC2-mediated barrier protection during malnutrition and helminth infection. J. Exp. Med. 2016, 213, 1409–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut-Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef]
- Christapher, P.V.; Parasuraman, S.; Asmawi, M.Z.; Murugaiyah, V. Acute and subchronic toxicity studies of methanol extract of Polygonum minus leaves in Sprague Dawley rats. Regul. Toxicol. Pharmacol. 2017, 86, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Truong, D.H.; Nguyen, D.H.; Ta, N.T.A.; Bui, A.V.; Do, T.H.; Nguyen, H.C. Evaluation of the Use of Different Solvents for Phytochemical Constituents, Antioxidants, and In Vitro Anti-Inflammatory Activities of Severinia buxifolia. J. Food Qual. 2019, 2019, 8178294. [Google Scholar] [CrossRef]
- Uddin, N.; Hasan, M.R.; Hasan, M.M.; Hossain, M.M.; Alam, M.R.; Hasan, M.R.; Islam, A.F.; Rahman, T.; Rana, M.S. Assessment of toxic effects of the methanol extract of Citrus macroptera Montr. Fruit via biochemical and hematological evaluation in female Sprague-Dawley rats. PLoS ONE 2014, 9, e111101. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Pereira, H.; Barreira, L.; Figueiredo, F.; Custódio, L.; Vizetto-Duarte, C.; Polo, C.; Rešek, E.; Engelen, A.; Varela, J. Polyunsaturated Fatty Acids of Marine Macroalgae: Potential for Nutritional and Pharmaceutical Applications. Mar. Drugs 2012, 10, 1920–1935. [Google Scholar] [CrossRef]
- Lopes, D.; Melo, T.; Rey, F.; Meneses, J.; Monteiro, F.L.; Helguero, L.A.; Abreu, M.H.; Lillebø, A.I.; Calado, R.; Domingues, M.R. Valuing Bioactive Lipids from Green, Red and Brown Macroalgae from Aquaculture, to Foster Functionality and Biotechnological Applications. Molecules 2020, 25, 3883. [Google Scholar] [CrossRef]





| Retention Time (min) | Compound | % of Area | |
|---|---|---|---|
| 1 | 13.495 | n-Hexadecanoic acid | 7.278 |
| 2 | 14.806 | 9-Octadecanoic acid | 3.744 |
| 3 | 14.974 | Octadecanoic acid | 15.841 |
| 4 | 18.674 | 1,4-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester | 2.466 |
| 5 | 20.802 | Vitamin E | 2.008 |
| 6 | 21.844 | Stigmasta-5,24(28)-dien-3-ol, (3.beta.,24Z)- | 21.153 |
| Retention Time (min) | Compound | % of Area | |
|---|---|---|---|
| 1 | 10.220 | 1,4-Cycloheptadiene, 6-(2-butynyl)- | 3.644 |
| 2 | 10.782 | 2,3-Dimethylanisole | 6.45 |
| 3 | 11.054 | Benzoic acid, 3-methyl | 3.603 |
| 4 | 11.216 | Heptadecan | 4.415 |
| 5 | 11.630 | Benzamide, 3-amino | 2.558 |
| 6 | 11.843 | Tetradecanoic acid | 5.265 |
| 7 | 12.419 | Neophytadiene | 6.068 |
| 8 | 12.671 | 4-Pyridinecarboxaldehyde N-oxide | 3.611 |
| 9 | 13.493 | n-Hexadecanoic acid | 38.686 |
| 10 | 14.651 | benzenesulfonamide, N-(2,5-dichlorophenyl)-4-methyl | 9.51 |
| 11 | 15.919 | 2-Heneicosanone | 2.522 |
| 12 | 20.815 | 2,2,3,3-Tetrafluoro-5-(1,1,2,2-tetrafluoroethoxy)-2,3-dihydrobenzofuran | 3.049 |
| 13 | 21.837 | 3-(Methylsulfanyl)-4-oxo-4,5,6,7-tetrahydro-2-benzothiophene-1-carboxylic acid, trimethylsilyl ester | 4.82 |
| Retention Time (min) | Compound | % of Area | |
|---|---|---|---|
| 1 | 10.388 | 1,4-Cycloheptadiene, 6-(2-butynyl)- | 3.644 |
| 2 | 11.578 | 2,3-Dimethylanisole | 6.45 |
| 3 | 11.856 | Benzoic acid, 3-methyl | 3.603 |
| 4 | 12.419 | Heptadecan | 4.415 |
| 5 | 13.480 | n-Hexadecanoic acid | 8.876 |
| 6 | 14.030 | cis-5,8,11,14,17-Eicosapentaenoic acid | 7.56 |
| 7 | 14.573 | Phytol | 2.747 |
| 8 | 14.722 | (6Z,9Z,12Z,15Z)-Methyl octadeca-6,9,12,15-tetraenoate | 11.451 |
| 9 | 14.767 | 9,12-Octadecadienoic acid (Z,Z)- | 4.796 |
| 10 | 14.819 | 9,12,15-Octadecatrienoic acid, (Z,Z,Z) | 16.811 |
| 11 | 15.951 | Arachidonic acid | 2.098 |
| 12 | 15.996 | cis-5,8,11,14,17-Eicosapentaenoic acid | 15.481 |
| 13 | 19.302 | Eicosapentaenoic Acid methyl ester | 1.11 |
| 14 | 20.803 | Vitamin E | 0.548 |
| 15 | 21.838 | Stigmasta-5,24(28)-dien-3-ol, (3.beta.,24Z)- | 4.126 |
| Retention Time (min) | Compound | % of Area | |
|---|---|---|---|
| 1 | 10.392 | Pentadecanal | 7.278 |
| 2 | 11.216 | Heptadecane | 3.744 |
| 3 | 12.44 | 2-Pentadecanone, 6,10,14-trimethyl | 15.841 |
| 4 | 13.48 | n-Hexadecanoic acid | 39.977 |
| 5 | 14.819 | 9-Octadecenoic acid | 12.005 |
| 6 | 20.815 | Cholesterol | 21.153 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, S.-H.; Lim, Y.; Kim, E.J.; Ko, Y.W.; Hong, I.-S.; Kim, S.; Jung, Y. Antarctic Marine Algae Extracts as a Potential Natural Resource to Protect Epithelial Barrier Integrity. Mar. Drugs 2022, 20, 562. https://doi.org/10.3390/md20090562
Ko S-H, Lim Y, Kim EJ, Ko YW, Hong I-S, Kim S, Jung Y. Antarctic Marine Algae Extracts as a Potential Natural Resource to Protect Epithelial Barrier Integrity. Marine Drugs. 2022; 20(9):562. https://doi.org/10.3390/md20090562
Chicago/Turabian StyleKo, Seong-Hee, YoonHee Lim, Eun Jae Kim, Young Wook Ko, In-Sun Hong, Sanghee Kim, and YunJae Jung. 2022. "Antarctic Marine Algae Extracts as a Potential Natural Resource to Protect Epithelial Barrier Integrity" Marine Drugs 20, no. 9: 562. https://doi.org/10.3390/md20090562
APA StyleKo, S.-H., Lim, Y., Kim, E. J., Ko, Y. W., Hong, I.-S., Kim, S., & Jung, Y. (2022). Antarctic Marine Algae Extracts as a Potential Natural Resource to Protect Epithelial Barrier Integrity. Marine Drugs, 20(9), 562. https://doi.org/10.3390/md20090562








