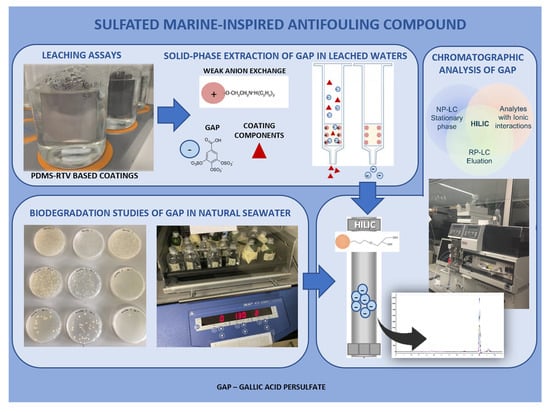
Quantification of a Sulfated Marine-Inspired Antifouling Compound in Several Aqueous Matrices: Biodegradation Studies and Leaching Assays from Polydimethylsiloxane Coatings
Abstract
:1. Introduction
2. Results
2.1. Development and Validation of the HILIC Method
2.2. Effect of Water pH on GAP Stability
2.3. Biodegradation of GAP in Natural Seawater
2.4. Leaching Assays
3. Materials and Methods
3.1. Materials
3.2. Chromatographic Conditions
3.3. Method Validation
3.4. Degradation Assays
3.4.1. Effect of Water pH on GAP Stability
3.4.2. Biodegradation of GAP in Natural Seawater (NSW)
3.5. GAP Incorporation in a Silicone-Based Commercial Coating
Leaching Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kornprobst, J.M.; Sallenave, C.; Barnathan, G. Sulfated compounds from marine organisms. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1998, 119, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Carvalhal, F.; Correia-da-Silva, M.; Sousa, E.; Pinto, M.; Kijjoa, A. SULFATION PATHWAYS: Sources and biological activities of marine sulfated steroids. J. Mol. Endocrinol. 2018, 61, T211. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, C.; Sousa, E.; Pinto, M.; Correia-da-Silva, M. An antifouling model from the sea: A review of 25 years of zosteric acid studies. Biofouling 2017, 33, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.; Correia-da-Silva, M.; Sousa, E.; Antunes, J.; Pinto, M.; Vasconcelos, V.; Cunha, I. Antifouling potential of nature-inspired sulfated compounds. Sci. Rep. 2017, 7, 42424. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Almeida, J.R.; Carvalhal, F.; Câmara, A.; Pereira, S.; Antunes, J.; Vasconcelos, V.; Pinto, M.; Silva, E.R.; Sousa, E.; et al. Overcoming environmental problems of biocides: Synthetic bile acid derivatives as a sustainable alternative. Ecotoxicol. Environ. Saf. 2020, 187, 109812. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, C.; Carvalhal, F.; Pereira, B.; Carvalho, S.; Sousa, E.; Pinto, M.M.M.; Calhorda, M.J.; Vasconcelos, V.; Almeida, J.R.; Silva, E.R.; et al. One Step Forward towards the Development of Eco-Friendly Antifouling Coatings: Immobilization of a Sulfated Marine-Inspired Compound. Mar. Drugs 2020, 18, 489. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, O.; Rijo, P.; Gomes, J.; Santos, R.; Monteiro, S.; Guedes, R.; Serralheiro, M.L.; Gomes, M.; Gomes, L.C.; Mergulhão, F.J.; et al. Antimicrobial Ceramic Filters for Water Bio-Decontamination. Coatings 2021, 11, 323. [Google Scholar] [CrossRef]
- Guo, Y.; Gaiki, S. Retention behavior of small polar compounds on polar stationary phases in hydrophilic interaction chromatography. J. Chromatogr. A 2005, 1074, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Tang, Y.H.; Yao, T.W.; Zeng, S. Separation of rutin nona(H-) and deca(H-) sulfonate sodium by ion-pairing reversed-phase liquid chromatography. J. Chromatogr. A 2004, 1036, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Virgiliou, C.; Sampsonidis, I.; Gika, H.; Raikos, N.; Theodoridis, G. Development and validation of a HILIC- MS/MS multi-targeted method for metabolomics applications. Electrophoresis 2015, 36, 2215–2225. [Google Scholar] [CrossRef] [PubMed]
- Jandera, P.; Hájek, T. Utilization of dual retention mechanism on columns with bonded PEG and diol stationary phases for adjusting the separation selectivity of phenolic and flavone natural antioxidants. J. Sep. Sci 2009, 32, 3603–3619. [Google Scholar] [CrossRef] [PubMed]
- Quiming, N.S.; Denola, N.L.; Ueta, I.; Saito, Y.; Tatematsu, S.; Jinno, K. Retention prediction of adrenoreceptor agonists and antagonists on a diol column in hydrophilic interaction chromatography. Anal. Chim. Acta 2007, 598, 41–50. [Google Scholar] [CrossRef]
- Buszewski, B.; Noga, S. Hydrophilic interaction liquid chromatography (HILIC)—A powerful separation technique. Anal. Bioanal. Chem. 2011, 402, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gonzalo, E.; García-Gómez, D. Hydrophilic Interaction Chromatography: Current Trends and Applications. In Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Bosch-Orea, C.; Sanchís, J.; Farré, M. Analysis of highly polar marine biotoxins in seawater by hydrophilic interaction liquid chromatography coupled to high resolution mass spectrometry. MethodsX 2021, 8, 101370. [Google Scholar] [CrossRef] [PubMed]
- Duarte, R.M.B.O.; Duarte, A.C. Chapter 9—Chromatography Coupled to Various Detectors as a Tool for Separation and Determination of Bioactive Compounds. In Comprehensive Analytical Chemistry; Rocha-Santos, T., Duarte, A.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 65, pp. 219–252. [Google Scholar]
- Li, H.; Liu, C.; Zhao, L.; Xu, D.; Zhang, T.; Wang, Q.; Cabooter, D.; Jiang, Z. A systematic investigation of the effect of sample solvent on peak shape in nano- and microflow hydrophilic interaction liquid chromatography columns. J. Chromatogr. A 2021, 1655, 462498. [Google Scholar] [CrossRef] [PubMed]
- ICH. Validation of Analytical Procedures: Text and Methodology Q2(R1). In Proceedings of the International Conference on Harmonization, Geneva, Switzerland, 2–5 March 2005. 17p. [Google Scholar]
- Petrović, M.; Hernando, M.D.; Díaz-Cruz, M.S.; Barceló, D. Liquid chromatography–tandem mass spectrometry for the analysis of pharmaceutical residues in environmental samples: A review. J. Chromatogr. A 2005, 1067, 1–14. [Google Scholar] [CrossRef]
- Huang, X.-Z.; Xu, Y.; Zhang, Y.-F.; Zhang, Y.; Wong, Y.H.; Han, Z.; Yin, Y.; Qian, P.-Y. Nontoxic piperamides and their synthetic analogues as novel antifouling reagents. Biofouling 2014, 30, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Willingham, G.L.; Jacobson, A.H. Designing an Environmentally Safe Marine Antifoulant. In Designing Safer Chemicals; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1996; Volume 640, pp. 224–233. [Google Scholar]
- ECHA. Guidance on Information Requirements and Chemical Safety Assessment; ECHA: Helsinki, Finland, 2017. [Google Scholar] [CrossRef]
- E895—89(2008); Standard Practice for Determination of Hydrolysis Rate Constants of Organic Chemicals in Aqueous Solutions (Withdrawn 2013). ASTM: West Conshohocken, PA, USA, 2008.
| Matrix | Range (µM) 1 | Linear Regression | R2 | LOD (µM) | LOQ (µM) |
|---|---|---|---|---|---|
| UPW | 30–600 | y = 2013.2x − 35215 | 0.9994 | 15 | 30 |
| NSW | y = 2607.9x + 7506.6 | 1 | 0.75 | 7.5 |
| Matrix | Concentration (µM) 1 | Accuracy (% ± SD) | Intra-Day Variability (RSD ± SD) | Inter-Day Variability (RSD ± SD) |
|---|---|---|---|---|
| UPW | 40 | 116.50 ± 3.1 | 3.9 ± 1.1 | 4.9 ± 0.4 |
| 100 | 103.06 ± 2.5 | 2.4 ± 0.7 | 2.9 ± 0.7 | |
| 500 | 99.93 ± 1.5 | 4.0 ± 2.1 | 2.9 ± 0.9 | |
| NSW | 40 | 113.25 ± 1.0 | 3.1 ± 1.7 | 4.8 ± 2.9 |
| 100 | 118.21 ± 1.7 | 3.0 ± 1.5 | 2.5 ± 1.1 | |
| 500 | 111.64 ± 2.2 | 3.7 ± 1.2 | 2.6 ± 1.0 |
| Stress Conditions 1 | Initial Concentration (%) | Concentration after 1 Day (%) | Concentration after 2 Days (%) | Concentration after 7 Days (%) |
|---|---|---|---|---|
| SNSW, pH 7.6 | 100 ± 1.7 | 97.4 ± 8.8 | 95.0 ± 4.0 | 94.8 ± 15.8 |
| UPW, pH 5 | 100 ± 5.2 | 95.5 ± 5.4 | 99.5 ± 1.6 | 98.6 ± 3.9 |
| UPW, pH 7 | 100 ± 3.0 | 98.3 ± 2.6 | 99.8 ± 1.5 | 101.3 ± 3.2 |
| UPW, pH 9 | 100 ± 8.8 | 97.6 ± 3.7 | 101.3 ± 3.2 | 103.9 ± 4.3 |
| Conditions | Initial Concentration (%)1 | Concentration after 1 Month (%)1 | Concentration after 2 Months (%)1 |
| Light and non-sterile | 100.0 ± 1.3 | 99.7 ± 1.8 | 110.9 ± 3.4 |
| Dark and non-sterile | 100.0 ± 0.5 | 100.0 ± 0.8 | 103.7 ± 1.5 |
| Light and sterile | 100.0 ± 0.5 | 100.3 ± 2.1 | 108.9 ± 3.6 |
| Dark and sterile | 100.0 ± 0.6 | 102.1 ± 1.6 | 115.8 ± 3.4 |
| Coating Matrix | GAP Content in Coating Formulation (wt.%) 1 | GAP Amount in Coated Plates (mg) 1 | Amount of Detected GAP in Leaching Waters (mg) 1 | Content of Released GAP from the Coating (%) 1 |
|---|---|---|---|---|
| PDMS-RTV | 0.56 ± 0.02 | 10.4 ± 0.5 | 0.037 ± 0.004 | 0.35 ± 0.027 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilas-Boas, C.; Gonçalves, V.; Marco, P.D.; Sousa, E.; Pinto, M.; Silva, E.R.; Tiritan, M.E.; Correia-da-Silva, M. Quantification of a Sulfated Marine-Inspired Antifouling Compound in Several Aqueous Matrices: Biodegradation Studies and Leaching Assays from Polydimethylsiloxane Coatings. Mar. Drugs 2022, 20, 548. https://doi.org/10.3390/md20090548
Vilas-Boas C, Gonçalves V, Marco PD, Sousa E, Pinto M, Silva ER, Tiritan ME, Correia-da-Silva M. Quantification of a Sulfated Marine-Inspired Antifouling Compound in Several Aqueous Matrices: Biodegradation Studies and Leaching Assays from Polydimethylsiloxane Coatings. Marine Drugs. 2022; 20(9):548. https://doi.org/10.3390/md20090548
Chicago/Turabian StyleVilas-Boas, Cátia, Virgínia Gonçalves, Paolo De Marco, Emília Sousa, Madalena Pinto, Elisabete R. Silva, Maria Elizabeth Tiritan, and Marta Correia-da-Silva. 2022. "Quantification of a Sulfated Marine-Inspired Antifouling Compound in Several Aqueous Matrices: Biodegradation Studies and Leaching Assays from Polydimethylsiloxane Coatings" Marine Drugs 20, no. 9: 548. https://doi.org/10.3390/md20090548
APA StyleVilas-Boas, C., Gonçalves, V., Marco, P. D., Sousa, E., Pinto, M., Silva, E. R., Tiritan, M. E., & Correia-da-Silva, M. (2022). Quantification of a Sulfated Marine-Inspired Antifouling Compound in Several Aqueous Matrices: Biodegradation Studies and Leaching Assays from Polydimethylsiloxane Coatings. Marine Drugs, 20(9), 548. https://doi.org/10.3390/md20090548