Coelastrella terrestris for Adonixanthin Production: Physiological Characterization and Evaluation of Secondary Carotenoid Productivity
Abstract
:1. Introduction
2. Results
2.1. Morphological and Physiological Characterization of C. terrestris WP154.1
2.1.1. Morphology
2.1.2. Pigment Composition
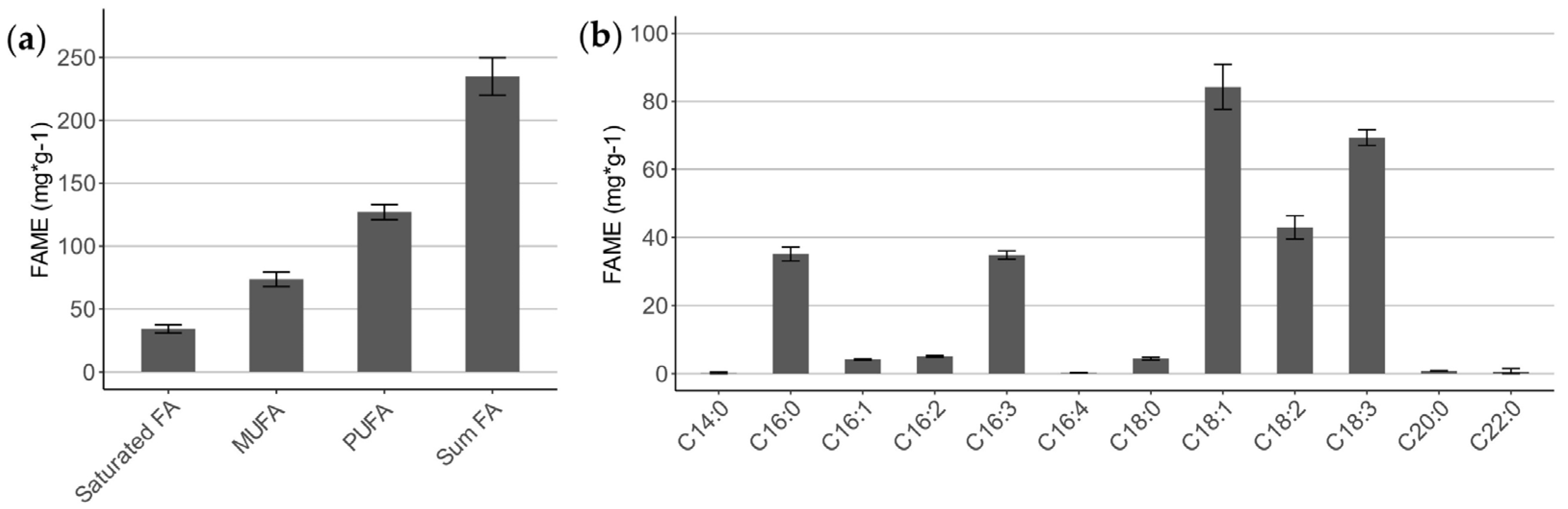
2.1.3. Fatty Acid Profile of Lipids
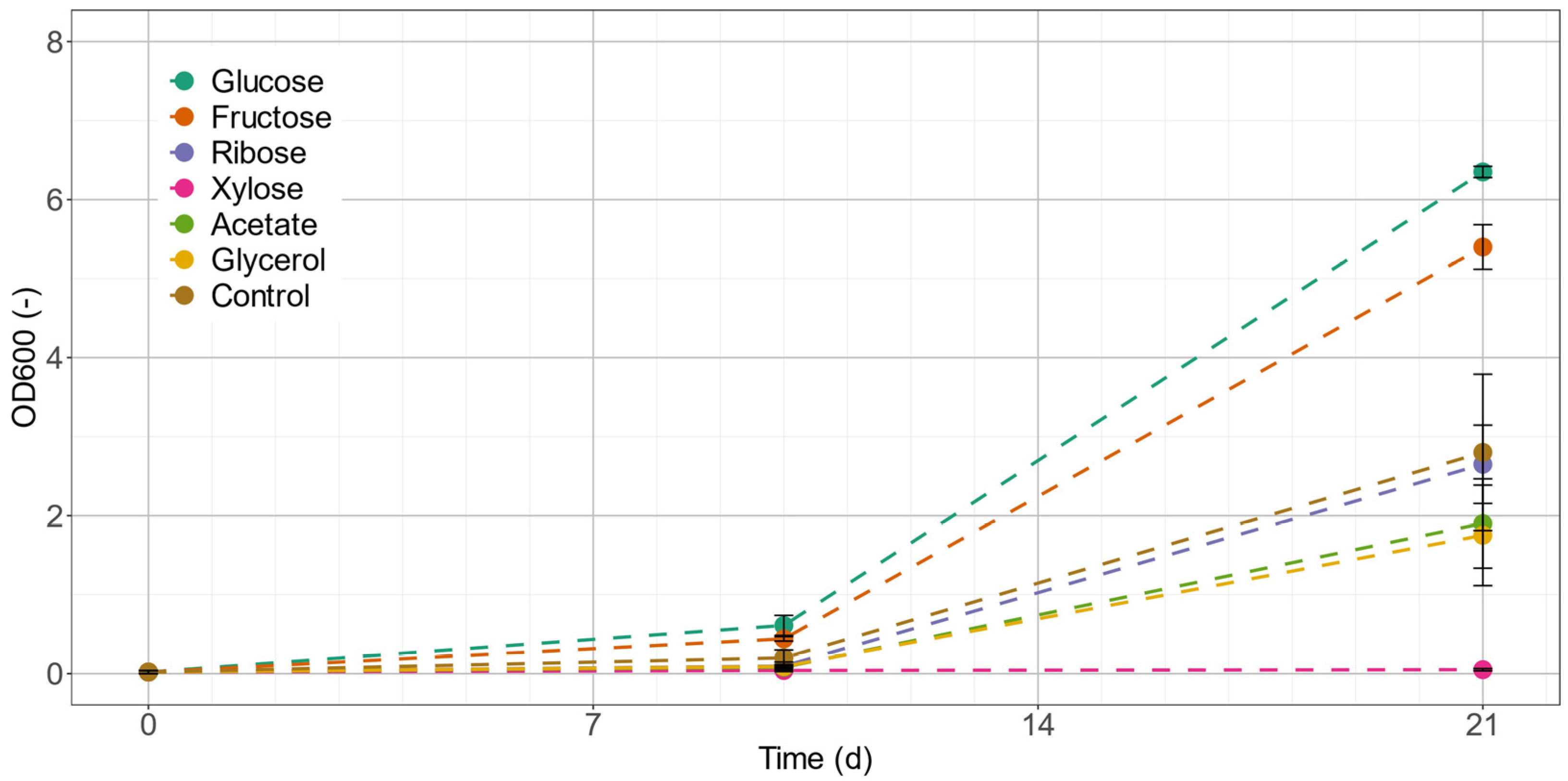
2.2. Carbon-Source Screening for Mixotrophic Growth
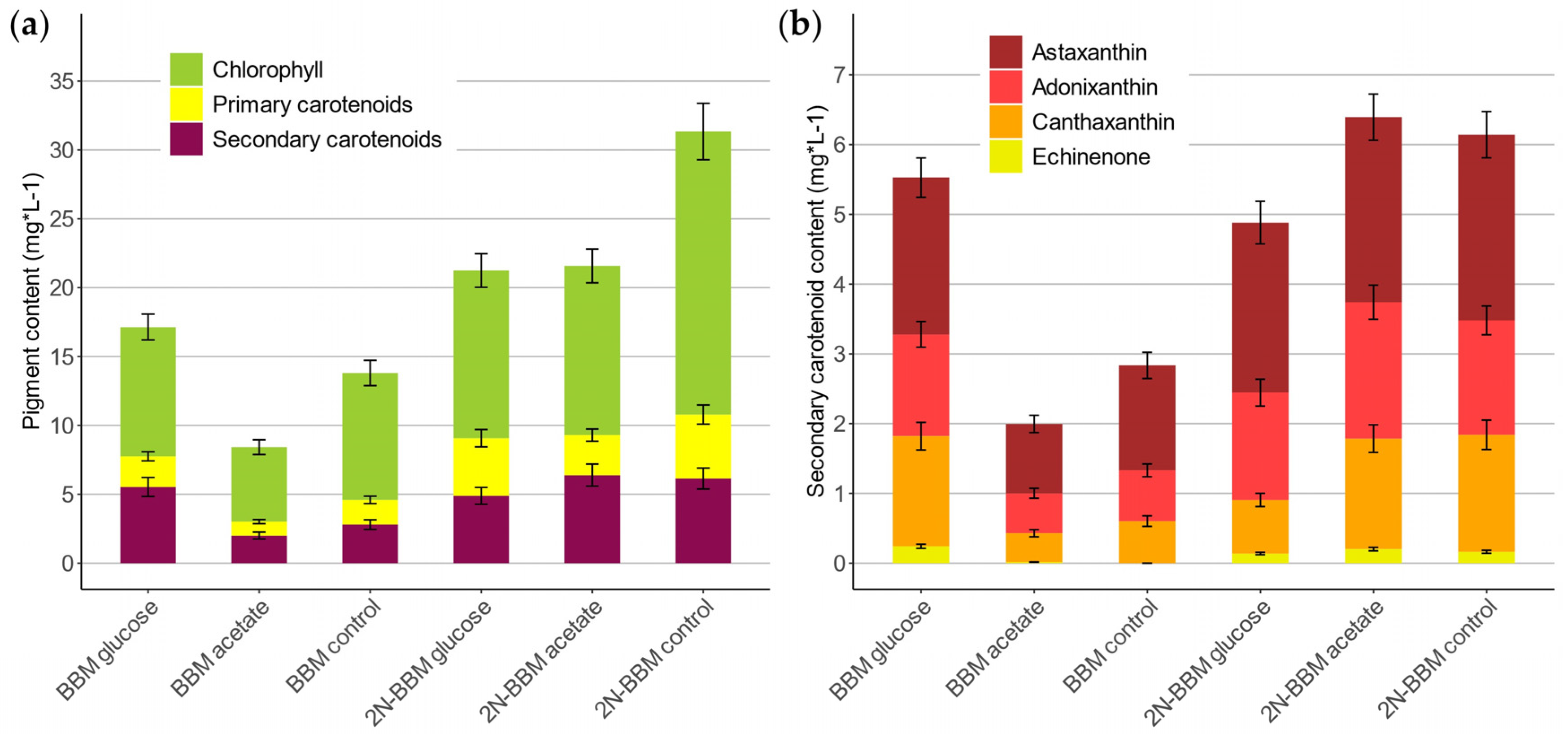
2.3. Scale-Up to 1.25 L Lab-Scale Photobioreactors
3. Discussion
3.1. Morphological and Physiological Characterization
3.2. Prelimary Experiments and Scale up to Photobioreactors
3.3. Biotechnological Potential of C. terrestris WP154.1
4. Materials and Methods
4.1. Sampling, Strain Isolation and Cultivation Medium
4.2. Strain Identification
4.3. Light Microscopy
4.4. Pigment Extraction, Identification and Quantification
4.5. Lipid Extraction, Transesterification and Quantification of Fatty Acids
4.6. C-Source Screening
4.7. Stirred Photobioreactor Cultivations
4.8. Quantification of Carbon-Source
4.9. Nitrate Quantification
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, J.; Saxena, R.C. An Introduction to Microalgae: Diversity and Significance. Diversity and Significance. Handb. Mar. Microalgae Biotechnol. Adv. 2015, 11–24. [Google Scholar] [CrossRef]
- Henriquez, V.; Escobar, C.; Galarza, J.; Gimpel, J. Carotenoids in Microalgae. Carotenoids Nat. 2016, 79, 219–237. [Google Scholar] [CrossRef]
- Doppler, P.; Spadiut, O. Introduction to autotrophic cultivation of microalgae in photobioreactors. In The Autotrophic Biorefinery; Kourist, R., Schmidt, S., Eds.; De Gruyter: Berlin, Germany, 2021; pp. 113–130. [Google Scholar] [CrossRef]
- Hu, J.; Nagarajan, D.; Zhang, Q.; Chang, J.S.; Lee, D.J. Heterotrophic cultivation of microalgae for pigment production: A review. Biotechnol. Adv. 2018, 36, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, G.; Meng, Y.; Wang, P.; Zhou, S.; Shang, H. Utilization of xylose as a carbon source for mixotrophic growth of Scenedesmus obliquus. Bioresour. Technol. 2014, 172, 180–185. [Google Scholar] [CrossRef]
- Lowrey, J.; Brooks, M.S.; McGinn, P.J. Heterotrophic and mixotrophic cultivation of microalgae for biodiesel production in agricultural wastewaters and associated challenges—a critical review. J. Appl. Phycol. 2015, 27, 1485–1498. [Google Scholar] [CrossRef]
- Molino, A.; Iovine, A.; Casella, P.; Mehariya, S.; Chianese, S.; Cerbone, A.; Rimauro, J.; Musmarra, D. Microalgae characterization for consolidated and new application in human food, animal feed and nutraceuticals. Int. J. Environ. Res. Public Health 2018, 15, 2436. [Google Scholar] [CrossRef] [Green Version]
- Varshney, P.; Mikulic, P.; Vonshak, A.; Beardall, J.; Wangikar, P.P. Extremophilic micro-algae and their potential contribution in biotechnology. Bioresour. Technol. 2015, 184, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Fan, C.; Chen, Y.; Hu, Z. The potential for microalgae as bioreactors to produce pharmaceuticals. Int. J. Mol. Sci. 2016, 17, 962. [Google Scholar] [CrossRef] [Green Version]
- Kumar, J.; Singh, D.; Tyagi, M.B.; Kumar, A. Applications in Biotechnology. In Cyanobacteria; Mishra, A.K., Tiwari, D.N., Rai, A.N., Eds.; Academic Press: Cambrige, MA, USA, 2019; pp. 327–346. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Demmig-Adams, B.; Stewart, J.J.; López-Pozo, M.; Polutchko, S.K.; Adams, W.W. Zeaxanthin, a Molecule for Photoprotection in Many Different Environments. Molecules 2020, 25, 5825. [Google Scholar] [CrossRef]
- Christaki, E.; Bonos, E.; Giannenasa, I.; Florou-Paneria, P. Functional properties of carotenoids originating from algae. J. Sci. Food Agric. 2013, 93, 5–11. [Google Scholar] [CrossRef]
- Varela, J.C.; Pereira, H.; Vila, M.; León, R. Production of carotenoids by microalgae: Achievements and challenges. Photosynth. Res. 2015, 125, 423–436. [Google Scholar] [CrossRef]
- Rico, M.; González, A.G.; Santana-Casiano, M.; González-Dávila, M.; Pérez-Almeida, N.; Suarez de Tangil, M. Production of Primary and Secondary Metabolites Using Algae. In Prospects and Challenges in Algal Biotechnology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–326. [Google Scholar] [CrossRef]
- Ambati, R.R.; Gogisetty, D.; Aswathanarayana, R.G.; Ravi, S.; Bikkina, P.N.; Bo, L.; Yuepeng, S. Industrial potential of carotenoid pigments from microalgae: Current trends and future prospects. Crit. Rev. Food Sci. Nutr. 2019, 59, 1880–1902. [Google Scholar] [CrossRef]
- Sun, X.M.; Ren, L.J.; Zhao, Q.Y.; Ji, X.J.; Huang, H. Microalgae for the production of lipid and carotenoids: A review with focus on stress regulation and adaptation. Biotechnol. Biofuels 2018, 11, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Q.; Li, H.; Zou, Y.; Liu, H.; Yang, L. Astaxanthin as a microalgal metabolite for aquaculture: A review on the synthetic mechanisms, production techniques, and practical application. Algal Res. 2021, 54, 102178. [Google Scholar] [CrossRef]
- Naguib, Y.M.A. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef]
- Iwata, S.; Imai, T.; Shimazawa, M.; Ishibashi, T.; Hayashi, M.; Hara, H.; Nakamura, S. Protective effects of the astaxanthin derivative, adonixanthin, on brain hemorrhagic injury. Brain Res. 2018, 1698, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T.; Yasui, H.; Ohmori, A.; Tokuda, H.; Suzuki, N.; Osawa, A.; Shindo, K.; Ishibashi, T. Anti-oxidative, anti-tumor-promoting, and anti-carcinogenic activities of adonirubin and adonixanthin. J. Oleo Sci. 2013, 62, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Reisigl, H. Zur Systematik und Ökologie alpiner Bodenalgen. Osterr. Bot. Z. 1964, 111, 402–499. [Google Scholar] [CrossRef]
- Hu, C.W.; Chuang, L.-T.; Yu, P.C.; Chen, C.N.N. Pigment production by a new thermotolerant microalga Coelastrella sp. F50. Food Chem. 2013, 138, 2071–2078. [Google Scholar] [CrossRef]
- Minhas, A.K.; Hodgson, P.; Barrow, C.J.; Sashidhar, B.; Adholeya, A. The isolation and identification of new microalgal strains producing oil and carotenoid simultaneously with biofuel potential. Bioresour. Technol. 2016, 211, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Song, H.; Liu, X.; Liu, B.; Hu, Z.; Liu, G. Morphology and molecular phylogeny of coccoid green algae Coelastrella sensu lato (Scenedesmaceae, Sphaeropeales), including the description of three new species and two new varieties. J. Phycol. 2019, 55, 1290–1305. [Google Scholar] [CrossRef] [PubMed]
- Goecke, F.; Noda, J.; Paliocha, M.; Gislerød, H.R. Revision of Coelastrella (Scenedesmaceae, Chlorophyta) and first register of this green coccoid microalga for continental Norway. World J. Microbiol. Biotechnol. 2020, 36, 149. [Google Scholar] [CrossRef]
- Zaytseva, A.; Chekanov, K.; Zaytsev, P.; Bakhareva, D.; Gorelova, O.; Kochkin, D.; Lobakova, E. Sunscreen effect exerted by secondary carotenoids and mycosporine-like amino acids in the aeroterrestrial chlorophyte Coelastrella rubescens under high light and UV-A irradiation. Plants 2021, 10, 2601. [Google Scholar] [CrossRef]
- Minyuk, G.; Chelebieva, E.; Chubchikova, I.; Dantsyuk, N.; Drobetskaya, I.; Sakhon, E.; Chekanov, K.; Solovchenko, A. Stress-induced secondary carotenogenesis in Coelastrella rubescens (Scenedesmaceae, Chlorophyta), a producer of value-added keto-carotenoids. Algae 2017, 32, 245–259. [Google Scholar] [CrossRef]
- Tschaikner, A.; Ingolic, E.; & Gärtner, G. Observations in a New Isolate of Coelastrella terrestris (Reisigl) Hegewald & Hanagata (Chlorophyta, Scenedesmaceae) from Alpine Soil (Tyrol, Austria). Phyton B. Aires 2007, 46, 237–245. [Google Scholar]
- Nicoletti, C.; Procházková, L.; Nedbalová, L.; Mócsai, R.; Altmann, F.; Holzinger, A.; Remias, D. Thorsmoerkia curvula gen. et spec. nov. (Trebouxiophyceae, Chlorophyta), a semi-terrestrial microalga from Iceland exhibits high levels of unsaturated fatty acids. J. Appl. Phycol. 2021, 33, 3671–3682. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Hu, H.Y.; Wu, Y.H.; Zhuang, L.L.; Xu, X.Q.; Wang, X.X.; Dao, G.H. Promising solutions to solve the bottlenecks in the large-scale cultivation of microalgae for biomass/bioenergy production. Renew. Sustain. Energy Rev. 2016, 60, 1602–1614. [Google Scholar] [CrossRef]
- Pang, N.; Xie, Y.; Oung HM, O.; Sonawane, B.V.; Fu, X.; Kirchhoff, H.; Cousins, A.B.; Chen, S. Regulation and stimulation of photosynthesis of mixotrophically cultured Haematococcus pluvialis by ribose. Algal Res. 2019, 39, 101443. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Liu, J.; Yang, N. A strategy for stimulating astaxanthin and lipid production in Haematococcus pluvialis by exogenous glycerol application under low light. Algal Res. 2020, 46, 101779. [Google Scholar] [CrossRef]
- Socher, M.L.; Löser, C.; Schott, C.; Bley, T.; Steingroewer, J. The challenge of scaling up photobioreactors: Modeling and approaches in small scale. Eng. Life Sci. 2016, 16, 598–609. [Google Scholar] [CrossRef]
- Doppler, P.; Kornpointner, C.; Halbwirth, H.; Remias, D.; Spadiut, O. Tetraedron minimum, first reported member of hydrodictyaceae to accumulate secondary carotenoids. Life 2021, 11, 107. [Google Scholar] [CrossRef]
- Da Silva Ferreira, V.; Sant’Anna, C. Impact of culture conditions on the chlorophyll content of microalgae for biotechnological applications. World J. Microbiol. Biotechnol. 2017, 33, 20. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, Y.; Schoefs, B. Secondary ketocarotenoid astaxanthin biosynthesis in algae: A multifunctional response to stress. Photosynth. Res. 2010, 106, 155–177. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Duan, C.; Yi, S.; Gao, Z.; Xiao, C.; Agathos, S.N.; Wang, G.; Li, J. Biotechnological production of astaxanthin from the microalga Haematococcus pluvialis. Biotechnol. Adv. 2020, 43, 107602. [Google Scholar] [CrossRef]
- Serna-Loaiza, S.; Zikeli, F.; Adamcyk, J.; Friedl, A. Towards a wheat straw biorefinery: Combination of Organosolv and Liquid Hot Water for the improved production of sugars from hemicellulose and lignin hydrolysis. Bioresour. Technol. Reports 2021, 14, 100667. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for high-value products towards human health and nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Iyer, G.; Nagle, V.; Gupte, Y.V.; Desai, S.; Iyer, M. Characterization of High Carotenoid Producing Coelastrella oocystiformis and its Anti-Cancer Potential. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 527–536. [Google Scholar]
- Tsuji, S.; Nakamura, S.; Maoka, T.; Yamada, T.; Imai, T.; Ohba, T.; Yako, T.; Hayashi, M.; Endo, K.; Saio, M.; et al. Antitumour effects of astaxanthin and adonixanthin on glioblastoma. Mar. Drugs 2020, 18, 474. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, S.; Devendran, S.; Tsai, P.C.; Dahms, H.U.; Ponnusamy, V.K. Potential of two-stage cultivation in microalgae biofuel production. Fuel 2019, 252, 339–349. [Google Scholar] [CrossRef]
- Tschaikner, A.; Ingolić, E.; Stoyneva, M.P.; Gärtner, G. Autosporulation in the soil alga Coelastrella terrestris (Chlorophyta, Scenedesmaceae, Scenedesmoideae). Phytol. Balc. 2007, 13, 29–34. [Google Scholar]
- Bolt’s Basal Medium BBM. Available online: http://cccryo.fraunhofer.de/sources/files/medien/BBM.pdf (accessed on 7 January 2022).
- Doppler, P.; Kriechbaum, R.; Singer, B.; Spadiut, O. Make microalgal cultures axenic again—A fast and simple workflow utilizing fluorescence-activated cell sorting. J. Microbiol. Methods 2021, 186, 106256. [Google Scholar] [CrossRef]
- Procházková, L.; Remias, D.; Řezanka, T.; Nedbalová, L. Ecophysiology of Chloromonas hindakii sp. Nov. (chlorophyceae), causing orange snow blooms at different light conditions. Microorganisms 2019, 7, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; McVeigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A comprehensive update on curation, resources and tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef] [PubMed]
- Remias, D.; Pichrtová, M.; Pangratz, M.; Lütz, C.; Holzinger, A. Ecophysiology, secondary pigments and ultrastructure of Chlainomonas sp. (Chlorophyta) from the European Alps compared with Chlamydomonas nivalis forming red snow. FEMS Microbiol. Ecol. 2016, 92, fiw030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remias, D.; Lütz, C. Characterisation of esterified secondary carotenoids and of their isomers in green algae: A HPLC approach. Arch. Hydrobiol. Suppl. Algol. Stud. 2007, 124, 85–94. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Doppler, P.; Gasser, C.; Kriechbaum, R.; Ferizi, A.; Spadiut, O. In Situ Quantification of Polyhydroxybutyrate in Photobioreactor Cultivations of Synechocystis sp. Using an Ultrasound-Enhanced ATR-FTIR Spectroscopy Probe. Bioengineering 2021, 8, 129. [Google Scholar] [CrossRef]

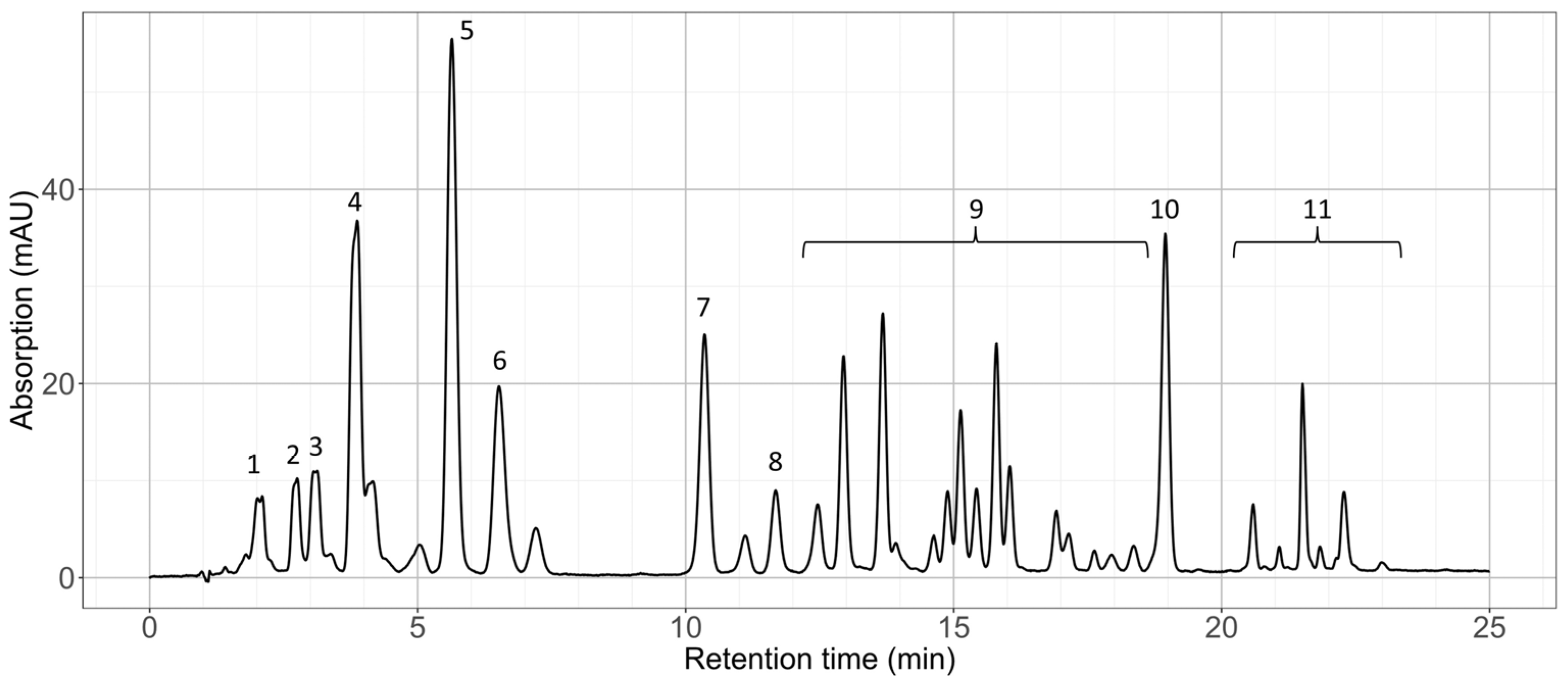

| Assigned Substance | Detected Mass (M + H)+ | Theoretical Mass (M + H)+ | Mass Error (ppm) | |
|---|---|---|---|---|
| 1 | Neoxanthin/violaxanthin 1 | 601.4242 | 601.4251 | 1.5 |
| 2 | Astaxanthin | 597.3930 | 597.3938 | 1.3 |
| 3 | Adonixanthin | 583.4343 | 583.4351 | 1.4 |
| 4 | Lutein/zeaxanthin 1 | 569.4355 | 569.4353 | 0.4 |
| 5 | Canthaxanthin | 565.4046 | 565.4040 | 1.1 |
| 6 | Chlorophyll b | 907.5218 | 907.5219 | 0.1 |
| 7 | Chlorophyll a | 893.5424 | 893.5426 | 0.2 |
| 8 | Echinenone | 551.4238 | 551.4247 | 1.6 |
| 9 | Astaxanthin and adonixanthin ME | |||
| 10 | β-carotene | 537.4441 | 537.4455 | 2.6 |
| 11 | Astaxanthin DE |
| Glucose | Fructose | Ribose | Xylose | Acetate | Glycerol | Control | |
|---|---|---|---|---|---|---|---|
| OD600 after 21 d (−) | 6.35 | 5.40 | 2.65 | 0.05 | 1.90 | 1.75 | 2.80 |
| µmax (d−1) | 0.34 | 0.31 | 0.16 | 0.07 | 0.14 | 0.15 | 0.23 |
| Interval (d) | BBM | 2N-BBM | |||||
|---|---|---|---|---|---|---|---|
| Glucose | Acetate | Control | Glucose | Acetate | Control | ||
| µmax (d−1) | 0–7 | 0.717 ± 0.089 | 0.561 ± 0.070 | 0.626 ± 0.078 | 0.757 ± 0.094 | 0.554 ± 0.069 | 0.681 ± 0.085 |
| µlimitation (d−1) | 7–21 | 0.111 ± 0.013 | 0.117 ± 0.014 | 0.126 ± 0.015 | 0.100 ± 0.012 | 0.197 ± 0.024 | 0.096 ± 0.012 |
| rSC (mg·L−1·d−1) | 0–7 | 0.184 ± 0.023 | 0.042 ± 0.005 | 0.035 ± 0.004 | 0.178 ± 0.022 | 0.047 ± 0.005 | 0.127 ± 0.015 |
| 7–21 | 0.302 ± 0.037 | 0.121 ± 0.015 | 0.182 ± 0.022 | 0.259 ± 0.032 | 0.432 ± 0.054 | 0.374 ± 0.046 | |
| qSC (mg·g−1·d−1) | 0–7 | 0.184 ± 0.023 | 0.070 ± 0.008 | 0.056 ± 0.007 | 0.242 ± 0.030 | 0.087 ± 0.010 | 0.137 ± 0.017 |
| 7–21 | 0.123 ± 0.015 | 0.067 ± 0.008 | 0.072 ± 0.009 | 0.097 ± 0.012 | 0.188 ± 0.023 | 0.128 ± 0.016 | |
| radonixanthin (mg·L−1·d−1) | 0–7 | 0.046 ± 0.005 | 0.011 ± 0.001 | 0.005 ± 0.000 | 0.049 ± 0.006 | 0.018 ± 0.002 | 0.056 ± 0.007 |
| 7–21 | 0.080 ± 0.010 | 0.034 ± 0.004 | 0.049 ± 0.006 | 0.085 ± 0.010 | 0.130 ± 0.016 | 0.089 ± 0.011 | |
| qadonixanthin (mg·g−1·d−1) | 0–7 | 0.046 ± 0.005 | 0.019 ± 0.002 | 0.009 ± 0.001 | 0.067 ± 0.008 | 0.034 ± 0.004 | 0.060 ± 0.007 |
| 7–21 | 0.033 ± 0.004 | 0.019 ± 0.002 | 0.019 ± 0.002 | 0.032 ± 0.004 | 0.056 ± 0.007 | 0.030 ± 0.003 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doppler, P.; Kriechbaum, R.; Käfer, M.; Kopp, J.; Remias, D.; Spadiut, O. Coelastrella terrestris for Adonixanthin Production: Physiological Characterization and Evaluation of Secondary Carotenoid Productivity. Mar. Drugs 2022, 20, 175. https://doi.org/10.3390/md20030175
Doppler P, Kriechbaum R, Käfer M, Kopp J, Remias D, Spadiut O. Coelastrella terrestris for Adonixanthin Production: Physiological Characterization and Evaluation of Secondary Carotenoid Productivity. Marine Drugs. 2022; 20(3):175. https://doi.org/10.3390/md20030175
Chicago/Turabian StyleDoppler, Philipp, Ricarda Kriechbaum, Maria Käfer, Julian Kopp, Daniel Remias, and Oliver Spadiut. 2022. "Coelastrella terrestris for Adonixanthin Production: Physiological Characterization and Evaluation of Secondary Carotenoid Productivity" Marine Drugs 20, no. 3: 175. https://doi.org/10.3390/md20030175
APA StyleDoppler, P., Kriechbaum, R., Käfer, M., Kopp, J., Remias, D., & Spadiut, O. (2022). Coelastrella terrestris for Adonixanthin Production: Physiological Characterization and Evaluation of Secondary Carotenoid Productivity. Marine Drugs, 20(3), 175. https://doi.org/10.3390/md20030175






