Echinochrome A Reverses Kidney Abnormality and Reduces Blood Pressure in a Rat Model of Preeclampsia
Abstract
1. Introduction

2. Results
2.1. Effects of Ech A on the Ang II-Induced PE Rat Model
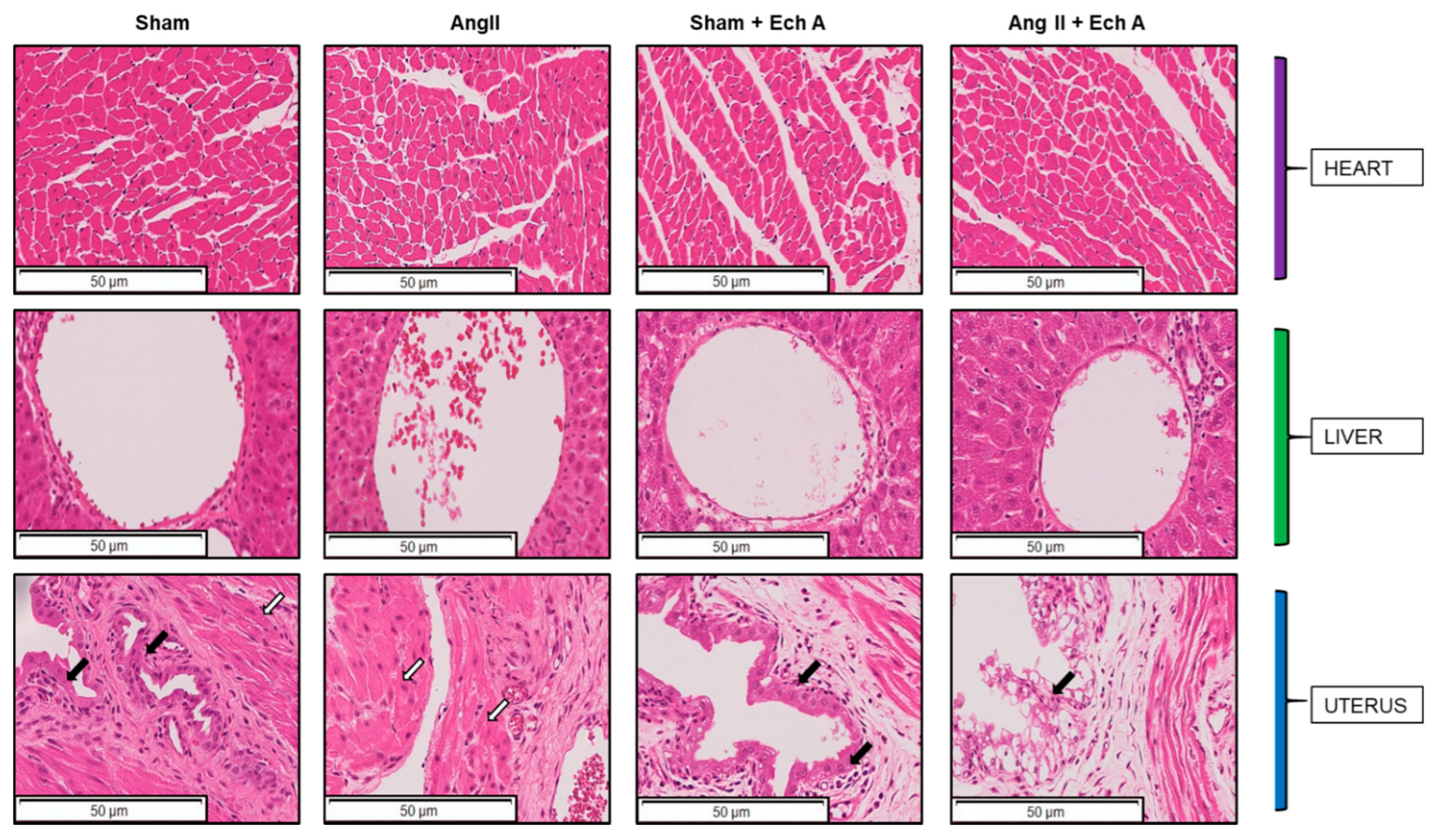
2.2. H and E Staining of Maternal Kidney Cortex in the Sham and Ang II Groups with and without Ech A
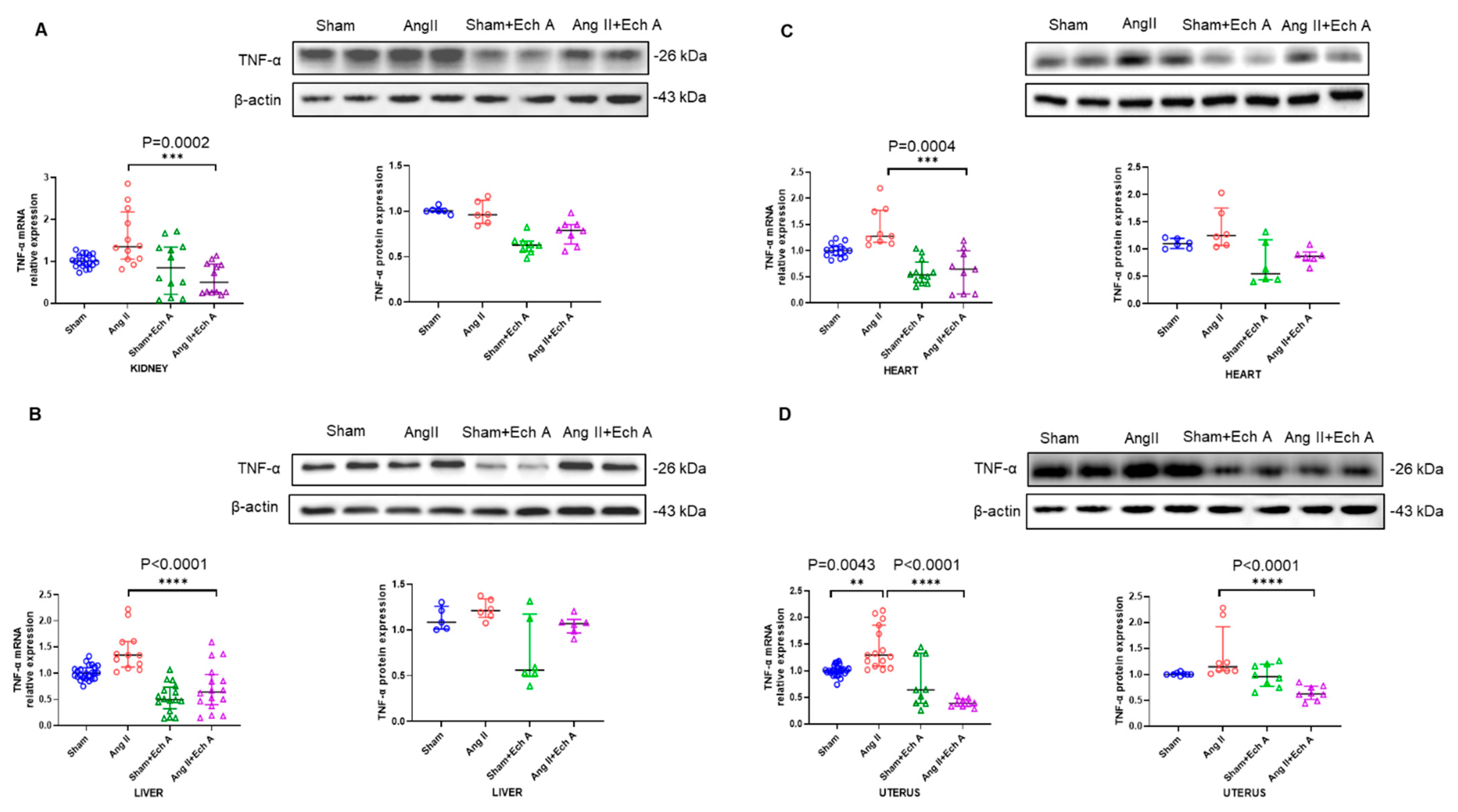
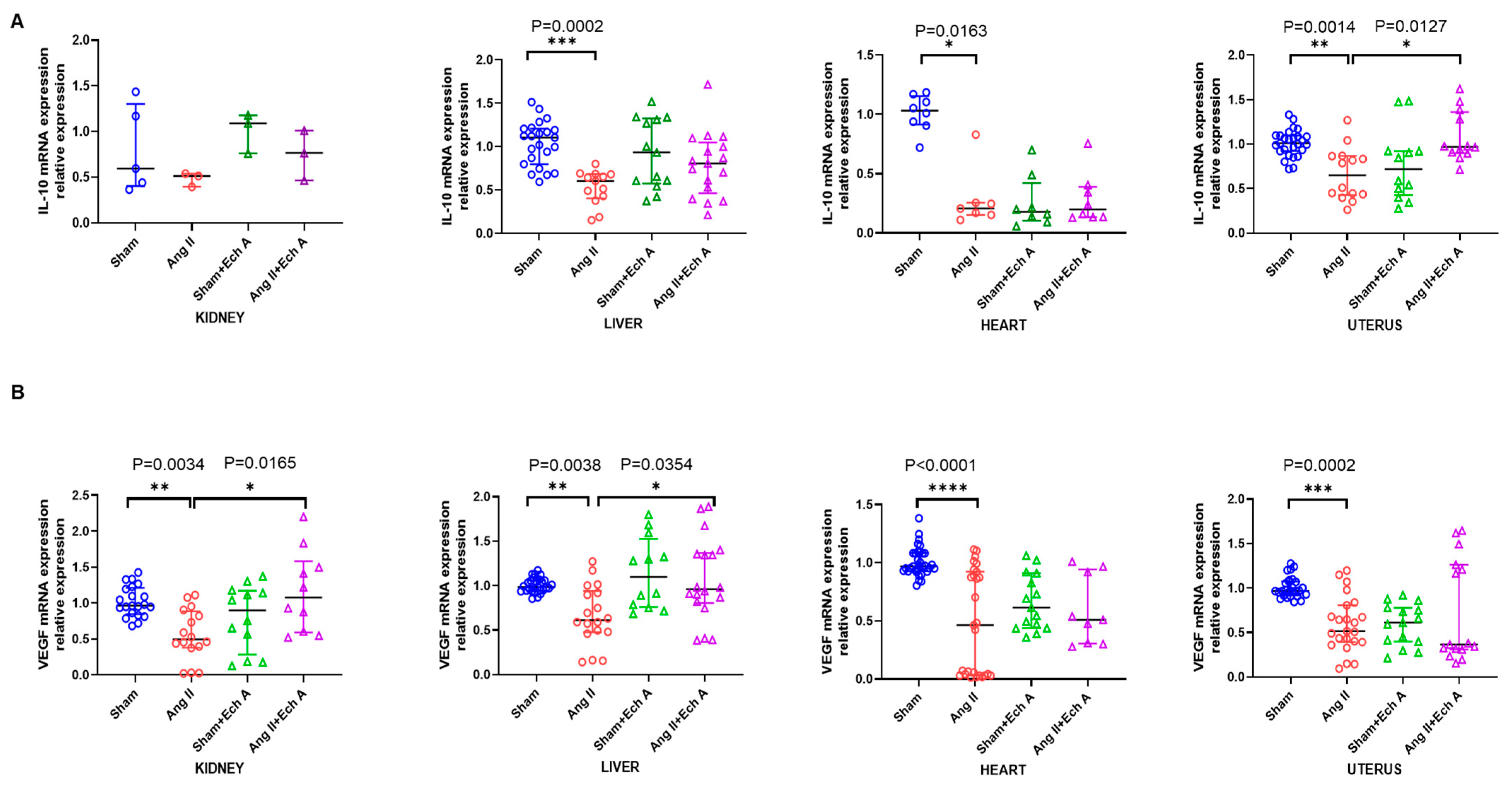
2.3. The mRNA and Protein Expressions of TNF–α, IL–10, and VEGF in the Ang II Group and Their Responses to Ech A Treatment
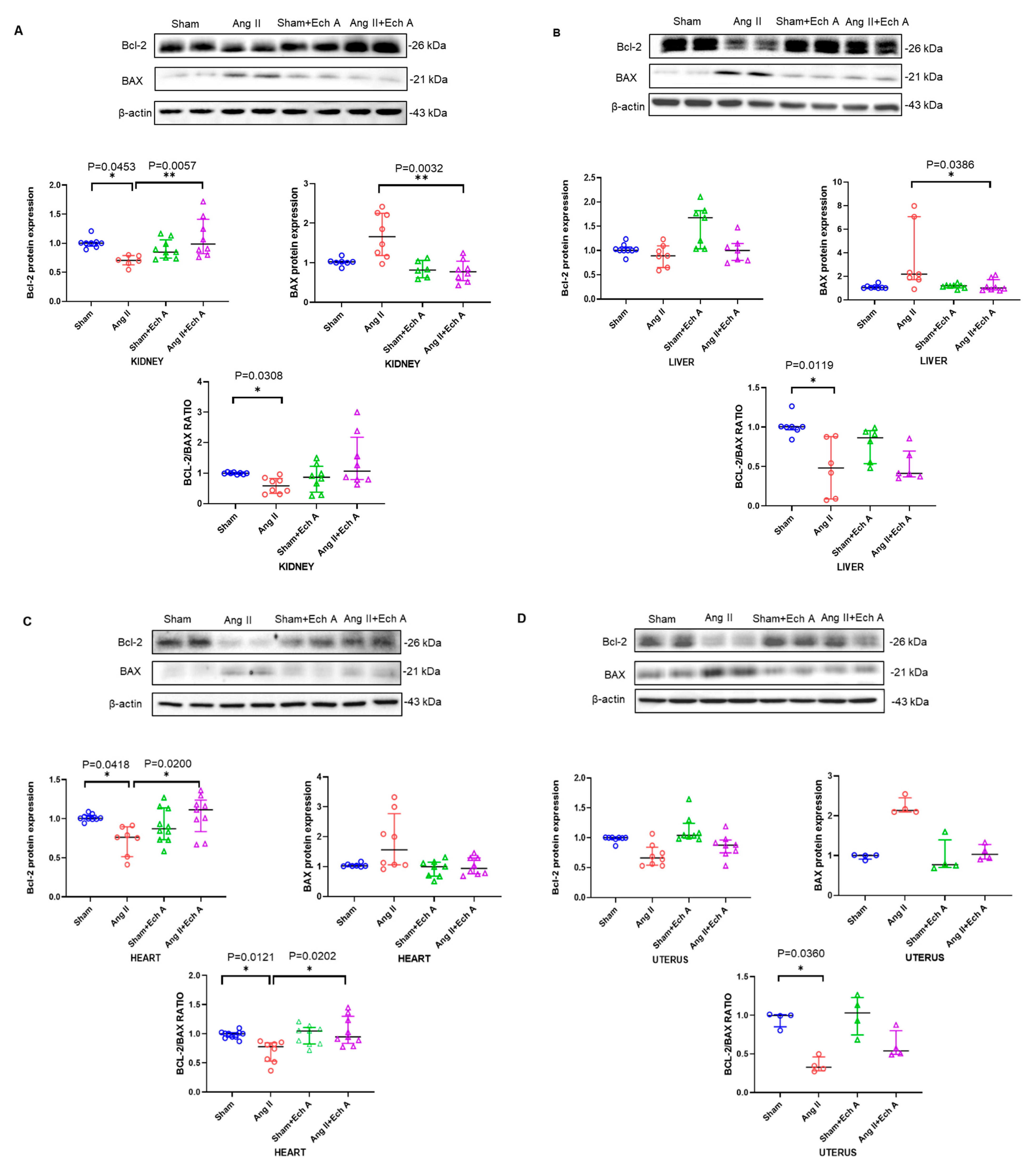
2.4. Bax, Bcl–2, and Their Ratios in the Kidneys, Livers, Hearts, and Uteruses of the Four Groups with and without Ech A Treatment
3. Discussion
4. Materials and Methods
4.1. Preeclampsia Rat Model
4.2. Measurement of Blood Pressure
4.3. H and E Staining
4.4. RNA Preparation and Quantitative Real-Time PCR
| TNF–α-RAT | CCAGGAGAAAGTCAGCCTCCT | F |
| TNF–α-RAT | TCAT ACCAGGGCTTGAGCTCA | R |
| IL–10-RAT | GCTGGACAACATACTGCTGA | F |
| IL–10-RAT | TGCTCCTTGAT TTCTGGG | R |
| VEGF-RAT | GCCCATGAAGTGGTGAAGTT | F |
| VEGF-RAT | ACTCCAGGGCTTCATCATTG | R |
| 18sRNA-RAT | CGCGGTTCTATTTTGTTGGT | F |
| 18sRNA-RAT | AGTCGGCATCGTTTATGGTC | R |
4.5. Western Blotting Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Song, G.; Lin, X.; Pang, X.; Meng, T. Upregulated unique long 16 binding protein 1 detected in preeclamptic placenta affects human extravillous trophoblast cell line (HTR-8/SVneo) invasion by modulating the function of uterine natural killer cells. Exp. Ther. Med. 2017, 13, 1447–1455. [Google Scholar] [CrossRef]
- Mistry, H.D.; Kurlak, L.O.; Mansour, Y.T.; Zurkinden, L.; Mohaupt, M.G.; Escher, G. Increased maternal and fetal cholesterol efflux capacity and placental CYP27A1 expression in pre-eclampsia. J. Lipid Res. 2017, 58, 1186–1195. [Google Scholar] [CrossRef]
- Shen, M.; Smith, G.N.; Rodger, M.; White, R.R.; Walker, M.C.; Wen, S.W.; Räisänen, S.H. Comparison of risk factors and outcomes of gestational hypertension and pre-eclampsia. PLoS ONE 2017, 12, e0175914. [Google Scholar] [CrossRef]
- Witlin, A.G.; Sibai, B.M. Magnesium sulfate therapy in preeclampsia and eclampsia. Obstet. Gynecol. 1998, 92, 883–889. [Google Scholar]
- American College of Obstetricians and Gynecologists. National High Blood Pressure Education Program Working Group Report on High Blood Pressure in Pregnancy. Am. J. Obstet. Gynecol. 2000, 183, S1–S22. [Google Scholar] [CrossRef]
- Sibai, B.M. Diagnosis and management of gestational hypertension and preeclampsia. Obstet. Gynecol. 2003, 102, 181–192. [Google Scholar]
- Sibai, B.M. Chronic hypertension in pregnancy. Obstet. Gynecol. 2002, 100, 369–377. [Google Scholar] [CrossRef]
- Freeman, D.J.; McManus, F.; Brown, E.A.; Cherry, L.; Norrie, J.; Ramsay, J.E.; Clark, P.; Walker, I.D.; Sattar, N.; Greer, I.A. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension 2004, 44, 708–714. [Google Scholar] [CrossRef]
- LaMarca, B.; Cornelius, D.; Wallace, K. Elucidating immune mechanisms causing hypertension during pregnancy. Physiology 2013, 28, 225–233. [Google Scholar] [CrossRef]
- Redman, C.W.; Sargent, I.L. Immunology of pre-eclampsia. Am. J. Reprod. Immunol. 2010, 63, 534–543. [Google Scholar] [CrossRef]
- Cornelius, D.C.; Hogg, J.P.; Scott, J.; Wallace, K.; Herse, F.; Moseley, J.; Wallukat, G.; Dechend, R.; LaMarca, B. Administration of interleukin-17 soluble receptor C suppresses TH17 cells, oxidative stress, and hypertension in response to placental ischemia during pregnancy. Hypertension 2013, 62, 1068–1073. [Google Scholar] [CrossRef]
- Irani, R.A.; Zhang, Y.; Zhou, C.C.; Blackwell, S.C.; Hicks, M.J.; Ramin, S.M.; Kellems, R.E.; Xia, Y. Autoantibody mediated angiotensin receptor activation contributes to preeclampsia through tumor necrosis factor-alpha signaling. Hypertension 2010, 55, 1246–1253. [Google Scholar] [CrossRef]
- LaMarca, B.B.; Cockrell, K.; Sullivan, E.; Bennett, W.; Granger, J.P. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension 2005, 46, 82–86. [Google Scholar] [CrossRef]
- Formby, B. Immunologic response in pregnancy: Its role in endocrine disorders of pregnancy and influence on the course of maternal autoimmune diseases. Endocrinol. Metab. Clin. N. Am. 1995, 24, 187–205. [Google Scholar] [CrossRef]
- Gadonski, G.; LaMarca, B.B.; Sullivan, E.; Bennett, W.; Chandler, D.; Granger, J.P. Hypertension produced by reductions in uterine perfusion in the pregnant rat: Role of interleukin 6. Hypertension 2006, 48, 711–716. [Google Scholar] [CrossRef]
- Keiser, S.D.; Veillon, E.W.; Parrish, M.R.; Bennett, W.; Cockrell, K.; Fournier, L.; Granger, J.P.; Martin, J.N., Jr.; Lamarca, B. Effects of 17-hydroxyprogesterone on tumor necrosis factor-alpha-induced hypertension during pregnancy. Am. J. Hypertens. 2009, 22, 1120–1125. [Google Scholar] [CrossRef]
- Lamarca, B.; Speed, J.; Ray, L.F.; Cockrell, K.; Wallukat, G.; Dechend, R.; Granger, J. Hypertension in response to IL-6 during pregnancy: Role of AT1-receptor activation. Int. J. Interf. Cytokine Mediat. Res. 2011, 201, 65–70. [Google Scholar] [CrossRef]
- Zhou, C.C.; Irani, R.A.; Dai, Y.; Blackwell, S.C.; Hicks, M.J.; Ramin, S.M.; Kellems, R.E.; Xia, Y. Autoantibody-mediated IL-6-dependent endothelin-1 elevation underlies pathogenesis in a mouse model of preeclampsia. J. Immunol. 2011, 186, 6024–6034. [Google Scholar] [CrossRef]
- Arriaga, P.L.; Jimenez, Z.L.; Vadillo, O.F.; Martinez, F.A.; Herrerias, C.T.; Hernandez, G.C. The predominant Th1 cytokine profile in maternal plasma of preeclamptic women is not reflected in the choriodecidual and fetal compartments. J. Soc. Gynecol. Investig. 2005, 12, 335–342. [Google Scholar] [CrossRef]
- Hennessy, A.; Pilmore, H.L.; Simmons, L.A.; Painter, D.M. A deficiency of placental IL 10 in preeclampsia. J. Immunol. 1999, 163, 3491–3495. [Google Scholar]
- Lamarca, B. The role of immune activation in contributing to vascular dysfunction and the pathophysiology of hypertension during preeclampsia. Minerva Ginecol. 2010, 62, 105–120. [Google Scholar]
- Anderson, H.A.; Mathieson, J.W.; Thomson, R.H. Distribution of spinochrome pigments in echinoids. Comp. Biochem. Physiol. 1969, 28, 333–345. [Google Scholar] [CrossRef]
- Kim, H.K.; Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A.; Han, J. Multifaceted Clinical Effects of Echinochrome. Mar. Drugs 2021, 19, 412. [Google Scholar] [CrossRef]
- Malyarenko, T.V.; Kicha, A.A.; Stonik, V.A.; Ivanchina, N.V. Sphingolipids of Asteroidea and Holothuroidea: Structures and Biological Activities. Mar. Drugs 2021, 19, 330. [Google Scholar] [CrossRef]
- Shikov, A.N.; Pozharitskaya, O.N.; Krishtopina, A.S.; Makarov, V.G. Naphthoquinone pigments from sea urchins: Chemistry and pharmacology. Phytochem. Rev. 2018, 17, 509–534. [Google Scholar] [CrossRef]
- Prokopov, I.A.; Kovaleva, E.L.; Minaeva, E.D.; Pryakhina, E.A.; Savin, E.V.; Gamayunova, A.V.; Pozharitskaya, O.N.; Makarov, V.G.; Shikov, A.N. Animal-derived medicinal products in Russia: Current nomenclature and specific aspects of quality control. J. Ethnopharmacol. 2019, 240, 111933. [Google Scholar] [CrossRef]
- Vasileva, E.A.; Mishchenko, N.P.; Zadorozhny, P.A.; Fedoreyev, S.A. New Aminonaphthoquinone from the Sea Urchins Strongylocentrotus pallidus and Mesocentrotus nudus. Nat. Prod. Commun. 2016, 11, 821–824. [Google Scholar] [CrossRef]
- Artyukov, A.A.; Zelepuga, E.A.; Bogdanovich, L.N.; Lupach, N.M.; Novikov, V.L.; Rutckova, T.A.; Kozlovskaya, E.P. Marine Polyhydroxynaphthoquinone, Echinochrome A: Prevention of Atherosclerotic Inflammation and Probable Molecular Targets. J. Clin. Med. 2020, 9, 1494. [Google Scholar] [CrossRef]
- Smith, L.C.; Ghosh, J.; Buckley, K.M.; Clow, L.A.; Dheilly, N.M.; Haug, T.; Henson, J.H.; Li, C.; Lun, C.M.; Majeske, A.J.; et al. Echinoderm immunity. Adv. Exp. Med. Biol. 2010, 708, 260–301. [Google Scholar]
- Cho, K.; Kang, M.C.; Parveen, A.; Yumnam, S.; Kim, S.Y. Anti-Inflammatory Effect of Chloroform Fraction of Pyrus Ussuriensis Maxim. Leaf Extract on 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis in nc/nga Mice. Nutrients 2019, 11, 276. [Google Scholar] [CrossRef]
- Yoon, C.S.; Kim, H.K.; Mishchenko, N.P.; Vasileva, E.A.; Fedoreyev, S.A.; Shestak, O.P.; Balaneva, N.N.; Novikov, V.L.; Stonik, V.A.; Han, J. The protective effects of Echinochrome A structural analogs against oxidative stress and doxorubicin in AC16 cardiomyocytes. Mol. Cell. Toxicol. 2019, 15, 407–414. [Google Scholar] [CrossRef]
- Mischenko, N.P.; Fedoreyev, S.A.; Pokhilo, N.D.; Anufriev, V.P.; Denisenko, V.A.; Glazunov, V.P. Echinamines A and B, first aminated hydroxynaphthazarins from the sea urchin Scaphechinus mirabilis. J. Nat. Prod. 2005, 68, 1390–1393. [Google Scholar] [CrossRef]
- Babenkova, I.V.; Teselkin Iu, O.; Makashova, N.V.; Guseva, M.R. Antioxidative activity of histochrome and some other drugs used in ophthalmology. Vestn. Oftalmol. 1999, 115, 22–24. [Google Scholar]
- Guseva, M.R.; Beslaneeva, M.B.; Mishchenko, N.P.; Khurai, A.R. The specific features of penetration of the antioxidant histochrome across the blood-ocular barrier: An experimental study. Vestn. Oftalmol. 2007, 123, 38–40. [Google Scholar]
- Mendilcioglu, I.; Karaveli, S.; Erdogan, G.; Simsek, M.; Taskin, O.; Ozekinci, M. Apoptosis and expression of Bcl-2, Bax, p53, caspase-3, and Fas, Fas ligand in placentas complicated by preeclampsia. Clin. Exp. Obstet. Gynecol. 2011, 38, 38–42. [Google Scholar]
- Lasukova, T.V.; Uskina, E.V.; Afanas’iev, S.A.; Ponomarenko, I.V.; Naryzhnaia, N.V.; Cherniavskii, A.M.; Lishmanov Iu, B. Effects of emoxipine and histochrome on lipid peroxidation and activity of serum MB-creatine phosphokinase in patients with ischemic heart disease during aortocoronary shunting. Eksp. Klin. Farmakol. 1997, 60, 51–53. [Google Scholar]
- Afanas’ev, S.A.; Lasukova, T.V.; Cherniavskii, A.M.; Vecherskii, I.; Ponomarenko, I.V. The effect of histochrome on the lipid peroxidation indices during the surgical treatment of patients with ischemic heart disease of different functional classes. Eksp. Klin. Farmakol. 1999, 62, 32–34. [Google Scholar]
- Makris, A.; Xu, B.; Yu, B.; Thornton, C.; Hennessy, A. Placental deficiency of interleukin-10 (IL-10) in preeclampsia and its relationship to an IL10 promoter polymorphism. Placenta 2006, 27, 445–451. [Google Scholar] [CrossRef]
- Chatterjee, P.; Chiasson, V.L.; Kopriva, S.E.; Young, K.J.; Chatterjee, V.; Jones, K.A.; Mitchell, B.M. Interleukin 10 deficiency exacerbates toll-like receptor 3-induced preeclampsia-like symptoms in mice. Hypertension 2011, 58, 489–496. [Google Scholar] [CrossRef]
- Markert, U.R.; Fitzgerald, J.S.; Seyfarth, L.; Heinzelmann, J.; Varosi, F.; Voigt, S.; Schleussner, E.; Seewald, H.-J. Lessons from reproductive immunology for other fields of immunology and clinical approaches. Chem. Immunol. Allergy. 2005, 89, 169–179. [Google Scholar]
- Bennett, W.; Lagoo-Deenadayalan, S.; Stopple, J.; Barber, W.; Hale, E.; Brackin, M.N.; Cowan, B.D. Cytokine expression by first-trimester human chorionic villi. Am. J. Reprod. Immunol. 1998, 40, 309–318. [Google Scholar] [CrossRef]
- Hanna, N.; Hanna, I.; Hleb, M.; Wagner, E.; Dougherty, J.; Balkundi, D.; Padbury, J.; Sharma, S. Gestational age-dependent expression of IL-10 and its receptor in human placental tissues and isolated cytotrophoblasts. J. Immunol. 2000, 164, 5721–5728. [Google Scholar] [CrossRef]
- Redman, C.W.; Sargent, I.L. Pre-eclampsia, the placenta and the maternal systemic inflammatory response—A review. Placenta 2003, 24, S21–S27. [Google Scholar] [CrossRef]
- Xu, B.; Nakhla, S.; Makris, A.; Hennessy, A. TNF-α inhibits trophoblast integration into endothelial cellular networks. Placenta 2011, 32, 241–246. [Google Scholar] [CrossRef]
- Bauer, S.; Pollheimer, J.; Hartmann, J.; Husslein, P.; Aplin, J.D.; Knöfler, M. Tumor necrosis factor-alpha inhibits trophoblast migration through elevation of plasminogen activator inhibitor-1 in firsttrimester villous explant cultures. J. Clin. Endocrinol. Metab. 2004, 89, 812–822. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, H.; Liu, J.; Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Zhang, Y. Echinochrome A Reverses Kidney Abnormality and Reduces Blood Pressure in a Rat Model of Preeclampsia. Mar. Drugs 2022, 20, 722. https://doi.org/10.3390/md20110722
Cui H, Liu J, Vasileva EA, Mishchenko NP, Fedoreyev SA, Stonik VA, Zhang Y. Echinochrome A Reverses Kidney Abnormality and Reduces Blood Pressure in a Rat Model of Preeclampsia. Marine Drugs. 2022; 20(11):722. https://doi.org/10.3390/md20110722
Chicago/Turabian StyleCui, Huixing, Junxian Liu, Elena A. Vasileva, Natalia P. Mishchenko, Sergey A. Fedoreyev, Valentin A. Stonik, and Yinhua Zhang. 2022. "Echinochrome A Reverses Kidney Abnormality and Reduces Blood Pressure in a Rat Model of Preeclampsia" Marine Drugs 20, no. 11: 722. https://doi.org/10.3390/md20110722
APA StyleCui, H., Liu, J., Vasileva, E. A., Mishchenko, N. P., Fedoreyev, S. A., Stonik, V. A., & Zhang, Y. (2022). Echinochrome A Reverses Kidney Abnormality and Reduces Blood Pressure in a Rat Model of Preeclampsia. Marine Drugs, 20(11), 722. https://doi.org/10.3390/md20110722







