Implication of Echinochrome A in the Plasticity and Damage of Intestinal Epithelium
Abstract
1. Introduction
2. Results
2.1. The Effect of Ech A on Mouse Intestinal Epithelial Homeostasis
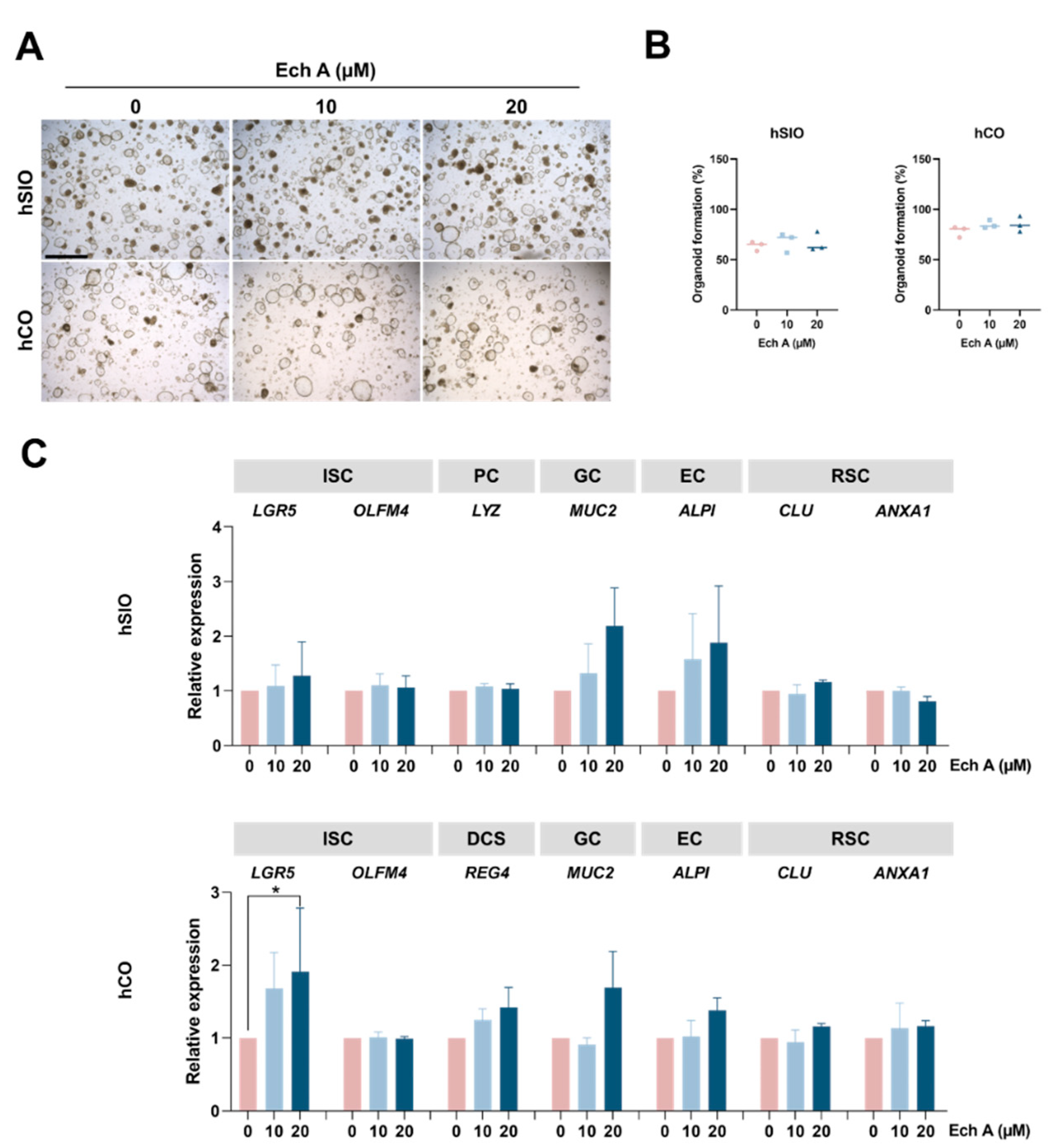
2.2. The Effect of Ech A on Human Epithelial Homeostasis
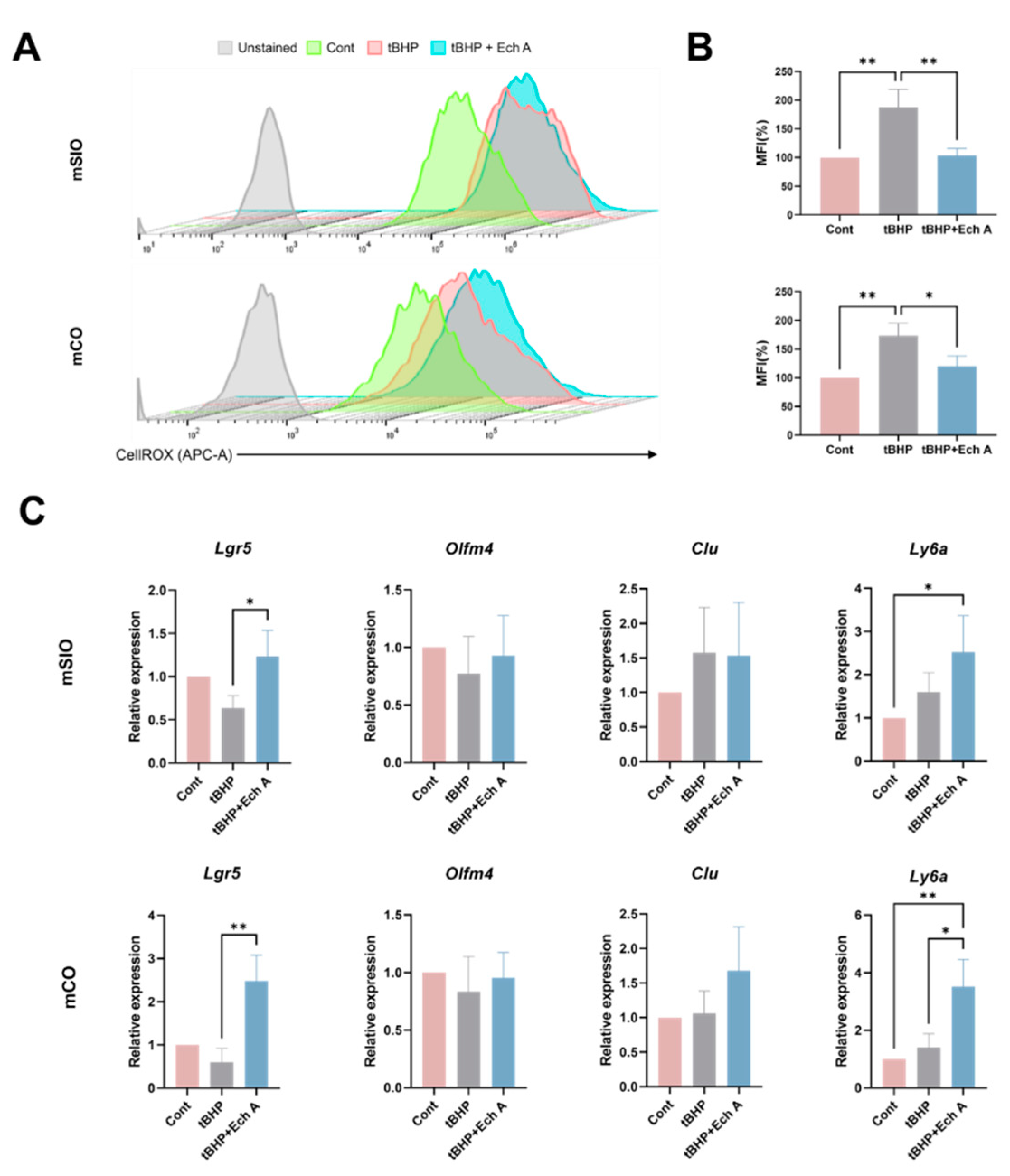
2.3. Antioxidative Ability of Ech A in Mouse Intestinal Organoids
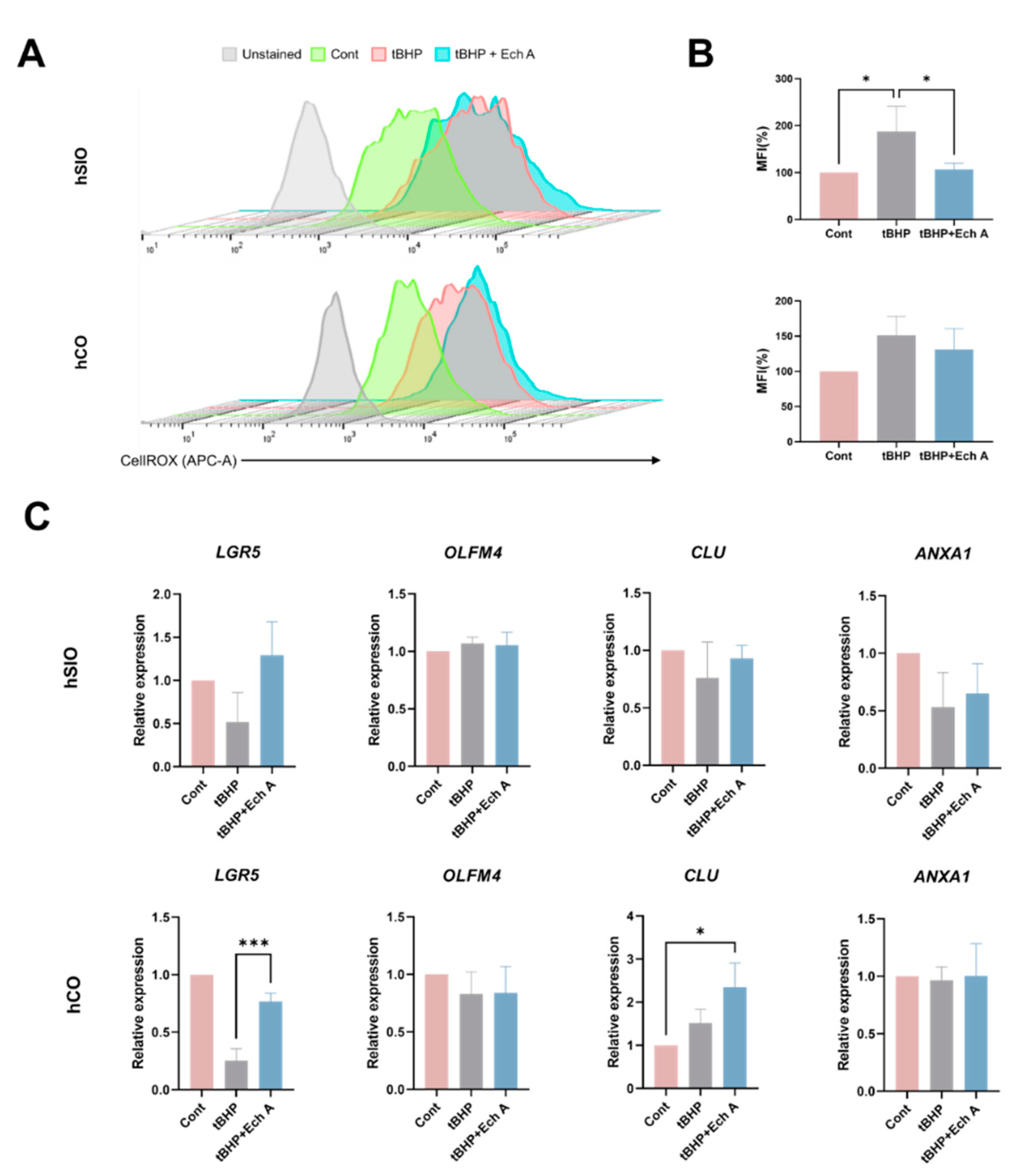
2.4. Antioxidative Ability of Ech A in Human Intestinal Organoids
3. Discussion
4. Materials and Methods
4.1. Preparation and Treatment of Ech A
4.2. Mouse Intestinal Organoid Culture
4.3. Human Intestinal Organoid Culture
4.4. Reverse Transcription and Quantitative Real-Time PCR (qPCR)
4.5. Flow Cytometric Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lebedev, A.V.; Ivanova, M.V.; Levitsky, D.O. Echinochrome, a naturally occurring iron chelator and free radical scavenger in artificial and natural membrane systems. Life Sci. 2005, 76, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Prokopov, I.A.; Kovaleva, E.L.; Minaeva, E.D.; Pryakhina, E.A.; Savin, E.V.; Gamayunova, A.V.; Pozharitskaya, O.N.; Makarov, V.G.; Shikov, A.N. Animal-derived medicinal products in Russia: Current nomenclature and specific aspects of quality control. J. Ethnopharmacol. 2019, 240, 111933. [Google Scholar] [CrossRef] [PubMed]
- Artyukov, A.A.; Zelepuga, E.A.; Bogdanovich, L.N.; Lupach, N.M.; Novikov, V.L.; Rutckova, T.A.; Kozlovskaya, E.P. Marine polyhydroxynaphthoquinone, Echinochrome A: Prevention of atherosclerotic inflammation and probable molecular targets. J. Clin. Med. 2020, 9, 1494. [Google Scholar] [CrossRef] [PubMed]
- Egorov, E.; Alekhina, V.; Volobueva, T.; Fedoreev, S.; Mishchenko, N.; Kol’tsova, E. Histochrome, a new antioxidant, in the treatment of ocular diseases. Vestn. Oftalmol. 1999, 115, 34–35. [Google Scholar] [PubMed]
- Zakirova, A.; Lebedev, A.; Kukharchuk, V.; Mishchenko, N.; Fedoreev, S. The antioxidant histochrome: Its effect on lipid peroxidation and the blood rheological properties in patients with unstable stenocardia. Ter. Arkh. 1996, 68, 12–14. [Google Scholar]
- Kim, H.K.; Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A.; Han, J. Multifaceted clinical effects of echinochrome. Mar. Drugs 2021, 19, 412. [Google Scholar] [CrossRef]
- Mischenko, N.; Fedoreev, S.; Zapara, T.; Ratushnyak, A. Effects of histochrom and emoxypin on biophysical properties of electroexitable cells. Bull. Exp. Biol. Med. 2009, 147, 196–200. [Google Scholar] [CrossRef]
- Lennikov, A.; Kitaichi, N.; Noda, K.; Mizuuchi, K.; Ando, R.; Dong, Z.; Fukuhara, J.; Kinoshita, S.; Namba, K.; Ohno, S.; et al. Amelioration of endotoxin-induced uveitis treated with the sea urchin pigment echinochrome in rats. Mol. Vis. 2014, 20, 171. [Google Scholar]
- Yun, H.R.; Ahn, S.W.; Seol, B.; Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Han, J.; Ko, K.S.; Rhee, B.D.; et al. Echinochrome A treatment alleviates atopic dermatitis-like skin lesions in NC/Nga mice via IL-4 and IL-13 suppression. Mar. Drugs 2021, 19, 622. [Google Scholar] [CrossRef]
- Oh, S.-J.; Seo, Y.; Ahn, J.-S.; Shin, Y.Y.; Yang, J.W.; Kim, H.K.; Han, J.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; et al. Echinochrome A reduces colitis in mice and induces in vitro generation of regulatory immune cells. Mar. Drugs 2019, 17, 622. [Google Scholar] [CrossRef]
- Fedoreyev, S.A.; Krylova, N.V.; Mishchenko, N.P.; Vasileva, E.A.; Pislyagin, E.A.; Iunikhina, O.V.; Lavrov, V.F.; Svitich, O.A.; Ebralidze, L.K.; Leonova, G.N. Antiviral and antioxidant properties of echinochrome A. Mar. Drugs 2018, 16, 509. [Google Scholar] [CrossRef] [PubMed]
- Sayed, D.A.; Soliman, A.M.; Fahmy, S.R. Echinochrome pigment as novel therapeutic agent against experimentally-induced gastric ulcer in rats. Biomed. Pharmacother. 2018, 107, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Park, G.-T.; Yoon, J.-W.; Yoo, S.-B.; Song, Y.-C.; Song, P.; Kim, H.-K.; Han, J.; Bae, S.-J.; Ha, K.-T.; Mishchenko, N.P.; et al. Echinochrome A treatment alleviates fibrosis and inflammation in bleomycin-induced scleroderma. Mar. Drugs 2021, 19, 237. [Google Scholar] [CrossRef]
- Shikov, A.N.; Pozharitskaya, O.N.; Krishtopina, A.S.; Makarov, V.G. Naphthoquinone pigments from sea urchins: Chemistry and pharmacology. Phytochem. Rev. 2018, 17, 509–534. [Google Scholar] [CrossRef]
- Shvilkin, A.; Serebriakov, L.; Tskitishvili, O.; Sadretdinov, S.; Kol’tsova, E.; Maksimov, O.; Mishchenko, N.; Novikov, V.; Levitskiĭ, D.; MIa, R. Effect of echinochrom on experimental myocardial reperfusion injury. Kardiologiia 1991, 31, 79–81. [Google Scholar] [PubMed]
- Buĭmov, G.; Maksimov, I.; Perchatkin, V.; Repin, A.; Afanas’ev, S.; Markov, V.; Karpov, R. Effect of the bioantioxidant histochrome on myocardial injury in reperfusion therapy on patients with myocardial infarction. Ter. Arkh. 2002, 74, 12–16. [Google Scholar]
- Gakhramanov, F.; Kerimov, K.; Dzhafarov, A. Use of natural antioxidants for the correction of changes in general and local parameters of lipid peroxidation and antioxidant defense system during experimental eye burn. Bull. Exp. Biol. Med. 2006, 142, 696–699. [Google Scholar] [CrossRef]
- Petrova, N.; Rascheskov, A.Y.; Bolgova, L.; Habibullina, N. Effectiveness of 0.02% pentahydroxyethylnaphtoquinone (hystochrome) in patients with active and fibrous stages of retinopathy of prematurity. Kazan Med. J. 2012, 93, 978–981. [Google Scholar] [CrossRef]
- Seol, J.E.; Ahn, S.W.; Seol, B.; Yun, H.R.; Park, N.; Kim, H.K.; Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A. Echinochrome A Protects against Ultraviolet B-induced Photoaging by Lowering Collagen Degradation and Inflammatory Cell Infiltration in Hairless Mice. Mar. Drugs 2021, 19, 550. [Google Scholar] [CrossRef]
- Seo, D.Y.; McGregor, R.A.; Noh, S.J.; Choi, S.J.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Han, J. Echinochrome A improves exercise capacity during short-term endurance training in rats. Mar. Drugs 2015, 13, 5722–5731. [Google Scholar] [CrossRef]
- Fahmy, S.R.; Sayed, D.A.; Soliman, A.M.; Almortada, N.Y.; Aal, W.E. Protective effect of Echinochrome against intrahepatic cholestasis induced by alpha-naphthylisothiocyanate in rats. Braz. J. Biol. 2019, 80, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, M.; Lebed’Ko, O.; Ryzhavskii, B.; Mishchenko, N. Effect of oral administration of echinochrome on lipopolysaccharide-induced lung injury in the immature Wistar rats. Eur. Respir. J. 2019, 54, PA2360. [Google Scholar]
- Sato, T.; Vries, R.G.; Snippert, H.J.; Van De Wetering, M.; Barker, N.; Stange, D.E.; Van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Stelzner, M.; Helmrath, M.; Dunn, J.C.; Henning, S.J.; Houchen, C.W.; Kuo, C.; Lynch, J.; Li, L.; Magness, S.T.; Martin, M.G. A nomenclature for intestinal in vitro cultures. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1359–G1363. [Google Scholar] [CrossRef]
- Seo, Y.; Oh, S.-J.; Ahn, J.-S.; Shin, Y.Y.; Yang, J.W.; Kim, H.-S. Implication of Porphyromonas gingivalis in colitis and homeostasis of intestinal epithelium. Lab. Anim. Res. 2019, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Regmi, S.; Seo, Y.; Ahn, J.-S.; Pathak, S.; Acharya, S.; Nguyen, T.T.; Yook, S.; Sung, J.-H.; Park, J.-B.; Kim, J.O.; et al. Heterospheroid formation improves therapeutic efficacy of mesenchymal stem cells in murine colitis through immunomodulation and epithelial regeneration. Biomaterials 2021, 271, 120752. [Google Scholar] [CrossRef] [PubMed]
- Zachos, N.C.; Kovbasnjuk, O.; Foulke-Abel, J.; In, J.; Blutt, S.E.; De Jonge, H.R.; Estes, M.K.; Donowitz, M. Human enteroids/colonoids and intestinal organoids functionally recapitulate normal intestinal physiology and pathophysiology. J. Biol. Chem. 2016, 291, 3759–3766. [Google Scholar] [CrossRef]
- Zhou, J.; Li, C.; Liu, X.; Chiu, M.C.; Zhao, X.; Wang, D.; Wei, Y.; Lee, A.; Zhang, A.J.; Chu, H. Infection of bat and human intestinal organoids by SARS-CoV-2. Nat. Med. 2020, 26, 1077–1083. [Google Scholar] [CrossRef]
- Fujii, M.; Clevers, H.; Sato, T. Modeling human digestive diseases with CRISPR-Cas9–modified organoids. Gastroenterology 2019, 156, 562–576. [Google Scholar] [CrossRef]
- Dutta, D.; Heo, I.; Clevers, H. Disease modeling in stem cell-derived 3D organoid systems. Trends Mol. Med. 2017, 23, 393–410. [Google Scholar] [CrossRef]
- Watanabe, S.; Kobayashi, S.; Ogasawara, N.; Okamoto, R.; Nakamura, T.; Watanabe, M.; Jensen, K.B.; Yui, S. Transplantation of intestinal organoids into a mouse model of colitis. Nat. Protoc. 2022, 17, 649–671. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.; Vilar, E.; Tsai, S.-Y.; Chang, K.; Amin, S.; Srinivasan, T.; Zhang, T.; Pipalia, N.H.; Chen, H.J.; Witherspoon, M. Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat. Med. 2017, 23, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Ayyaz, A.; Kumar, S.; Sangiorgi, B.; Ghoshal, B.; Gosio, J.; Ouladan, S.; Fink, M.; Barutcu, S.; Trcka, D.; Shen, J.; et al. Single-cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature 2019, 569, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Yui, S.; Azzolin, L.; Maimets, M.; Pedersen, M.T.; Fordham, R.P.; Hansen, S.L.; Larsen, H.L.; Guiu, J.; Alves, M.R.P.; Rundsten, C.F.; et al. YAP/TAZ-Dependent Reprogramming of Colonic Epithelium Links ECM Remodeling to Tissue Regeneration. Cell Stem Cell 2018, 22, 35–49.e37. [Google Scholar] [CrossRef]
- Qu, M.; Xiong, L.; Lyu, Y.; Zhang, X.; Shen, J.; Guan, J.; Chai, P.; Lin, Z.; Nie, B.; Li, C.; et al. Establishment of intestinal organoid cultures modeling injury-associated epithelial regeneration. Cell Res. 2021, 31, 259–271. [Google Scholar] [CrossRef]
- Workman, M.J.; Gleeson, J.P.; Troisi, E.J.; Estrada, H.Q.; Kerns, S.J.; Hinojosa, C.D.; Hamilton, G.A.; Targan, S.R.; Svendsen, C.N.; Barrett, R.J. Enhanced utilization of induced pluripotent stem cell–derived human intestinal organoids using microengineered chips. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 669–677.e662. [Google Scholar] [CrossRef]
- Yamashita, T.; Inui, T.; Yokota, J.; Kawakami, K.; Morinaga, G.; Takatani, M.; Hirayama, D.; Nomoto, R.; Ito, K.; Cui, Y. Monolayer platform using human biopsy-derived duodenal organoids for pharmaceutical research. Mol. Ther. Methods Clin. Dev. 2021, 22, 263–278. [Google Scholar] [CrossRef]
- Sun, H.; Chow, E.C.; Liu, S.; Du, Y.; Pang, K.S. The Caco-2 cell monolayer: Usefulness and limitations. Expert Opin. Drug Metab. Toxicol. 2008, 4, 395–411. [Google Scholar] [CrossRef]
- Artegiani, B.; Clevers, H. Use and application of 3D-organoid technology. Hum. Mol. Genet. 2018, 27, R99–R107. [Google Scholar] [CrossRef]
- Puschhof, J.; Pleguezuelos-Manzano, C.; Martinez-Silgado, A.; Akkerman, N.; Saftien, A.; Boot, C.; de Waal, A.; Beumer, J.; Dutta, D.; Heo, I. Intestinal organoid cocultures with microbes. Nat. Protoc. 2021, 16, 4633–4649. [Google Scholar] [CrossRef]
- Zietek, T.; Rath, E.; Haller, D.; Daniel, H. Intestinal organoids for assessing nutrient transport, sensing and incretin secretion. Sci. Rep. 2015, 5, 16831. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Kim, S.; Cho, S.-W. Gastrointestinal tract modeling using organoids engineered with cellular and microbiota niches. Exp. Mol. Med. 2020, 52, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; Van Es, J.H.; Kuipers, J.; Kujala, P.; Van Den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Kim, K.-H.; Yoo, B.-C.; Ku, J.-L. Induction of LGR5 by H2O2 treatment is associated with cell proliferation via the JNK signaling pathway in colon cancer cells. Int. J. Oncol. 2012, 41, 1744–1750. [Google Scholar] [CrossRef][Green Version]
- Myant, K.B.; Cammareri, P.; McGhee, E.J.; Ridgway, R.A.; Huels, D.J.; Cordero, J.B.; Schwitalla, S.; Kalna, G.; Ogg, E.-L.; Athineos, D. ROS production and NF-κB activation triggered by RAC1 facilitate WNT-driven intestinal stem cell proliferation and colorectal cancer initiation. Cell stem cell 2013, 12, 761–773. [Google Scholar] [CrossRef]
- Morris, O.; Jasper, H. Reactive Oxygen Species in intestinal stem cell metabolism, fate and function. Free Radic. Biol. Med. 2021, 166, 140–146. [Google Scholar] [CrossRef]
- Nath, A.; Chakrabarti, P.; Sen, S.; Barui, A. Reactive oxygen species in modulating intestinal stem cell dynamics and function. Stem Cell Rev. Rep. 2022, 18, 2328–2350. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Wu, A.; Yu, B.; Zhang, K.; Xu, Z.; Wu, D.; He, J.; Luo, J.; Luo, Y.; Yu, J.; Zheng, P. Transmissible gastroenteritis virus targets Paneth cells to inhibit the self-renewal and differentiation of Lgr5 intestinal stem cells via Notch signaling. Cell Death Dis. 2020, 11, 40. [Google Scholar] [CrossRef]
- Beumer, J.; Clevers, H. Regulation and plasticity of intestinal stem cells during homeostasis and regeneration. Development 2016, 143, 3639–3649. [Google Scholar] [CrossRef]
- Wang, S.; Kai, L.; Zhu, L.; Xu, B.; Chen, N.; Valencak, T.G.; Wang, Y.; Shan, T. Cathelicidin-WA Protects Against LPS-Induced Gut Damage Through Enhancing Survival and Function of Intestinal Stem Cells. Front. Cell Dev. Biol. 2021, 9, 685363. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Ho, S.B. Intestinal goblet cells and mucins in health and disease: Recent insights and progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.-B.; Pimentel-Nunes, P.; Roncon-Albuquerque, R.; Leite-Moreira, A. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hepatol. Int. 2010, 4, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cavallaro, P.M.; Kim, B.-M.; Liu, T.; Wang, H.; Kühn, F.; Adiliaghdam, F.; Liu, E.; Vasan, R.; Samarbafzadeh, E. A role for intestinal alkaline phosphatase in preventing liver fibrosis. Theranostics 2021, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Detel, D.; Baticic, L.; Varljen, J. The influence of age on intestinal dipeptidyl peptidase IV (DPP IV/CD26), disaccharidases, and alkaline phosphatase enzyme activity in C57BL/6 mice. Exp. Aging Res. 2007, 34, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Tuin, A.; Poelstra, K.; de Jager-Krikken, A.; Bok, L.; Raaben, W.; Velders, M.P.; Dijkstra, G. Role of alkaline phosphatase in colitis in man and rats. Gut 2009, 58, 379–387. [Google Scholar] [CrossRef]
- Ramasamy, S.; Nguyen, D.D.; Eston, M.A.; Nasrin Alam, S.; Moss, A.K.; Ebrahimi, F.; Biswas, B.; Mostafa, G.; Chen, K.T.; Kaliannan, K. Intestinal alkaline phosphatase has beneficial effects in mouse models of chronic colitis. Inflamm. Bowel Dis. 2011, 17, 532–542. [Google Scholar] [CrossRef]
- Van der Sluis, M.; De Koning, B.A.; De Bruijn, A.C.; Velcich, A.; Meijerink, J.P.; Van Goudoever, J.B.; Büller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef]
- Park, G.B.; Kim, M.J.; Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Han, J.; Lee, H.S.; Kim, D.; Jeong, J.Y. Echinochrome A Promotes Ex Vivo Expansion of Peripheral Blood-Derived CD34(+) Cells, Potentially through Downregulation of ROS Production and Activation of the Src-Lyn-p110delta Pathway. Mar. Drugs 2019, 17, 526. [Google Scholar] [CrossRef]
- Jang, J.; Jung, Y.; Chae, S.; Chung, S.I.; Kim, S.M.; Yoon, Y. WNT/beta-catenin pathway modulates the TNF-alpha-induced inflammatory response in bronchial epithelial cells. Biochem Biophys Res. Commun 2017, 484, 442–449. [Google Scholar] [CrossRef]
- Ma, B.; Hottiger, M.O. Crosstalk between Wnt/beta-Catenin and NF-kappaB Signaling Pathway during Inflammation. Front. Immunol. 2016, 7, 378. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ma, B.; Zhu, H.; Bai, J.; Liu, L.; Li, X.; Cai, J.; Wang, B.; Wang, L.; Pang, Y.; et al. PI3K/Akt and Wnt/beta-catenin Signaling Cross-regulate NF-kappaB Signaling in TNF-alpha-induced Human Lgr5(+) Intestinal Stem Cells. Anticancer Res. 2022, 42, 3325–3340. [Google Scholar] [CrossRef] [PubMed]
- El Homsi, M.; Ducroc, R.; Claustre, J.; Jourdan, G.; Gertler, A.; Estienne, M.; Bado, A.; Scoazec, J.Y.; Plaisancie, P. Leptin modulates the expression of secreted and membrane-associated mucins in colonic epithelial cells by targeting PKC, PI3K, and MAPK pathways. Am. J. Physiol. Gastrointest Liver Physiol. 2007, 293, G365–G373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; An, L.Y.; Hu, W.X.; Li, Z.Y.; Qiang, Y.Y.; Zhao, B.Y.; Han, T.S.; Wu, C.C. Mechanism of endometrial MUC2 in reproductive performance in mice through PI3K/AKT signaling pathway after lipopolysaccharide treatment. Ecotoxicol. Environ. Saf. 2022, 231, 113177. [Google Scholar] [CrossRef]
- Mishchenko, N.P.; Fedoreev, S.A.; Bagirova, V. Histochrome: A new original domestic drug. Pharm. Chem. J. 2003, 37, 48–52. [Google Scholar] [CrossRef]
- Talalaeva, O.; Mishchenko, N.; Briukhanov, V.; IaF, Z.; Lampatov, V.; Dvornikova, L. Identification of histochrome metabolism products in urine for studying drug pharmacokinetics. Eksperimental’naia I Klin. Farmakol. 2014, 77, 29–32. [Google Scholar]
- Sokolova, E.V.; Menzorova, N.I.; Davydova, V.N.; Kuz’mich, A.S.; Kravchenko, A.O.; Mishchenko, N.P.; Yermak, I.M. Effects of carrageenans on biological properties of echinochrome. Mar. Drugs 2018, 16, 419. [Google Scholar] [CrossRef]
- Yermak, I.M.; Gorbach, V.I.; Glazunov, V.P.; Kravchenko, A.O.; Mishchenko, N.P.; Pimenova, E.A.; Davydova, V.N. Liposomal form of the echinochrome-carrageenan complex. Mar. Drugs 2018, 16, 324. [Google Scholar] [CrossRef]

| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Mouse | ||
| Alpi | CTGCCAAGAAGCTGCAGCCCA | GGCTAGGGGTGTCTCCGGTCC |
| Clu | GCTGCTGATCTGGGACAATG | ACCTACTCCCTTGAGTGGACA |
| Gapdh | GGAAGGGCTCATGACCAC | GCAGGGATGATGTTCTGG |
| Lgr5 | GGGAGCGTTCACGGGCCTTC | GGTTGGCATCTAGGCGCAGGG |
| Lyz | CGTTGTGAGTTGGCCAGAA | GCTAAACACACCCAGTCAGC |
| Ly6a | GAAAGAGCTCAGGGACTGGAGTGTT | TTAGGAGGGCAGATGGGTAAGCAA |
| Muc2 | TGCCCAGAGAGTTTGGAGAG | CCTCACATGTGGTCTGGTTG |
| Olfm4 | ATTCGCTATGGCCAAGGAGG | GAGGGGCCGATTCACATCAA |
| Reg4 | AACCTGCCTGTGTGGATTGG | GTTCATCTCAGCGCAATGCC |
| Tff3 | TAATGCTGTTGGTGGTCCTG | CAGCCACGGTTGTTACACTG |
| Human | ||
| ALPI | TCCTGCCGTTGGACCTTCA | GGCCTGCTTGGTCTTCCTTA |
| ANXA1 | CGA AAC AAT GCA CAG CGT CA | TCA GTG TTT CAT CCA GGG GC |
| CLU | GTTGCTTTTGCACCTACGGG | GAGCAGCAGAGTCGAGTGTT |
| GAPDH | GTCTCCTCTGACTTCAACAGCG | ACCACCCTGTTGCTGTAGCCAA |
| LGR5 | GGAGTTACGTCTTGCGGGAA | CAGGCCACTGAAACAGCTTG |
| LYZ | GCCTAGCACTCTGACCTAGC | GTTCTGGCCAACTCACACCT |
| MUC2 | CAGCACCGATTGCTGAGTTG | GCTGGTCATCTCAATGGCAG |
| OLFM4 | TCAGCTCAACTGGAGAGGGT | GCCATAGGTGATCCGCAACT |
| REG4 | TGGTTGCCAAACAGAATGCC | GGCCAGTGCCAGAGATCTAA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, J.-S.; Shin, Y.Y.; Oh, S.-J.; Song, M.-H.; Kang, M.-J.; Park, S.Y.; Nguyen, P.T.; Nguyen, D.K.; Kim, H.K.; Han, J.; et al. Implication of Echinochrome A in the Plasticity and Damage of Intestinal Epithelium. Mar. Drugs 2022, 20, 715. https://doi.org/10.3390/md20110715
Ahn J-S, Shin YY, Oh S-J, Song M-H, Kang M-J, Park SY, Nguyen PT, Nguyen DK, Kim HK, Han J, et al. Implication of Echinochrome A in the Plasticity and Damage of Intestinal Epithelium. Marine Drugs. 2022; 20(11):715. https://doi.org/10.3390/md20110715
Chicago/Turabian StyleAhn, Ji-Su, Ye Young Shin, Su-Jeong Oh, Min-Hye Song, Min-Jung Kang, So Yeong Park, Phuong Thao Nguyen, Dang Khoa Nguyen, Hyoung Kyu Kim, Jin Han, and et al. 2022. "Implication of Echinochrome A in the Plasticity and Damage of Intestinal Epithelium" Marine Drugs 20, no. 11: 715. https://doi.org/10.3390/md20110715
APA StyleAhn, J.-S., Shin, Y. Y., Oh, S.-J., Song, M.-H., Kang, M.-J., Park, S. Y., Nguyen, P. T., Nguyen, D. K., Kim, H. K., Han, J., Vasileva, E. A., Mishchenko, N. P., Fedoreyev, S. A., Stonik, V. A., Seo, Y., Lee, B.-C., & Kim, H.-S. (2022). Implication of Echinochrome A in the Plasticity and Damage of Intestinal Epithelium. Marine Drugs, 20(11), 715. https://doi.org/10.3390/md20110715







