Laminaria japonica Peptides Suppress Liver Cancer by Inducing Apoptosis: Possible Signaling Pathways and Mechanism
Abstract
1. Introduction
2. Results
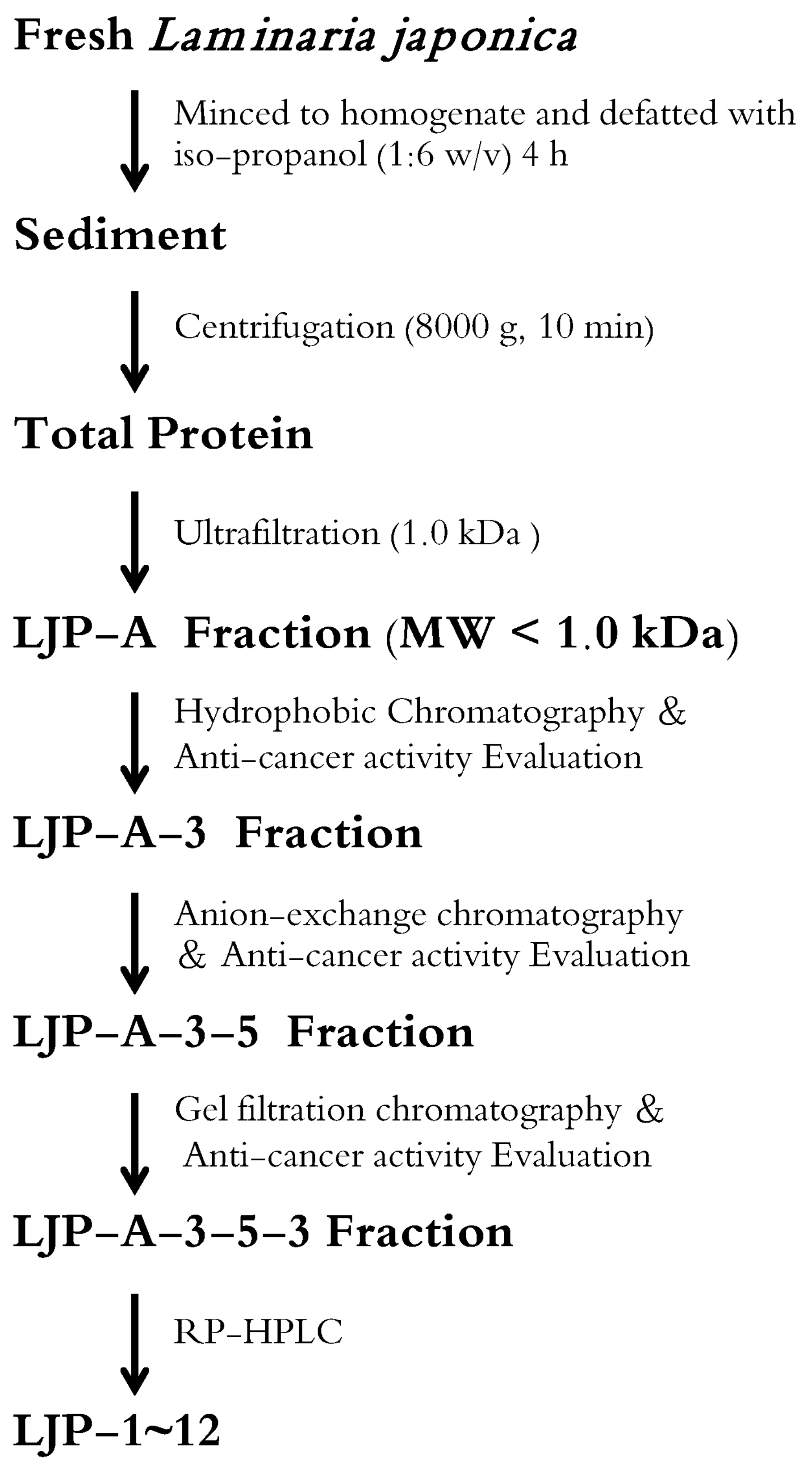
2.1. Isolation and Analysis of LJPs
2.2. LJPs Inhibited HCC Cell Growth In Vitro
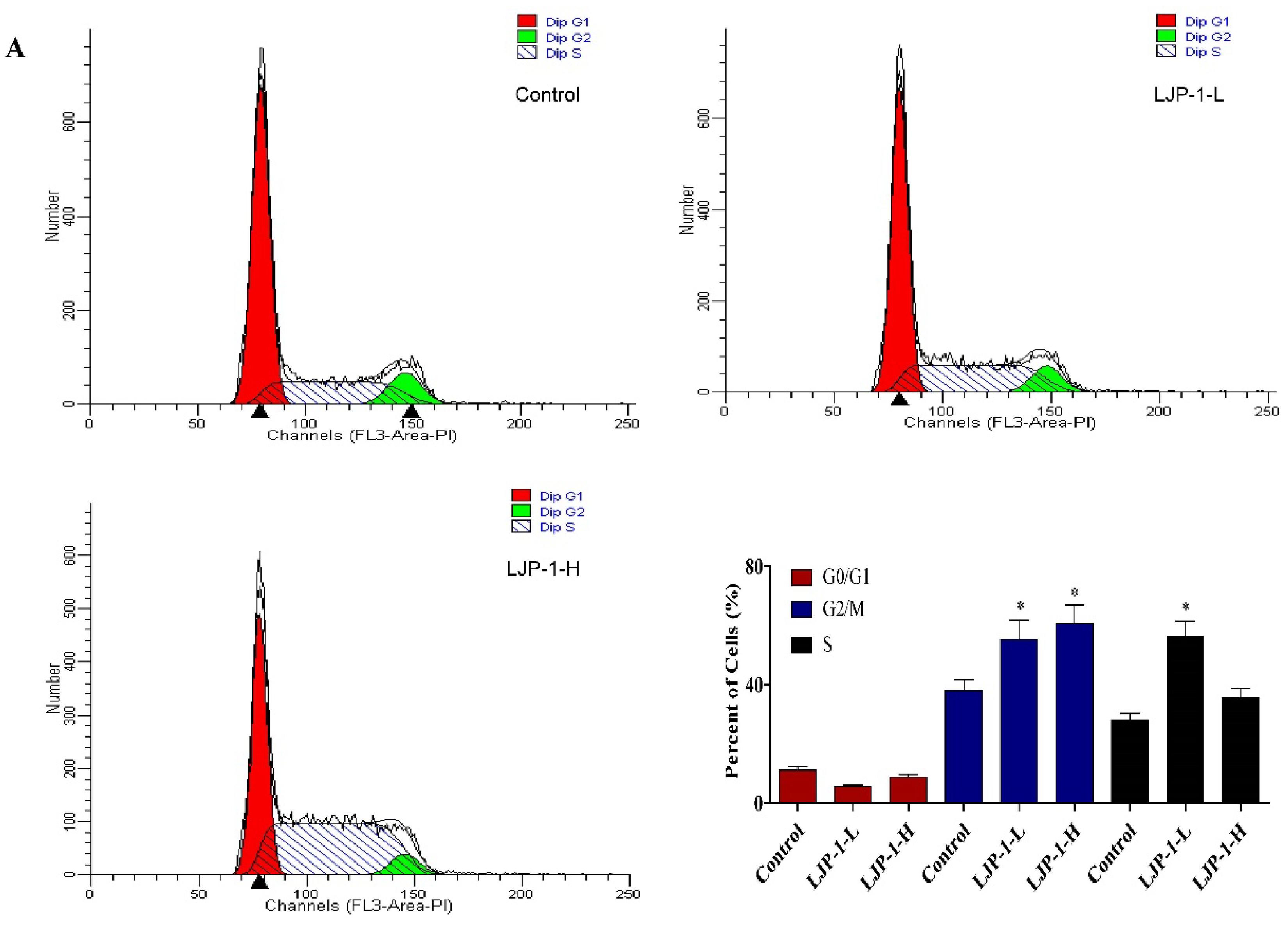
2.3. LJP-1 Induced Cell Cycle Arrest by Regulating Cyclin Expression
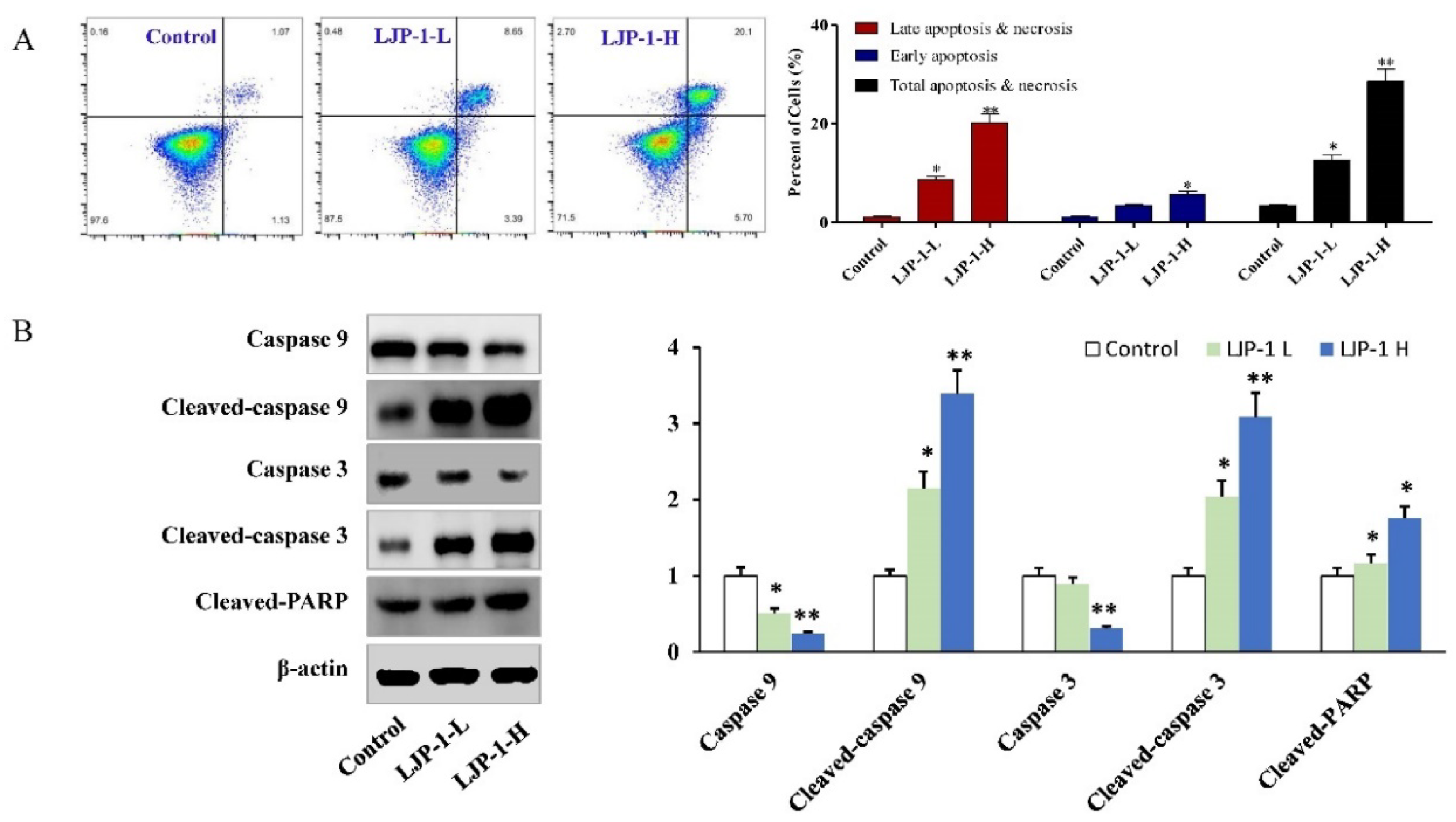
2.4. LJP-1 Induced Caspase-Dependent Apoptosis in H22 Cells
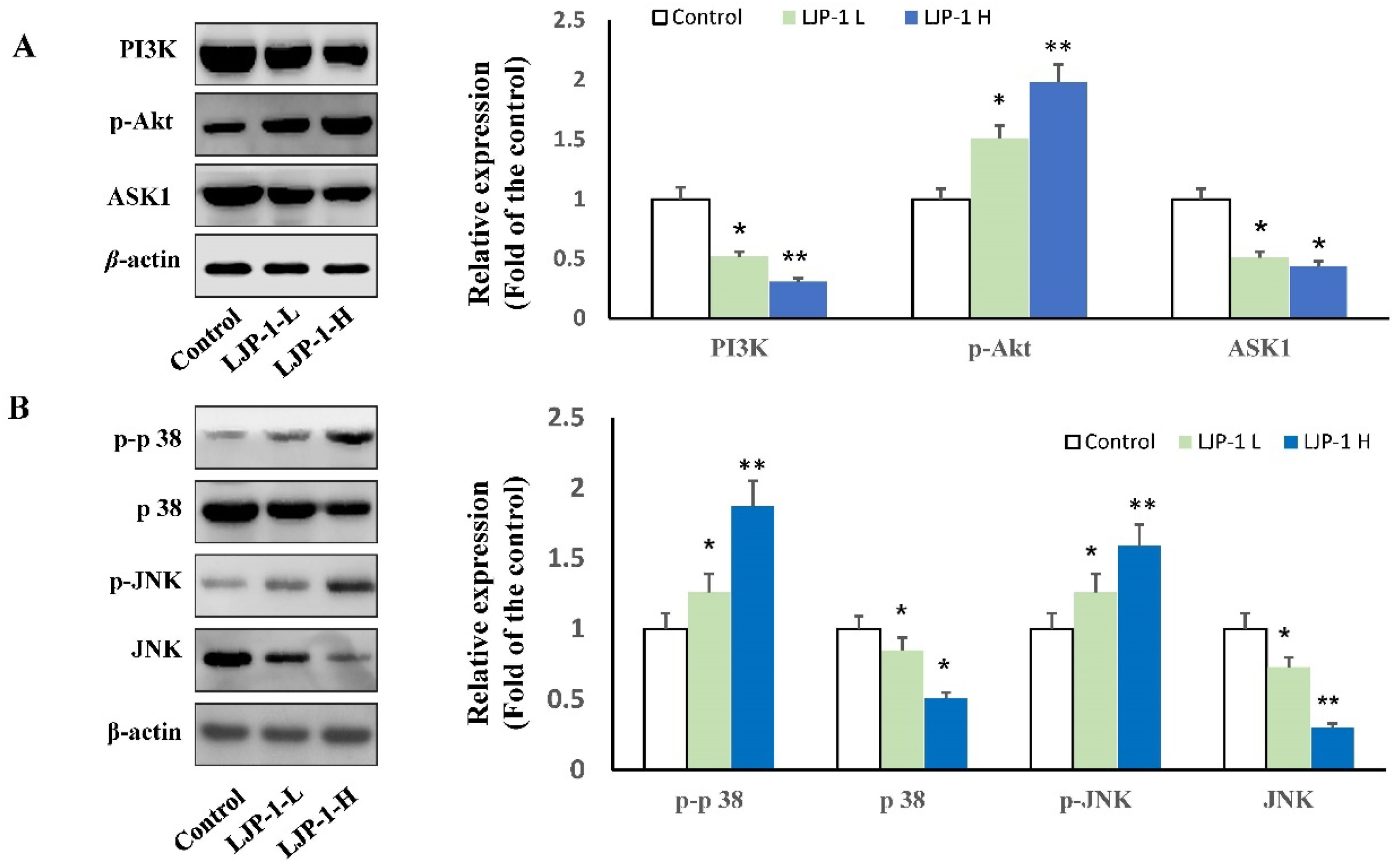
2.5. LJP-1 Induced Apoptosis of H22 Cells by Regulating p38-MAPK and PI3K/AKT Pathways
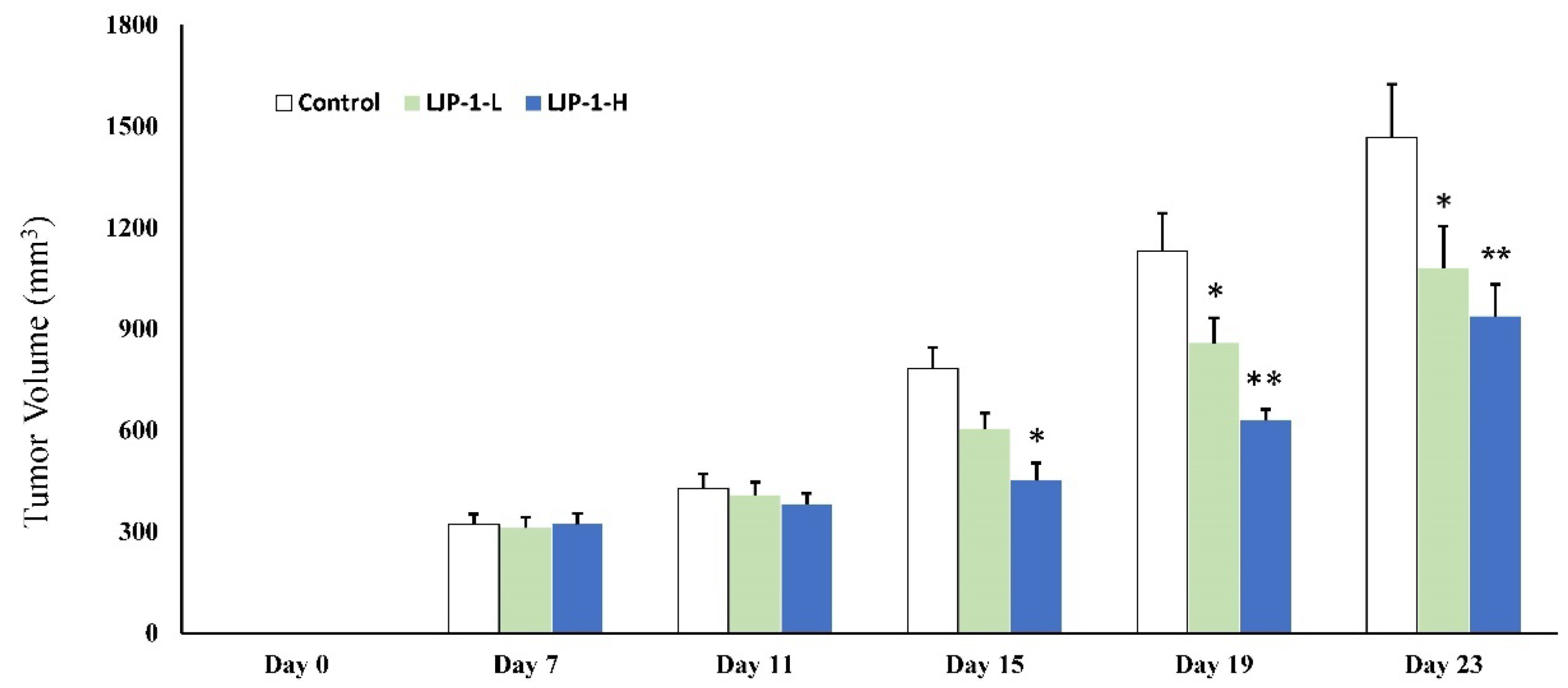
2.6. LJP-1 Inhibited Tumor Growth In Vivo
2.7. LJP-1 Induced Cell Apoptosis and Tumore Necrosis
3. Discussion
4. Materials and Methods
4.1. Materials and Cell Culture
4.2. Isolation and Amino Acid Sequence Analysis of LJPs
4.2.1. Hydrophobic Chromatography
4.2.2. Anion-Exchange Chromatography
4.2.3. Gel Filtration Chromatography
4.2.4. RP-HPLC and HPLC-ESI-MS Analysis
4.2.5. Summary of Key Steps
4.3. Cell Apoptosis and Cell Cycle Assays
4.4. Western Blot Analysis
4.5. Immunization of Liver-Cancer-bearing Mice
4.6. Evaluation of Tumor Apoptosis and Hematoxylin and Eosin (H&E) Staining
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wild, C.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention; IARC Press: Lyon, France, 2020. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Bank, T.; Malnassy, G.; Arteaga, M.; Shang, N.; Dalheim, A.; Ding, X.; Cotler, S.J.; Denning, M.F.; Nishimura, M.I.; et al. Inhibition of insulin-like growth factor 1 receptor enhances the efficacy of sorafenib in inhibiting hepatocellular carcinoma cell growth and survival. Hepatol. Commun. 2018, 2, 732–746. [Google Scholar] [CrossRef] [PubMed]
- Kaseb, A.O. Beyond the basics: The differential effects of demographics and hepatitis status on treatment outcome in hepatocellular carcinoma. Oncology 2013, 85, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Maduro, J.H.; Pras, E.; Willemse, P.H.B.; Vries, E.G.E.D. Acute and long-term toxicity following radiotherapy alone or in combination with chemotherapy for locally advanced cervical cancer. Cancer Treat. Rev. 2004, 29, 471–488. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, H.; Hu, M.; Huang, T.; Hu, Y.; Sang, N.; Zhao, Y. Recent progress in treatment of hepatocellular carcinoma. Am. J. Cancer Res. 2020, 10, 2993–3036. [Google Scholar]
- Rodrigues, T.; Sieglitz, F.; Bernardes, G.J.L. Natural product modulators of transient receptor potential (TRP) channels as potential anti-cancer agents. Chem. Soc. Rev. 2016, 45, 6130–6137. [Google Scholar] [CrossRef]
- Mabate, B.; Daub, C.D.; Malgas, S.; Edkins, A.L.; Pletschke, B.I. Fucoidan Structure and Its Impact on Glucose Metabolism: Implications for Diabetes and Cancer Therapy. Mar. Drugs 2021, 19, 30. [Google Scholar] [CrossRef]
- Jin, X.; Zhao, T.; Shi, D.; Ye, M.B.; Yi, Q. Protective role of fucoxanthin in diethylnitrosamine-induced hepatocarcinogenesis in experimental adult rats. Drug Dev. Res. 2019, 80, 209–217. [Google Scholar] [CrossRef]
- Duan, Y.; Li, J.; Jing, X.; Ding, X.; Yu, Y.; Zhao, Q. Fucoidan Induces Apoptosis and Inhibits Proliferation of Hepatocellular Carcinoma via the p38 MAPK/ERK and PI3K/Akt Signal Pathways. Cancer Manag. Res. 2020, 12, 1713–1723. [Google Scholar] [CrossRef]
- Du, X.; Xiao, S.; Luo, Q.; Liu, X.; Liu, J. Laminaria japonica cyclic peptides exert anti-colorectal carcinoma effects through apoptosis induction in vitro and in vivo. J. Pept. Sci. 2022, 28, e3385. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.K. Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.O.; Chauhan, P.S.; Arukha, A.P.; Chavda, V.; Dubey, A.; Yadav, D. The Therapeutic Potential of the Anticancer Activity of Fucoidan: Current Advances and Hurdles. Mar. Drugs 2021, 19, 265. [Google Scholar] [CrossRef]
- Hsu, W.J.; Lin, M.H.; Kuo, T.C.; Chou, C.M.; Mi, F.L.; Cheng, C.H.; Lin, C.W. Fucoidan from Laminaria japonica exerts antitumor effects on angiogenesis and micrometastasis in triple-negative breast cancer cells. Int. J. Biol. Macromol. 2020, 149, 600–608. [Google Scholar] [CrossRef]
- Lin, Y.; Qi, X.; Liu, H.; Xue, K.; Xu, S.; Tian, Z. The anti-cancer effects of fucoidan: A review of both in vivo and in vitro investigations. Cancer Cell Int. 2020, 20, 154. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Escrig, A.; Goni Cambrodon, I. Nutritional evaluation and physiological effects of edible seaweeds. Arch. Latinoam. Nutr. 1999, 49, 114–120. [Google Scholar] [PubMed]
- Oshima, E. Medicinal Uses of Seaweed in Traditional Chinese Medicine. In Traditional Chinese Medicine: Scientific Basis for Its Use; Royal Society of Chemistry, Thomas Graham House: Cambridge, UK, 2013; p. 238. [Google Scholar]
- Huang, H.; Fang, J.; Fan, X.; Miyata, T.; Hu, X.; Zhang, L.; Zhang, L.; Cui, Y.; Liu, Z.; Wu, X. Advances in Molecular Mechanisms for Traditional Chinese Medicine Actions in Regulating Tumor Immune Responses. Front. Pharmacol. 2020, 11, 1009. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.X.; Wang, Z.C.; Huang, Y.; Ohishi, H.; Wu, R.J.; Shao, Y.; Wang, H.; Qin, M.Y.; Wu, Z.L.; Li, Y.Y.; et al. Fucoxanthin extracted from Laminaria Japonica inhibits metastasis and enhances the sensitivity of lung cancer to Gefitinib. J. Ethnopharmacol. 2021, 265, 113302. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.K. Fucoidans from Marine Algae as Potential Matrix Metalloproteinase Inhibitors. Adv. Food Nutr. Res. 2014, 72, 177–193. [Google Scholar]
- Duarte, M.E.R.; Cardoso, M.A.; Noseda, M.D.; Cerezo, A.S. Structural studies on fucoidans from the brown seaweedSargassum stenophyllum. Carbohydr. Res. 2001, 333, 281–293. [Google Scholar] [CrossRef]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and Anti-Inflammatory Effects of Fucoidan: A Review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef]
- Go, H.; Hwang, H.J.; Nam, T.J. Glycoprotein extraction from Laminaria japonica promotes IEC-6 cell proliferation. Int. J. Mol. Med. 2009, 24, 819–824. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cho, D.-M.; Kim, D.-S.; Lee, D.-S.; Kim, H.-R.; Pyeun, J.-H. Trace components and functional saccharides in seaweed-1-changes in proximate composition and trace elements according to the harvest season and places. Korean J. Fish. Aquat. Sci. 1995, 28, 49–59. [Google Scholar]
- Han, M.H.; Kim, G.Y.; Moon, S.K.; Kim, W.J.; Nam, T.J.; Choi, Y.H. Apoptosis induction by glycoprotein isolated from Laminaria japonica is associated with down-regulation of telomerase activity and prostaglandin E2 synthesis in AGS human gastric cancer cells. Int. J. Oncol. 2011, 38, 577–584. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Go, H.; Hwang, H.J.; Nam, T.J. A glycoprotein from Laminaria japonica induces apoptosis in HT-29 colon cancer cells. Toxicol. Vitr. 2010, 24, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Frezza, C. The role of mitochondria in the oncogenic signal transduction. Int. J. Biochem. Cell Biol. 2014, 48, 11–17. [Google Scholar] [CrossRef]
- Malumbres, M.; Carnero, A. Cell cycle deregulation: A common motif in cancer. Prog. Cell Cycle Res. 2003, 5, 5–18. [Google Scholar]
- Scaglione-Sewell, B.A.; Bissonnette, M.; Skarosi, S.; Abraham, C.; Brasitus, T. A vitamin D3 analog induces a G1-phase arrest in CaCo2 Cells by inhibiting Cdk2 and Cdk6: Roles of cyclin E, p21(Waf1), and p27(Kip1). Endocrinology 2000, 141, 3931–3939. [Google Scholar] [CrossRef]
- Hall, M.; Bates, S.; Peters, G. Evidence for different modes of action of cyclin-dependent kinase inhibitors: p15 and p16 bind to kinases, p21 and p27 bind to cyclins. Oncogene 1995, 11, 1581–1588. [Google Scholar]
- Estaquier, J.; Vallette, F.; Vayssiere, J.L.; Mignotte, B. The mitochondrial pathways of apoptosis. Adv. Exp. Med. Biol. 2012, 942, 157–183. [Google Scholar] [CrossRef]
- Choudhary, G.S.; Al-Harbi, S.; Almasan, A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. In Apoptosis and Cancer; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–9. [Google Scholar] [CrossRef]
- Kong, A.N.; Yu, R.; Chen, C.; Mandlekar, S.; Primiano, T. Signal transduction events elicited by natural products: Role of MAPK and caspase pathways in homeostatic response and induction of apoptosis. Arch. Pharm. Res. 2000, 23, 1–16. [Google Scholar] [CrossRef]
- Noorolyai, S.; Shajari, N.; Baghbani, E.; Sadreddini, S.; Baradaran, B. The relation between PI3K/AKT signalling pathway and cancer. Gene 2019, 698, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, H.O.; Huang, H.; Tsung, A. Autophagy: Dual Response in the Development of Hepatocellular Carcinoma. Cells 2019, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.Y.; Kim, L.E.; Jeong, H.; Yeo, B.K.; Lee, J.-W.; Nam, H.; Ha, S.; An, H.-K.; Park, H.; Jung, S. GSK3B induces autophagy by phosphorylating ULK1. Exp. Mol. Med. 2021, 53, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Chao, X.; Williams, J.; Fulte, S.; Li, T.; Yang, L.; Ding, W.X. Autophagy in liver diseases: A review. Mol. Asp. Med. 2021, 82, 100973. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pras, E.; Fernandez-Iglesias, A.; Gracia-Sancho, J.; Perez-Del-Pulgar, S. Cell Death in Hepatocellular Carcinoma: Pathogenesis and Therapeutic Opportunities. Cancers 2021, 14, 48. [Google Scholar] [CrossRef]
- Sun, E.J.; Wankell, M.; Palamuthusingam, P.; McFarlane, C.; Hebbard, L. Targeting the PI3K/Akt/mTOR Pathway in Hepatocellular Carcinoma. Biomedicines 2021, 9, 1639. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Zhou, C.; Mei, W.; Zeng, C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front. Oncol. 2022, 12, 819128. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef]
- Stec, J.; Szczotka, M.; Kuźmak, J. Cytotoxicity of feed-borne mycotoxins to animal cell lines in vitro using the MTT assay. Bull. Vet. Inst. Pulawy 2007, 51, 679–684. [Google Scholar]
- Liu, J.; Zhou, F.; Zhang, L.; Wang, H.; Chen, H. DMXAA-pyranoxanthone hybrids enhance inhibition activities against human cancer cells with multi-target functions. Eur. J. Med. Chem. 2017, 143, 1768. [Google Scholar] [CrossRef]
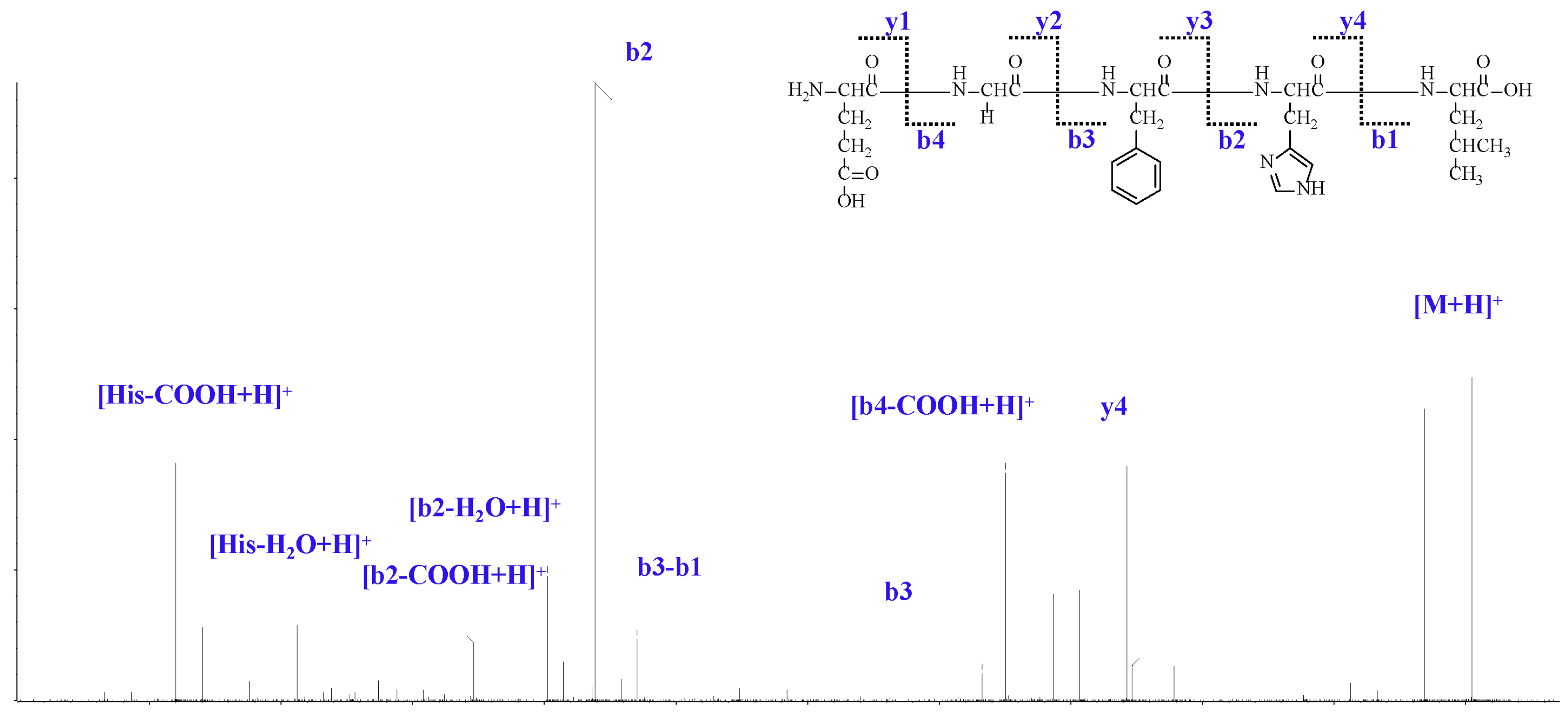


| Comp. | Amino Acid Sequence | IC50 (mM) | ||
|---|---|---|---|---|
| HuH7 | HepG2 | H22 | ||
| LJP-1 | EGFHL | 0.48 ± 0.05 | 0.45 ± 0.05 | 0.36 ± 0.03 |
| LJP-2 | LWEHSH | 2.25 ± 0.22 | 1.71 ± 0.13 | 0.62 ± 0.05 |
| LJP-3 | FSHRGH | 1.42 ± 0.12 | >4.0 | 1.70 ± 0.15 |
| LJP-4 | EGHGF | 0.62 ± 0.07 | 0.65 ± 0.07 | 0.68 ± 0.07 |
| LJP-5 | FSTHGG | 2.44 ± 0.22 | 2.78 ± 0.26 | 1.49 ± 0.13 |
| LJP-6 | FKEHGY | >4.0 | 3.06 ± 0.31 | >4.0 |
| LJP-7 | HAGYSWA | 2.53 ± 0.24 | 1.66 ± 0.11 | 1.44 ± 0.12 |
| LJP-8 | FSHTYV | 0.44 ± 0.04 | 0.63 ± 0.05 | 0.76 ± 0.08 |
| LJP-9 | FEHSG | 0.53 ± 0.05 | 0.69 ± 0.08 | 0.51 ± 0.07 |
| LJP-10 | HASWEH | 3.52 ± 0.35 | 2.69 ± 0.22 | 3.53 ± 0.34 |
| LJP-11 | YEHSHG | 0.49 ± 0.05 | 0.61 ± 0.07 | 0.70 ± 0.07 |
| LJP-12 | TFKHG | 0.41 ± 0.04 | 0.60 ± 0.08 | 0.72 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Li, Y.; Guo, W.; Liu, J.; Lao, W.; Hu, P.; Lin, Y.; Chen, H. Laminaria japonica Peptides Suppress Liver Cancer by Inducing Apoptosis: Possible Signaling Pathways and Mechanism. Mar. Drugs 2022, 20, 704. https://doi.org/10.3390/md20110704
Wu Y, Li Y, Guo W, Liu J, Lao W, Hu P, Lin Y, Chen H. Laminaria japonica Peptides Suppress Liver Cancer by Inducing Apoptosis: Possible Signaling Pathways and Mechanism. Marine Drugs. 2022; 20(11):704. https://doi.org/10.3390/md20110704
Chicago/Turabian StyleWu, Yingzi, Yuanhui Li, Wenhai Guo, Jie Liu, Weiguo Lao, Penghui Hu, Yiguang Lin, and Hongjie Chen. 2022. "Laminaria japonica Peptides Suppress Liver Cancer by Inducing Apoptosis: Possible Signaling Pathways and Mechanism" Marine Drugs 20, no. 11: 704. https://doi.org/10.3390/md20110704
APA StyleWu, Y., Li, Y., Guo, W., Liu, J., Lao, W., Hu, P., Lin, Y., & Chen, H. (2022). Laminaria japonica Peptides Suppress Liver Cancer by Inducing Apoptosis: Possible Signaling Pathways and Mechanism. Marine Drugs, 20(11), 704. https://doi.org/10.3390/md20110704







