Abstract
Two new cytotoxic twelve-membered macrolides, sporiolides A (1) and B (2), were isolated from the cultured broth of a fungus Cladosporium sp., which was separated from an Okinawan marine brown alga Actinotrichia fragilis, and the structures were elucidated by spectroscopic data. Sporiolides A (1) and B (2) exhibited cytotoxicity against murine lymphoma L1210 cells. Spoliolide A (1) showed antifungal activity against Cryptococcus neoformans and Neurospora crassa.
Introduction
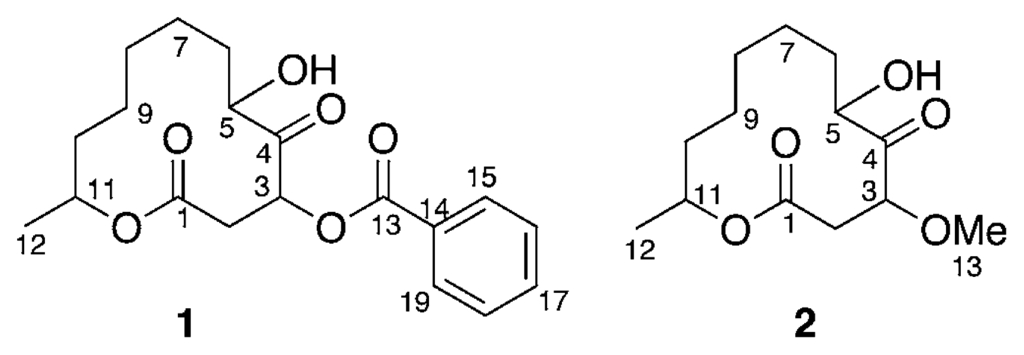
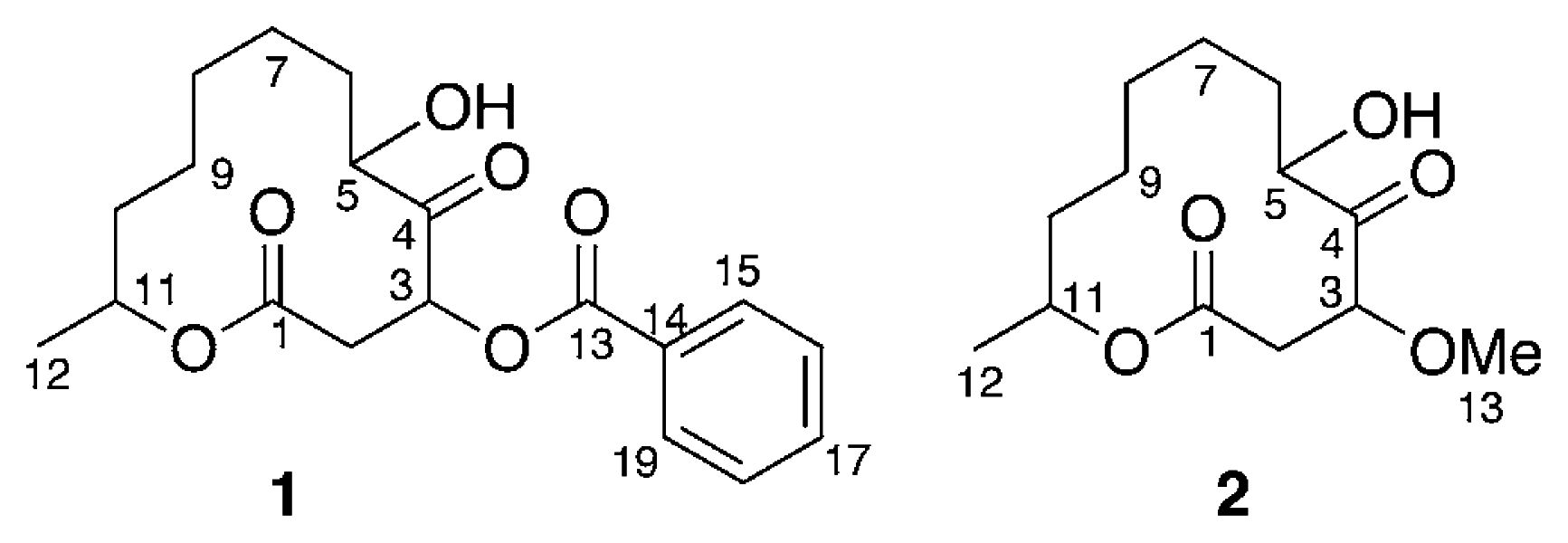
Marine microorganisms such as bacteria, fungi, and microalgae have proven to be a rich source of structurally novel and biologically active secondary metabolites [1]. In our search for new substances from marine microorganisms [2], two new cytotoxic twelve-membered macrolides, sporiolides A (1) and B (2), were isolated from the cultured broth of a fungus Cladosporium sp., which was separated from an Okinawan marine brown alga Actinotrichia fragilis. In this paper we describe the isolation and structure elucidation of 1 and 2.
Results and Discussion
The fungus Cladosporium sp. (L037) was separated from the brown alga collected off Seragaki Beach, Okinawa Island, and grown in SC broth [starch (1%) and casein (0.1%) in 50% sea water, pH 7.4] at 28°C for 14 days. The filtrate of the cultured broth (10 L) was extracted with EtOAc (1 L × 2). The EtOAc-soluble portions (58 mg) were subjected to a silica gel column (hexane/EtOAc, 70:30) followed by C18 reversed-phase HPLC (Develosil ODS-5, Nomura Chemical, 1.0 × 25 cm: flow rate 2.5 mL/min; UV detection at 254 nm; eluent: MeOH/H2O, 70:30) to give sporiolides A (1, 2.7 mg) and B (2, 11.5 mg) together with a known related macrolide, cladospolide D [3] (7.0 mg). On the other hand, other known compounds, cladospolide A [4–6] (5.0 mg), iso-cladospolide B [7] (2.0 mg), and seco-patulolide C [7] (3.0 mg), were isolated from the EtOAc extract of the mycelium.
Sporiolide A (1) {[α]D25 −14° (c 0.2, MeOH)} was obtained as colorless amorphous solid. The molecular weight of 1 was elucidated to be 348 Dalton on the basis of FABMS data that showed the pseudomolecular ion at m/z 371 (M+Na)+. The molecular formula, C19H24O6, of 1 was established by HRFABMS data [m/z 371.1483, (M+Na)+, Δ +1.2 mmu]. The IR spectrum suggested the presence of hydroxy (3426 cm−1), unsaturated ester and/or ketone carbonyl (1724 cm−1) groups. The UV absorptions at 237 (9200) and 209 (11700) nm indicated the presence of benzoyl chromophore. The 1H NMR (Table 1) spectrum of 1 showed proton signals due to a benzoyl group [δH 8.05 (2H, m), 7.56 (1H, m), and 7.43 (2H, m)].

Table 1.
1H and 13C NMR Data of Sporiolides A (1) and B (2) in CDCl3.
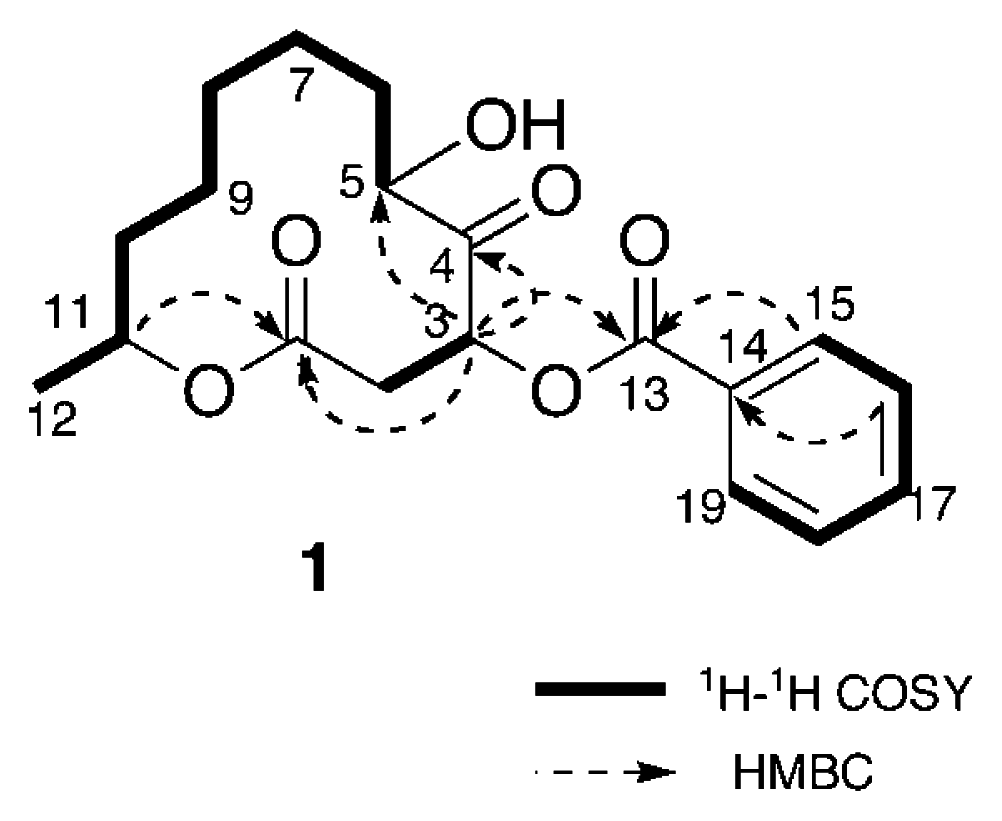
Analysis of the 1H-1H COSY spectrum (Figure 1) revealed connectivities of C-2 to C-3 and C-5 to C-12. HMBC correlations of H-3 (δH 5.90) to C-1 (δC 168.8), C-4 (δC 207.1), and C-5 (δC 76.0) and H-11 (δH 4.89) to C-1 indicated that 1 possessed a twelve-membered macrocyclic lactone with a ketone group at C-4 and a hydroxy at C-5. An HMBC correlation between H-3 to C-13 (δC 165.5) revealed that the benzoyl group was attached to C-3. Thus, the structure of sporiolide A was assigned as 1, which corresponded to be a 3-O-benzoyl form of pandangolide 1 [7].
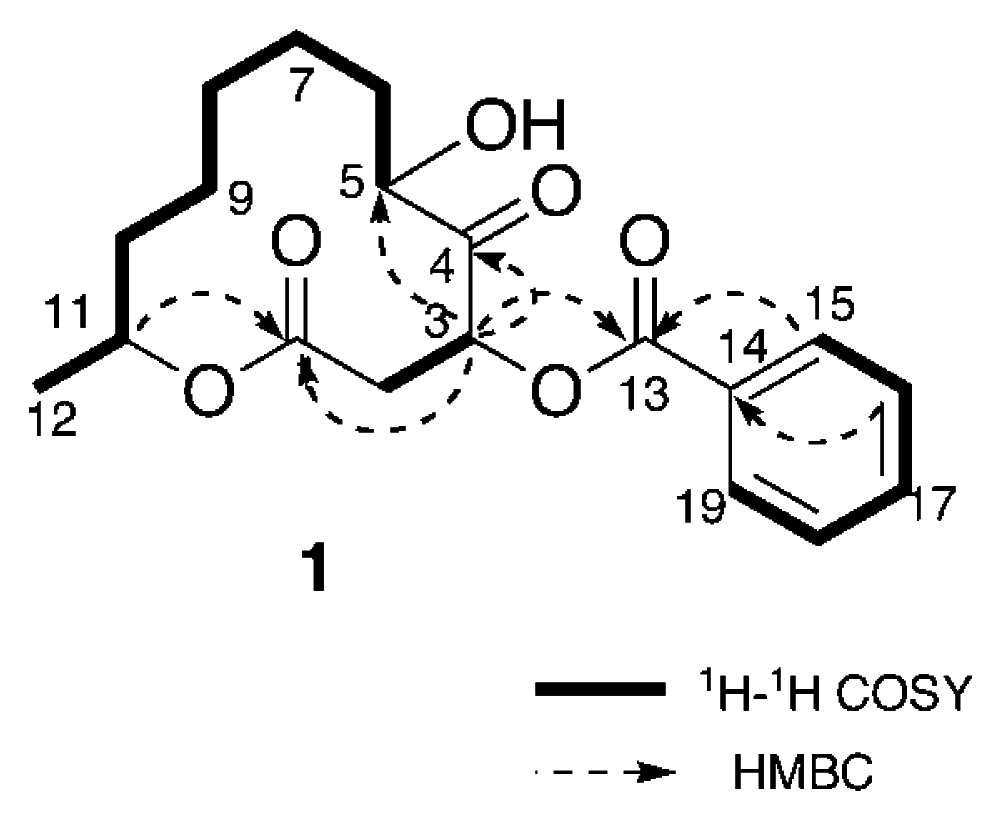
Figure 1.
Selected 2D NMR correlations for sporiolide A (1)
Sporiolide B (2) {[α]D25 −33° (c 0.3, MeOH)} was obtained as colorless amorphous solid. The molecular weight of 2 was elucidated by m/z 281(M+Na)+ in the positive mode FABMS. The molecular formula, C13H22O5, of 2 was established by HRFABMS data (m/z 281.1367 [M+Na]+, Δ+ 0.2mmu). The IR spectrum suggested the presence of hydroxy (3429 cm−1), unsaturated ester and/or ketone carbonyl (1710 cm−1) groups. Detailed analysis of 1H, 13C, and 2D NMR data revealed that the structure of 2 was similar to that of 1, except for functional group at C-3. An HMBC correlation of H-3 (δH 4.46) to C-13 (δC 58.2, MeO) indicated the presence of a methoxy group at C-3. Thus, the structure of sporiolide B (2) was elucidated to be a 3-O-methoxy form of pandangolide 1 [7].
Sporiolides A (1) and B (2) were new twelve-membered macrocyclic lactones from the cultured broth of a marine-derived fungus Cladosporium sp. [8], although similar twelve-membered macrocyclic lactone such as cladospolide A has been obtained from a terrestrial fungus Cladosporium sp. and more recently, cladospolide D [3], iso-cladospolide B, seco-patulolide C, and pandangolides 1 and 2 have been isolated from an unidentified marine fungus [6,7], while pandagolides 2 and 3 were isolated from a marine-derived fungus Cladosporium herbarum [9]. Sporiolides A (1) and B (2) exhibited cytotoxicity against murine lymphoma L1210 cells with IC50 values of 0.13 and 0.81 μg/mL, respectively. Sporiolide A (1) showed antifungal activity against Candida albicans, Cryptococcus neoformans, Aspergillus niger, and Neurospora crassa and antibacterial activity against Micrococcus luteus, while sporiolide B (2) had antibacterial activity against Micrococcus luteus (Table 2).

Table 2.
Antimicrobial Activity of Sporiolides A (1) and B (2).
Experimental
General
Optical rotations were measured on a JASCO DIP-1000 polarimeter. The IR and UV spectra were recorded on a JASCO FT/IR-5300 and a Shimadzu UV-1600PC spectrophotometer, respectively. CD spectra were measured on a JASCO J-720 spectropolarimeter. NMR spectra were recorded on a Bruker AMX-600 spectrometer. FAB mass spectrum was obtained on a JEOL HX-110 spectrometer using nitrobenzyl alcohol as a matrix.
Fungal Material and Fermentation
The fungus Cladosporium sp. (L037) was separated from the brown alga Actinotrichia fragilis, which was collected off Seragaki Beach at Okinawa Island. Subcultures of the organism are deposited at Graduate School of Pharmaceutical Sciences, Hokkaido University. The fungus was grown in SC broth [starch (1%) and casein (0.1%) in 50% sea water, pH 7.4] at 28°C for 14 days. The cultured broth (10 L) was filtered.
Extraction and Separation
The filtrate of the cultured broth (10 L) was extracted with EtOAc (1 L × 2). The EtOAc-soluble portions (58 mg) were subjected to a silica gel column (hexane/EtOAc, 70:30) followed by C18 reversed-phase HPLC [Develosil ODS-5, Nomura Chemical, 1.0 × 25 cm: flow rate 2.5 mL/min; UV detection at 254 nm; eluent: MeOH/H2O, 70:30] to give sporiolides A (1, 2.7 mg) and B (2, 11.5 mg) together with cladospolide D (7.0 mg). On the other hand, cladospolide A, isocladospolide B, and seco-patulolide C were isolated from the EtOAc extract of the mycelium.
Spectral Data
Sporiolide A (1): colorless amorphous solid; [α]D25 −14° (c 0.2, MeOH)}; UV (MeOH) λmax 237 (ɛ9200) and 209 (11700) nm; IR (KBr) νmax 3426, 1724, and 1633 cm−1; 1H and 13C NMR (Table 1); FABMS m/z 371 [M+Na]+; HRFABMS m/z 371.1483 [M+Na]+ (calcd for C19H24O6Na, 371.1471).
Sporiolide B (2): colorless amorphous solid; [α]D25 −33° (c 0.3, MeOH); IR (KBr) νmax 3429, 1710, and 1646 cm−1; 1H and 13C NMR (Table 1); FABMS m/z 281 [M+Na]+; HRFABMS m/z 281.1367 [M+Na]+ (calcd for C13H22O5Na, 281.1365).
Acknowledgments
This work was partly supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
- Sample Availability: Samples are available from the authors.
Reference and Notes
- Blunt, J. W.; Copp, B. R.; Munro, M. H. G.; Northcote, P. T.; Prinsep, P. R. Nat. Prod. Rep 2004, 21, 1–49, and references cited therein.
- Tsuda, M.; Mugishima, T. K.; Komatsu, T.; Sone, T.; Tanaka, M.; Mikami, Y.; Shiro, M.; Hirai, M.; Ohizumi, Y.; Kobayashi, J. Tetrahedron 2003, 59, 3227–3230, and references cited therein.
- Zhang, H.; Tomoda, H.; Tabata, N.; Miura, H.; Namikoshi, M.; Yamaguchi, Y.; Masuma, R.; Omura, S. J. Antibiot 2001, 54, 635–641.
- Hirota, A.; Sakai, H.; Isogai, A. Agric. Biol. Chem 1985, 43, 731–735.
- Hirota, A.; Sakai, H.; Isogai, A.; Kitano, Y.; Ashida, T.; Hirota, H.; Takahashi, T. Agric. Biol. Chem 1985, 49, 903–904.
- Hirota, H.; Hirota, A.; Sakai, H.; Isogai, A.; Takahashi, T. Bull. Chem. Soc. Jpn 1985, 58, 2147–2148.
- Smith, C. J.; Abbanat, D.; Bernan, V. S.; Maiese, W. M.; Greenstein, M.; Jompa, J.; Tahir, A.; Ireland, C. M. J. Nat. Prod 2000, 63, 142–145.
- Since sporiolides A (1) and B (2) were unstable and were not obtained from the extracts of recultivation, stereochemistry of 1 and 2 was not determined.
- Jadulco, R.; Proksch, P.; Wray, V.; Sudarsono Berg, A.; Gräfe, U. J. Nat. Prod 2001, 64, 527–530.
© 2004 by MDPI Reproduction is permitted for noncommercial purposes.