Precursor-Directed Biosynthesis Mediated Amplification of Minor Aza Phenylpropanoid Piperazines in an Australian Marine Fish-Gut-Derived Fungus, Chrysosporium sp. CMB-F214
Abstract
:1. Introduction
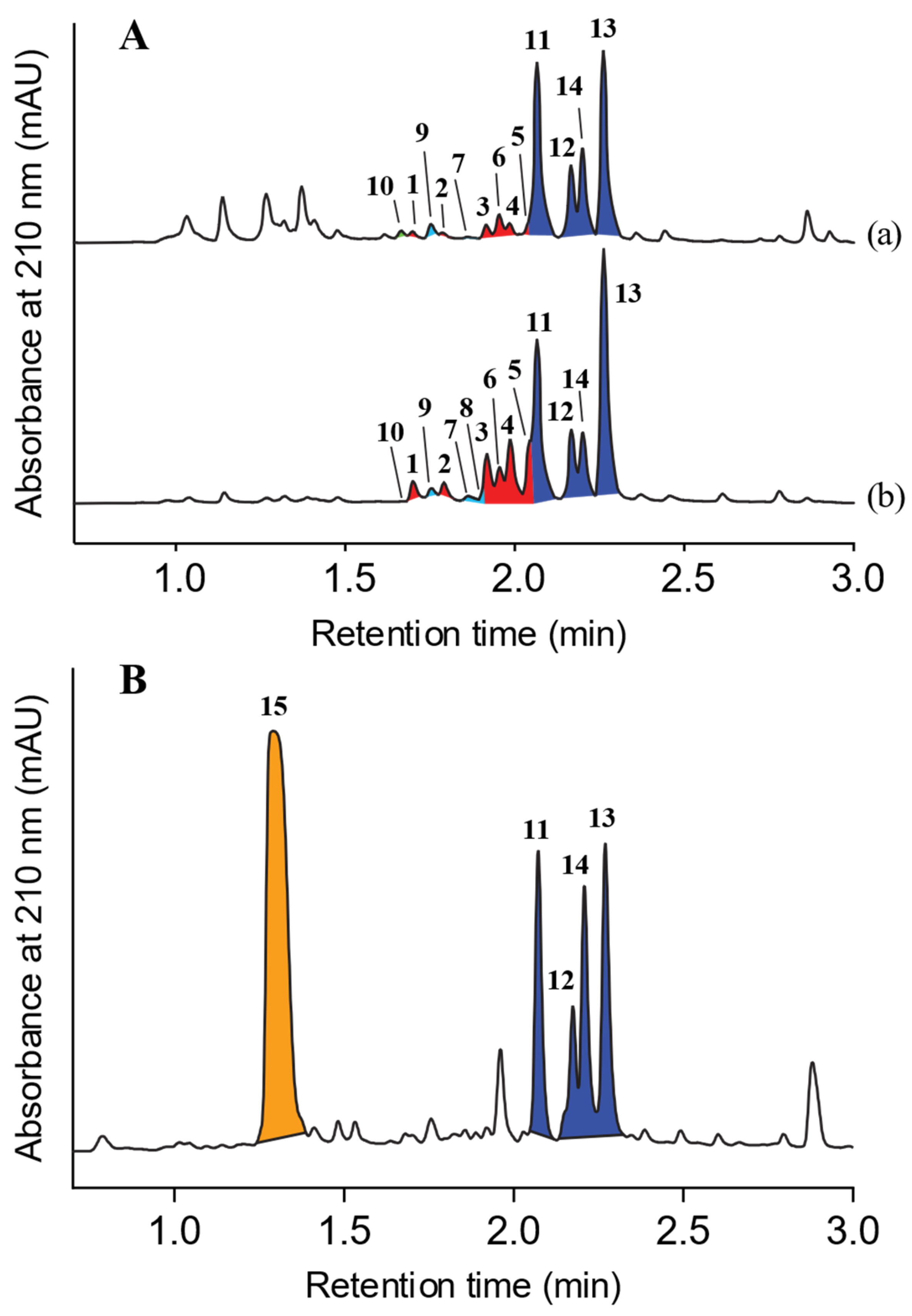
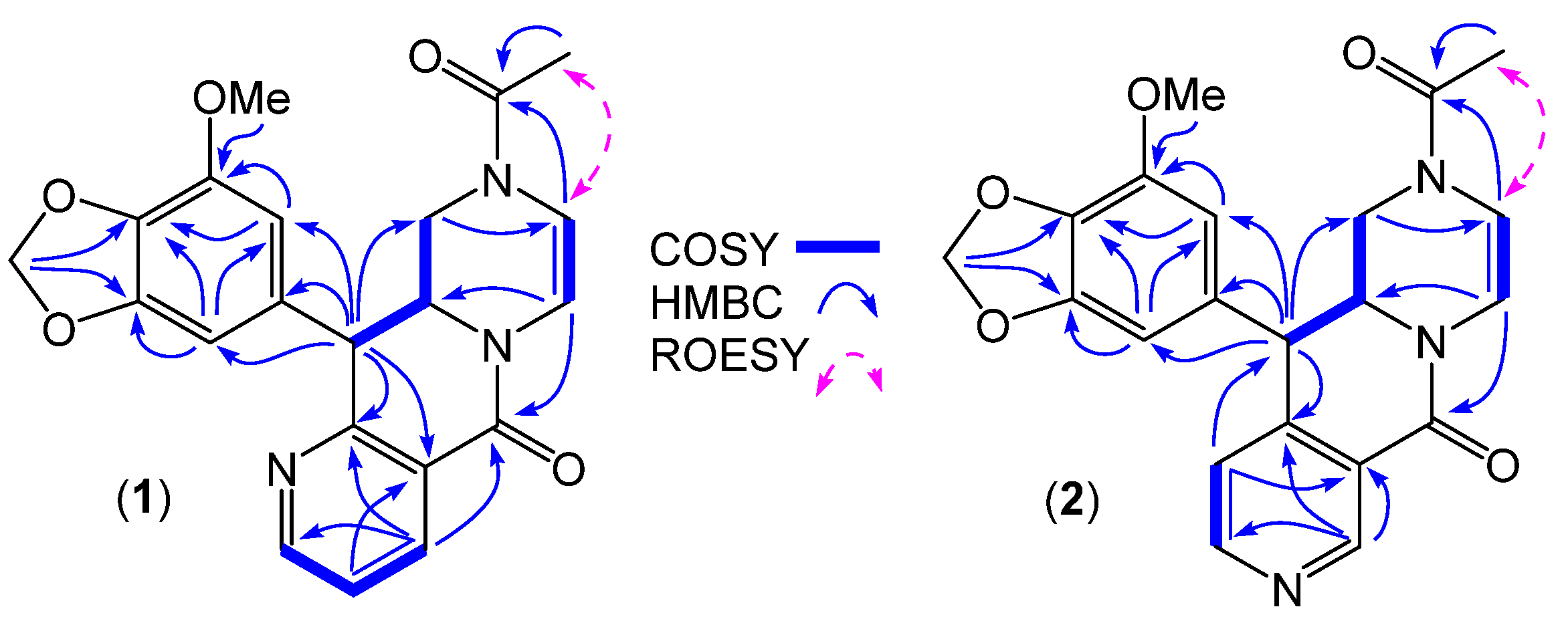
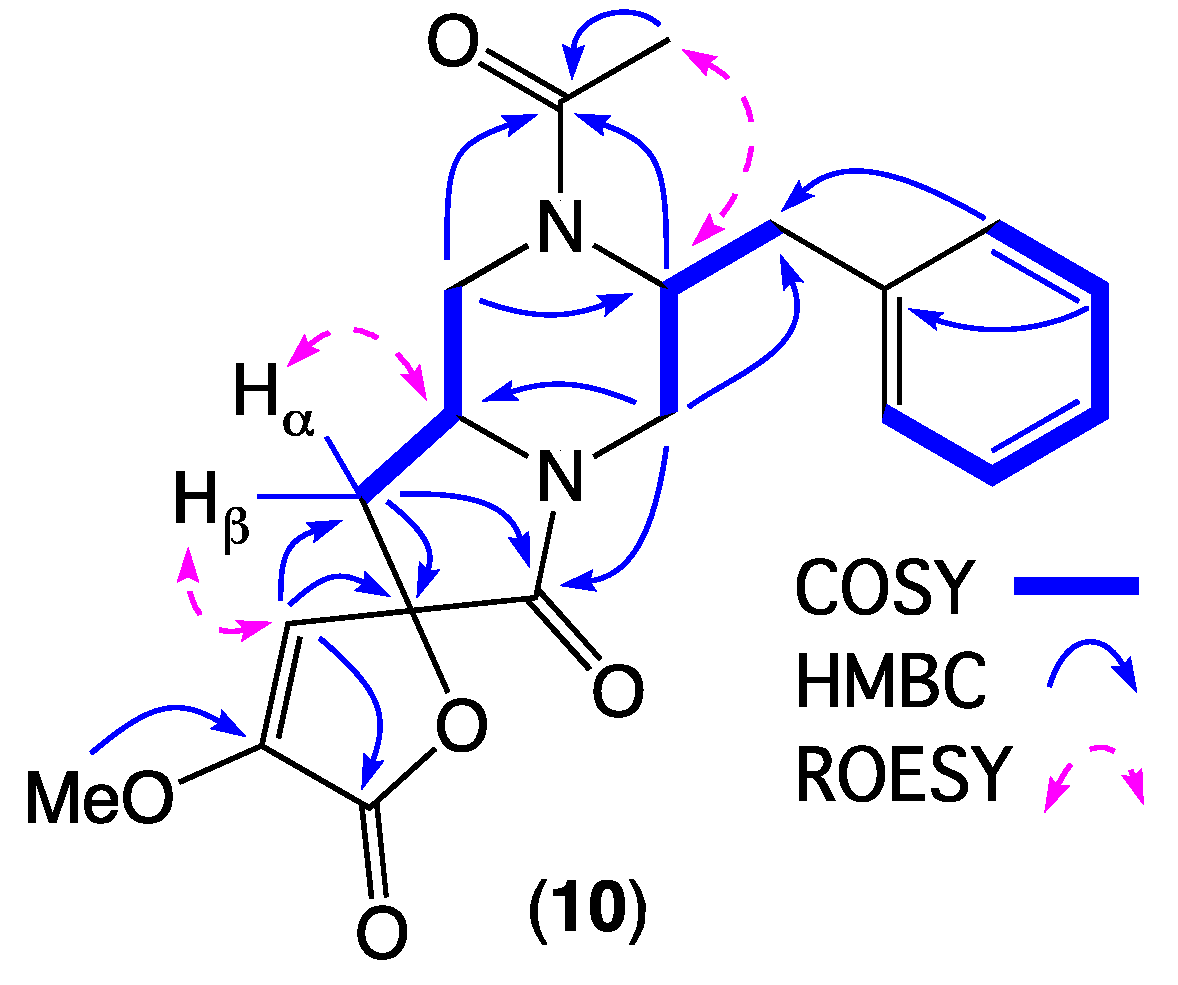
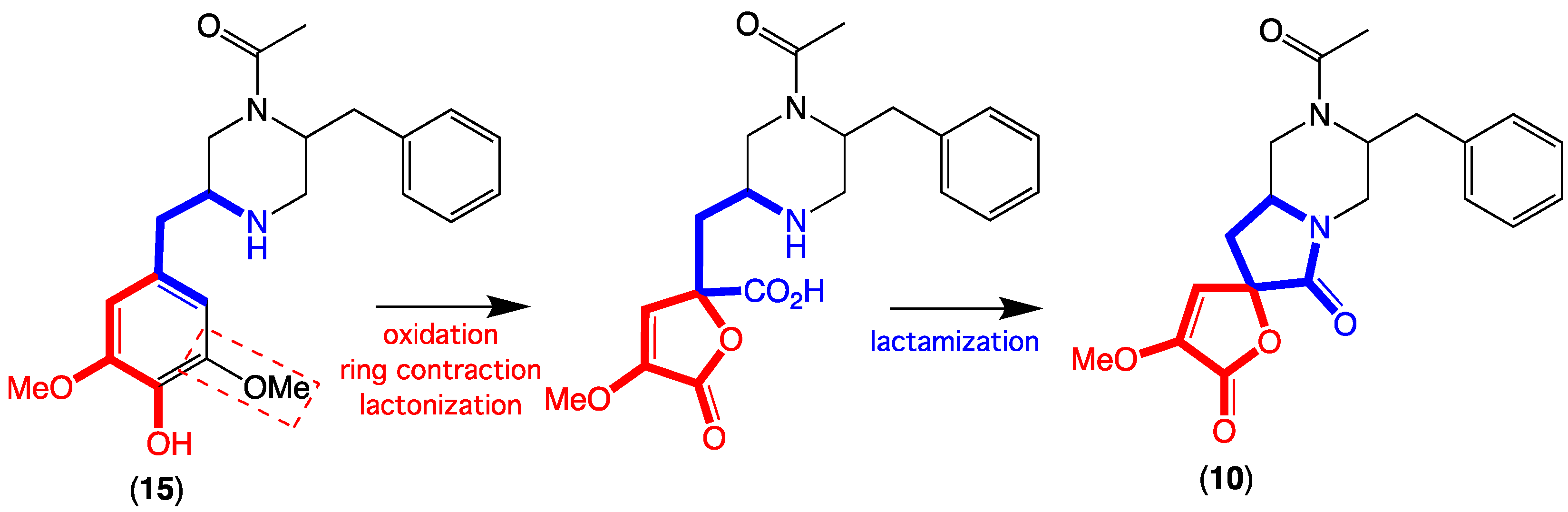
2. Results and Discussion
3. Materials and Methods
3.1. Chrysosporium sp. CMB-F214 Collection, Isolation and Taxonomy
3.2. Chrysosporium sp. CMB-F214 Media MATRIX Study
3.3. Analytical Precursor-Directed (Nicotinate) Feeding Study
3.4. M2 Agar Scale-up Culture and Production of Chrysosporazine Q (15)
3.5. Chrysosporium sp. CMB-F214 Culture Supplemented with Sodium Nicotinate Leading to Amplified Production of the Minor Natural Products, Azachrysosporazines 1–6, New Chrysosporazines 7–9 and Spirochrysosporazine A (10)
3.6. Metabolites’ Characterisation
- Azachrysosporazine A1 (1); yellow oil; [α]D21.2 − 88.4 (c 0.083, MeOH); NMR (600 MHz, DMSO-d6) see Table 1 and Table 2 and Table S1, Figures S4–S7; ESI(+)MS m/z 394 [M + H]+; HRESI(+)MS m/z 394.1399 [M + H]+ (calcd for C21H20N3O5, 394.1397).
- Azachrysosporazine A2 (2); yellow oil; [α]D21.2 − 55.0 (c 0.1, MeOH); NMR (600 MHz, DMSO-d6) see Table 1 and Table 2 and Table S2, Figures S8–S12; ESI(+)MS m/z 394 [M + H]+; HRESI(+)MS m/z 394.1404 [M + H]+ (calcd for C21H20N3O5, 394.1397).
- Azachrysosporazine B1 (3); yellow oil; [α]D21.2 + 43.1 (c 0.116, MeOH); NMR (600 MHz, DMSO-d6) see Table 1 and Table 2 and Table S3, Figures S13–S18; ESI(+)MS m/z 500 [M + H]+; HRESI(+)MS m/z 500.1831 [M + H]+ (calcd for C28H26N3O6, 500.1816).
- Azachrysosporazine C1 (4); yellow oil; [α]D21.2 − 29.6 (c 0.125, MeOH); NMR (600 MHz, DMSO-d6) see Table 1 and Table 2 and Table S4, Figures S19–S24; ESI(+)MS m/z 486 [M + H]+; HRESI(+)MS m/z 486.2035 [M + H]+ (calcd for C28H28N3O5, 486.2023).
- Azachrysosporazine C2 (5); yellow oil; [α]D21.2 − 36.3 (c 0.166, MeOH); NMR (600 MHz, DMSO-d6) see Table 1 and Table 2 and Table S5, Figures S25–S30; ESI(+)MS m/z 486 [M + H]+; HRESI(+)MS m/z 486.2035 [M + H]+ (calcd for C28H28N3O5, 486.2023).
- Azachrysosporazine D1 (6); yellow oil; [α]D22.2 + 19.3 (c 0.033, MeOH); NMR (600 MHz, DMSO-d6) see Figures S31–S32; ESI(+)MS m/z 488 [M + H]+; HRESI(+)MS m/z 488.2180 [M + H]+ (calcd for C28H30N3O5, 488.2180).
- Chrysosporazine N (7); yellow oil; [α]D21.2 + 5.3 (c 0.166, MeOH); NMR (600 MHz, DMSO-d6) see Table 3 and Table 4 and Table S6, Figures S33–S38; ESI(+)MS m/z 501 [M + H]+; HRESI(+)MS m/z 501.2014 [M + H]+ (calcd for C29H29N2O6, 501.2020).
- Chrysosporazine O (8); yellow oil; [α]D21.2 − 86.8 (c 0.185, MeOH); NMR (600 MHz, DMSO-d6) see Table 3 and Table 4 and Table S7, Figures S39–S44; ESI(+)MS m/z 501 [M + H]+; HRESI(+)MS m/z 501.2019 [M + H]+ (calcd for C29H29N2O6, 501.2020).
- Chrysosporazine P (9); yellow oil; [α]D22.2 + 18.4 (c 0.08, MeOH); NMR (600 MHz, DMSO-d6) see Figures S45–S46; ESI(+)MS m/z 489 [M + H]+; HRESI(+)MS m/z 489.2394 [M + H]+ (calcd for C29H33N2O5, 489.2384).
- Spirochrysosporazine A (10); yellow oil; [α]D21.0 − 76.6 (c 0.154, MeOH); NMR (600 MHz, DMSO-d6) see Table 3 and Table 4 and Table S8, Figures S47–S52; ESI(+)MS m/z 371 [M + H]+; HRESI(+)MS m/z 393.1422 [M + Na]+ (calcd for C20H22N2O5Na, 393.1421).
- Chrysosporazine Q (15); White powder; [α]D22.5 − 28.2 (c 0.286, MeOH); NMR (600 MHz, DMSO-d6) see Table 3 and Table 4 and Table S9, Figures S53–S58; ESI(+)MS m/z 385 [M + H]+; HRESI(+)MS m/z 385.2122 [M + H]+ (calcd for C22H29N2O4, 385.2122).
3.7. Acid Hydrolysis of Azachrysosporazine D1 (6) and Chrysosporazine P (9)
3.8. Antibacterial Assay
3.9. Antifungal Assay
3.10. Cytotoxicity Assay
3.11. MDR Reversal (Doxorubicin) Assay (P-glycoprotein Inhibition Assay)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Elbanna, A.H.; Khalil, Z.G.; Bernhardt, P.V.; Capon, R.J. Scopularides Revisited: Molecular Networking Guided Exploration of Lipodepsipeptides in Australian Marine Fish Gastrointestinal Tract-Derived Fungi. Mar. Drugs 2019, 17, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, O.G.; Khalil, Z.G.; Capon, R.J. Prolinimines: N-Amino-l-Pro-Methyl Ester (Hydrazine) Schiff Bases from a Fish Gastrointestinal Tract-Derived Fungus, Trichoderma Sp. CMB-F563. Org. Lett. 2018, 20, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, O.G.; Khalil, Z.G.; Capon, R.J. N-Amino-l-Proline Methyl Ester from an Australian Fish Gut-Derived Fungus: Challenging the Distinction between Natural Product and Artifact. Mar. Drugs. 2021, 19, 151. [Google Scholar] [CrossRef] [PubMed]
- Elbanna, A.H.; Khalil, Z.G.; Bernhardt, P.V.; Capon, R.J. Chrysosporazines A-E: P-Glycoprotein Inhibitory Piperazines from an Australian Marine Fish Gastrointestinal Tract-Derived Fungus, Chrysosporium Sp. CMB-F214. Org. Lett. 2019, 21, 8097–8100. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, O.G.; Salim, A.A.; Khalil, Z.G.; Elbanna, A.H.; Bernhardt, P.V.; Capon, R.J. Chrysosporazines F-M: P-Glycoprotein Inhibitory Phenylpropanoid Piperazines from an Australian Marine Fish Derived Fungus, Chrysosporium Sp. CMB-F294. J. Nat. Prod. 2020, 83, 497–504. [Google Scholar] [CrossRef] [PubMed]




| Position | δH, mult (J in Hz) | (2) δH, mult (J in Hz) | (3) δH, mult (J in Hz) | (4) δH, mult (J in Hz) | (5) δH, mult (J in Hz) |
|---|---|---|---|---|---|
| 1 | a. 4.26, ddd (13.3,3.0, 1.2) | a. 4.21, ddd (13.1,3.0, 1.2) | a. 3.98, dd (12.9, 3.8) | a. 4.29, dd (13.8, 3.8) | a. 4.19, dd (13.8, 3.9) |
| b. 2.99, dd (13.3, 9.8) | b. 2.97, dd (13.1, 9.8) | b. 3.51 b | b. 3.04 a, dd (13.8, 11.4) | b. 2.99, dd (13.8, 11.0) | |
| 2 | 4.47, ddd (12.3, 9.8, 3.0) | 4.49, ddd (12.1, 9.8, 3.0) | 4.21, ddd (9.8, 5.4, 3.8) | 3.88, ddd (11.4, 10.0, 3.8) | 3.91, ddd (11.2, 11.0, 3.9) |
| 3 | 4.51, d (12.3) | 4.46, d (12.1) | 4.46, d (5.4) | 4.49, d (10.0) | 4.46, d (11.2) |
| 5 | 6.57, d (1.4) | 6.68, d (0.9) | 6.31, d (1.4) | 6.49, d (1.4) | 6.64, d (1.3) |
| 9 | 6.65, d (1.4) | 6.74, d (0.9) | 6.55, d (1.4) | 6.63, d (1.4) | 6.73, d (1.3) |
| 1′ | 6.80, d (6.8) | 6.81, d (6.8) | a. 4.79, dd (14.1, 1.3) | a. 4.56, dd (13.4, 1.1) | a. 4.56, dd (13.5, 1.2) |
| - | - | b. 3.51 b | b. 3.00, m | b. 2.95, dd (13.5, 4.1) | |
| 2′ | 6.55, dd (6.8, 1.2) | 6.56, dd (6.8, 1.2) | 5.89, dd (5.7, 1.3) | 4.25, m | 4.27, m |
| 3′ | - | - | - | a. 3.03 a, m | a. 3.06, dd (13.4, 8.8) |
| - | - | - | b. 2.90, dd (13.4, 5.8) | b. 2.93, dd (13.4, 5.8) | |
| 5′/9′ | - | - | 7.97, m | 7.26, m | 7.26, m |
| 6′/8′ | - | - | 7.56, m | 7.30, m | 7.30, m |
| 7′ | - | - | 7.68, m | 7.22, m | 7.23, m |
| 4″ | - | 6.66, d (5.0) | - | - | 6.72, br s |
| 5′′ | 8.56, dd (4.8, 1.8) | 8.59, d (5.0) | 8.64, dd (4.7, 1.8) | 8.60, dd (4.7, 1.8) | 8.60, br s |
| 6′′ | 7.43, ddd (7.8, 4.8, 0.8) | - | 7.44, dd (7.8, 4.7) | 7.45, dd (7.8, 4.7) | - |
| 7′′ | 8.26, dd (7.8, 1.8) | 9.03, s | 8.18, dd (7.8, 1.8) | 8.18, dd (7.8, 1.8) | 9.08, br s |
| NCOCH3 | 2.10, s | 2.10, s | 2.00, s | 1.60, s | 1.59, s |
| 6-OCH2 | 6.02/6.00, Abq | 6.06/6.05, Abq | 5.95/5.94, Abq | 5.99/5.99, Abq | 6.05, br s |
| 8-OCH3 | 3.80, s | 3.81, s | 3.78, s | 3.80, s | 3.82, s |
| Position | (1) δC, Type | (2) δC, Type | (3) δC, Type | (4) δC, Type | (5) δC, Type |
|---|---|---|---|---|---|
| 1 | 42.3, CH2 | 42.1, CH2 | 47.3, CH2 | 40.1 c, CH2 | 39.8 c, CH2 |
| 2 | 56.9, CH | 56.5 a, CH | 59.2, CH | 58.0, CH | 56.9, CH |
| 3 | 48.8, CH | 45.4, CH | 47.2, CH | 48.8, CH | 45.7, CH |
| 4 | 132.8, C | 131.1, C | 135.3, C | 134.6, C | 132.6, C |
| 5 | 103.4, CH | 103.0 b, CH | 102.0, CH | 103.1, CH | 103.0, CH |
| 6 | 148.5, C | 149.0, C | 148.6, C | 148.5, C | 148.9, C |
| 7 | 134.0, C | 134.6, C | 133.8, C | 133.8, C | 134.4, C |
| 8 | 143.1, C | 143.6, C | 143.1, C | 143.1, C | 143.5, C |
| 9 | 110.1, CH | 109.6 b, CH | 108.6, CH | 109.6, CH | 109.5, CH |
| 1′ | 106.7, CH | 106.4, CH | 42.6, CH2 | 45.0, CH2 | 44.5, CH2 |
| 2′ | 112.6, CH | 112.7, CH | 54.3, CH | 54.6, CH | 54.3, CH |
| 3′ | - | - | 197.6, C | 34.9, CH2 | 34.8, CH2 |
| 4′ | - | - | 134.7, C | 138.2, C | 138.2, C |
| 5′/9′ | - | - | 128.2, CH | 129.4, CH | 129.4, CH |
| 6′/8′ | - | - | 128.9, CH | 128.4, CH | 128.4, CH |
| 7′ | - | - | 133.5, CH | 126.5, CH | 126.5, CH |
| 1″ | 158.5, C | 157.4, C | 161.9, C | 163.5, C | 162.9, C |
| 2″ | 123.2, C | 123.1, C | 122.8, C | 122.9, C | 123.0, C |
| 3″ | 158.6, C | 149.8, C | 158.1, C | 158.4, C | 149.3, C |
| 4″ | - | 121.5, CH | - | - | 121.5, CH |
| 5″ | 152.4, CH | 152.5, CH | 153.0, CH | 152.5, CH | 152.4, CH |
| 6″ | 122.9, CH | - | 123.1, CH | 122.7, CH | - |
| 7″ | 135.4, CH | 147.9, CH | 135.6, CH | 135.5, CH | 148.3, CH |
| 1-NCOCH3 | 166.7, C | 166.7, C | 170.5, C | 168.4, C | 168.3, C |
| 1-NCOCH3 | 20.8, CH3 | 20.8, CH3 | 21.4, CH3 | 20.7, CH3 | 20.6, CH3 |
| 6-OCH2 | 101.2, CH2 | 101.6, CH2 | 101.3, CH2 | 101.2, CH2 | 101.5, CH2 |
| 8-OCH3 | 56.4, CH3 | 56.5 a, CH3 | 56.4, CH3 | 56.4, CH3 | 56.4, CH3 |
| Position | (7) δH, mult (J in Hz) | (8) δH, mult (J in Hz) | (10) δH, mult (J in Hz) | (15) δH, mult (J in Hz) |
|---|---|---|---|---|
| 1 | a. 3.77, dd (13.6, 4.1) | a. 3.68, dd (14.3, 4.1) | a. 4.61, dd (13.3, 4.3) | a. 4.48, dd (14.5, 3.2) |
| b. 3.43 a | b. 3.61, dd (14.3, 6.8) | b. 2.77 b, dd (13.3, 11.7) | b. 2.92 b, m | |
| 2 | 4.27, ddd (12.4, 9.0, 4.1) | 4.47, ddd (12.4, 6.8, 4.1) | 3.60, m | 3.27, m |
| 3 | 4.36, d (9.0) | 4.25, d (12.4) | α. 2.51 a, dd (14.8, 7.2) | a. 2.88, dd (13.0, 6.2) |
| - | - | β. 2.39, dd (14.8, 6.3) | b. 2.85, dd (13.0, 8.0) | |
| 5 | 6.54, s | 6.61, s | - | 6.55, br s |
| 7 | - | - | 6.65, s | - |
| 9 | 6.54, s | 6.61, s | - | 6.55, br s |
| 1′ | a. 4.76, dd(14.3, 1.9) | a. 4.37, dd (14.1, 7.1) | a. 3.79 c, d (13.4) | a. 3.29, m |
| b. 3.52, dd (14.3, 5.5) | b. 3.98, dd (14.1, 4.5) | b. 3.18, dd (13.4, 4.4) | b. 3.25, m | |
| 2′ | 5.83, dd (5.5, 1.9) | 5.67, dd (7.1, 4.5) | 4.21, m | 4.30, m |
| 3′ | - | - | a. 2.93, dd (13.2, 6.9) | a. 3.21, dd (13.7, 10.4) |
| - | - | b. 2.80 b, dd (13.2, 8.5) | b. 2.93 b, m | |
| 5′/9′ | 7.97, dd (8.1, 1.0) | 8.03, dd (8.4, 1.3) | 7.24 m | 7.25 m |
| 6′/8′ | 7.55, dd (8.1, 7.6) | 7.58, dd (7.8, 7.6) | 7.29, m | 7.31, m |
| 7′ | 7.67, t (7.6) | 7.70, t (7.8) | 7.23, m | 7.25, m |
| 4″ | 6.82, d (8.7) | 6.65, d (7.8) | - | - |
| 5′′ | 7.45, ddd (8.7, 7.4, 1.5) | 7.42, ddd (7.8, 7.4, 1.3) | - | - |
| 6′′ | 7.35, dd (7.7, 7.4) | 7.35, dd (7.6, 7.4) | - | - |
| 7′′ | 7.85, dd (7.7, 1.5) | 7.85, dd (7.6, 1.3) | - | - |
| NCOCH3 | 1.91, s | 1.83, s | 1.69, s | 1.46, s |
| 6-OCH3 | 3.69, s | 3.74, s | 3.80 c, s | 3.76, s |
| 8-OCH3 | 3.69, s | 3.74, s | - | 3.76, s |
| 7-OH | 8.43, br s | 8.53, br s | - | 8.03, br s |
| 1′-NH | - | - | - | 9.06, m |
| Position | (7) δC, Type | (8) δC, Type | (10) δC, Type | (15) δC, Type |
|---|---|---|---|---|
| 1 | 46.0, CH2 | 44.6, CH2 | 41.1, CH2 | 37.1, CH2 |
| 2 | 58.5, CH | 57.7, CH | 51.1, CH | 55.9, CH |
| 3 | 45.4, CH | 46.9, CH | 32.6, CH2 | 36.1, CH2 |
| 4 | 130.1, C | 128.0, C | 85.0, C | 125.1, C |
| 5 | 106.3, CH | 106.6, CH | 166.1, C | 106.6, CH |
| 6 | 148.3, C | 148.5, C | 147.0, C | 148.0, C |
| 7 | 134.7 a, C | 135.0, C | 117.9, CH | 134.6, C |
| 8 | 148.3, C | 148.5, C | - | 148.0, C |
| 9 | 106.3, CH | 106.6, CH | - | 106.6, CH |
| 1′ | 40.9, CH2 | 38.8, CH2 | 43.0, CH2 | 45.9, CH2 |
| 2′ | 55.1, CH | 57.1, CH | 53.9, CH | 52.3, CH |
| 3′ | 196.6, C | 196.3, C | 34.7, CH2 | 34.7, CH2 |
| 4′ | 134.7 a, C | 134.6, C | 137.9, C | 137.6, C |
| 5′/9′ | 128.2, CH | 128.2, CH | 129.4, CH | 129.3, CH |
| 6′/8′ | 128.9, CH | 129.1, CH | 128.4, CH | 128.5, CH |
| 7′ | 133.5, CH | 133.8, CH | 126.6, CH | 126.8, CH |
| 1″ | 162.8, C | 162.5, C | 166.7, C | - |
| 2″ | 127.4, C | 127.5 a, C | - | - |
| 3″ | 140.9, C | 142.0, C | - | - |
| 4″ | 127.7, CH | 127.1 b, CH | - | - |
| 5″ | 132.4, CH | 132.3, CH | - | - |
| 6″ | 127.0, CH | 127.1 b, CH | - | - |
| 7″ | 127.5, CH | 127.5 a, CH | - | - |
| 1-NCOCH3 | 169.9, C | 169.1, C | 168.7, C | 168.4, C |
| 1-NCOCH3 | 21.2, CH3 | 20.9, CH3 | 21.0, CH3 | 20.3, CH3 |
| 6-OCH3 | 56.1, CH3 | 56.2, CH3 | 58.4, CH3 | 55.9, CH3 |
| 8-OCH3 | 56.1, CH3 | 56.2, CH3 | - | 55.9, CH3 |
| SW620 Ad300 | SW620 | ||||
|---|---|---|---|---|---|
| Treatment | IC50 a (µM) | FR b | GS c | Treatment | IC50 a (µM) |
| Doxorubicin | 5.75 | 57.5 | 1.0 | Doxorubicin | 0.10 |
| +1 (2.5 µM) | 0.60 | 6.0 | 9.5 | 1 | >30 |
| +2 (2.5 µM) | 0.84 | 8.4 | 6.8 | 2 | >30 |
| +3 (2.5 µM) | 0.28 | 2.8 | 20.5 | 3 | >30 |
| +4 (2.5 µM) | 0.27 | 2.7 | 21.3 | 4 | >30 |
| +5 (2.5 µM) | 0.29 | 2.9 | 19.8 | 5 | >30 |
| +6 (2.5 µM) | 3.55 | 35.5 | 1.62 | 6 | >30 |
| +7 (2.5 µM) | 7.05 | 70.5 | 0.81 | 7 | >30 |
| +8 (2.5 µM) | 5.15 | 51.5 | 1.11 | 8 | >30 |
| +9 (2.5 µM) | 5.08 | 50.8 | 1.13 | 9 | >30 |
| +10 (2.5 µM) | 4.13 | 41.3 | 1.39 | 10 | >30 |
| +11 (2.5 µM) | 0.80 | 8.0 | 7.18 | 11 | >30 |
| +12 (2.5 µM) | 0.22 | 2.2 | 26.1 | 12 | >30 |
| +13 (2.5 µM) | 0.31 | 3.0 | 18.5 | 13 | >30 |
| +14 (2.5 µM) | 4.36 | 43.6 | 1.32 | 14 | >30 |
| +15 (2.5 µM) | 4.50 | 45.0 | 1.27 | 15 | >30 |
| +verapamil (2.5 µM) | 0.71 | 7.0 | 8.1 | doxorubicin + verapamil | 0.092 |
| verapamil | >30 | -- | -- | verapamil | >30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbanna, A.H.; Agampodi Dewa, A.; Khalil, Z.G.; Capon, R.J. Precursor-Directed Biosynthesis Mediated Amplification of Minor Aza Phenylpropanoid Piperazines in an Australian Marine Fish-Gut-Derived Fungus, Chrysosporium sp. CMB-F214. Mar. Drugs 2021, 19, 478. https://doi.org/10.3390/md19090478
Elbanna AH, Agampodi Dewa A, Khalil ZG, Capon RJ. Precursor-Directed Biosynthesis Mediated Amplification of Minor Aza Phenylpropanoid Piperazines in an Australian Marine Fish-Gut-Derived Fungus, Chrysosporium sp. CMB-F214. Marine Drugs. 2021; 19(9):478. https://doi.org/10.3390/md19090478
Chicago/Turabian StyleElbanna, Ahmed H., Amila Agampodi Dewa, Zeinab G. Khalil, and Robert J. Capon. 2021. "Precursor-Directed Biosynthesis Mediated Amplification of Minor Aza Phenylpropanoid Piperazines in an Australian Marine Fish-Gut-Derived Fungus, Chrysosporium sp. CMB-F214" Marine Drugs 19, no. 9: 478. https://doi.org/10.3390/md19090478
APA StyleElbanna, A. H., Agampodi Dewa, A., Khalil, Z. G., & Capon, R. J. (2021). Precursor-Directed Biosynthesis Mediated Amplification of Minor Aza Phenylpropanoid Piperazines in an Australian Marine Fish-Gut-Derived Fungus, Chrysosporium sp. CMB-F214. Marine Drugs, 19(9), 478. https://doi.org/10.3390/md19090478









