Use of Quorum Sensing Inhibition Strategies to Control Microfouling
Abstract
1. Introduction
2. Results
2.1. Selection of Marine Bacterial Biofilm-Forming Strains and Validation of xCELLigence® Screening Method
2.2. AHL Production by Marine Bacterial Biofilm-Forming Strains
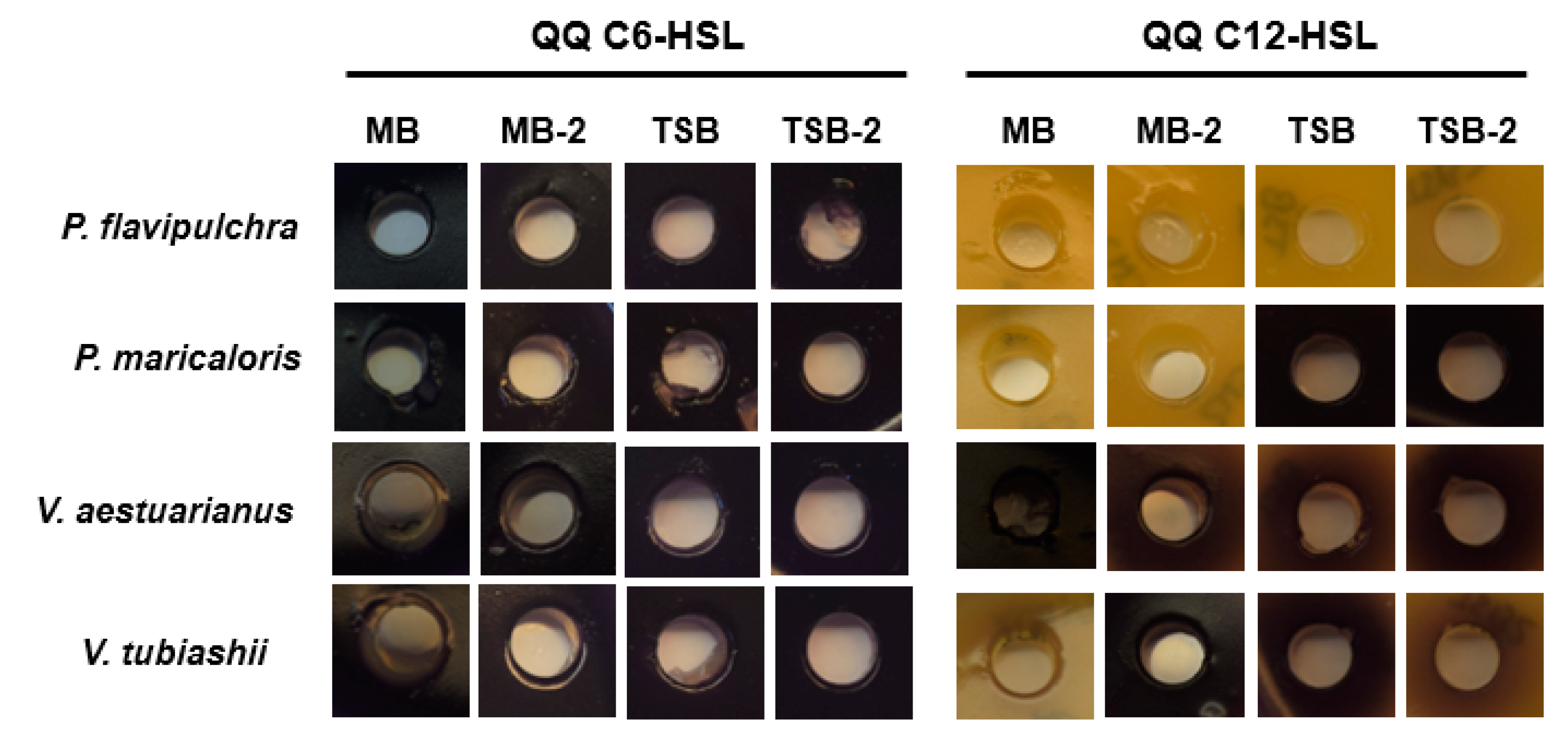
2.3. Characterization of Quorum Quenching Activity of Marine Bacterial Biofilm-Forming Strains
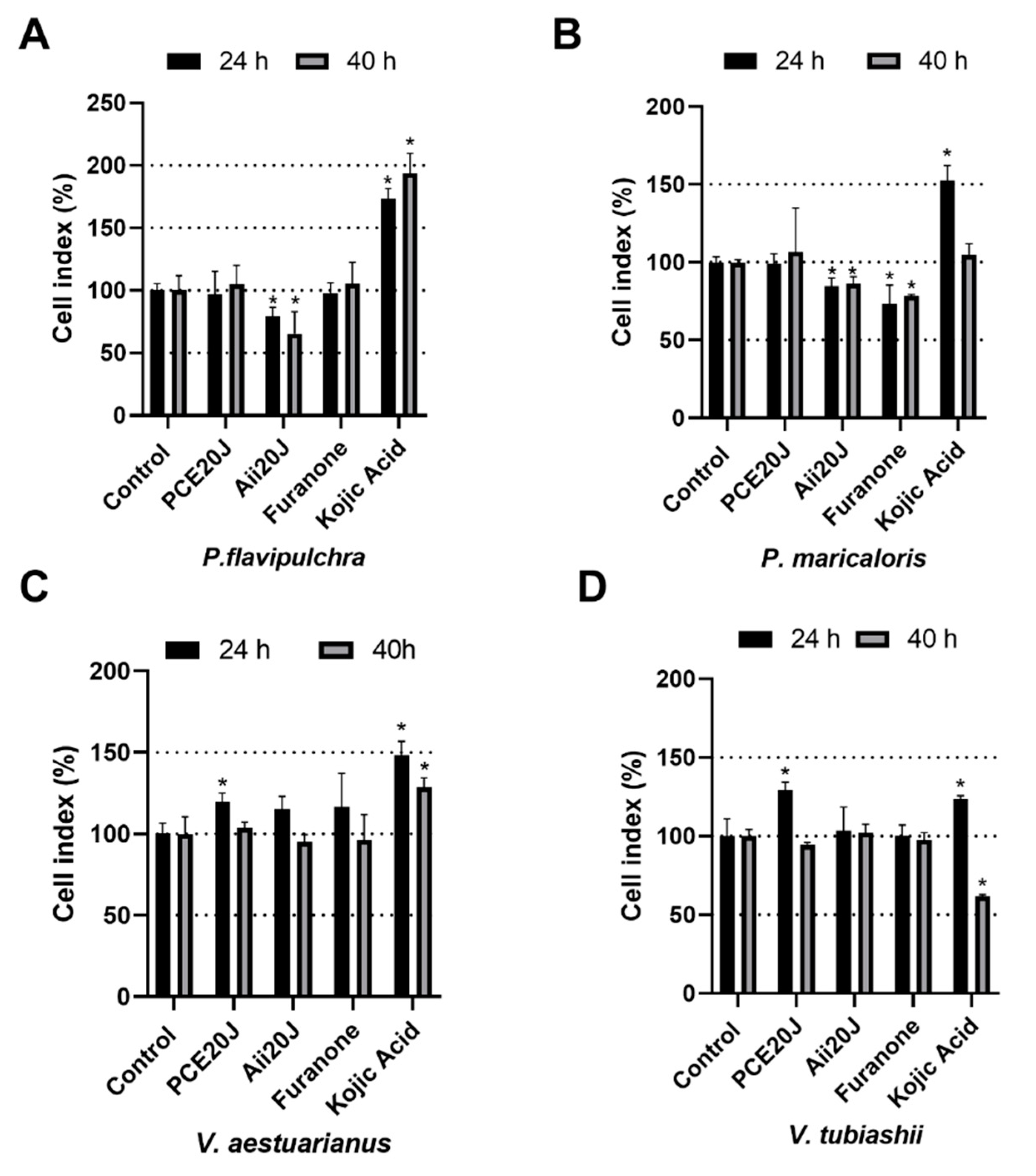
2.4. Effect of QS Inhibitors and QQ Enzymes on Biofilm Formation Measured using xCELLigence®
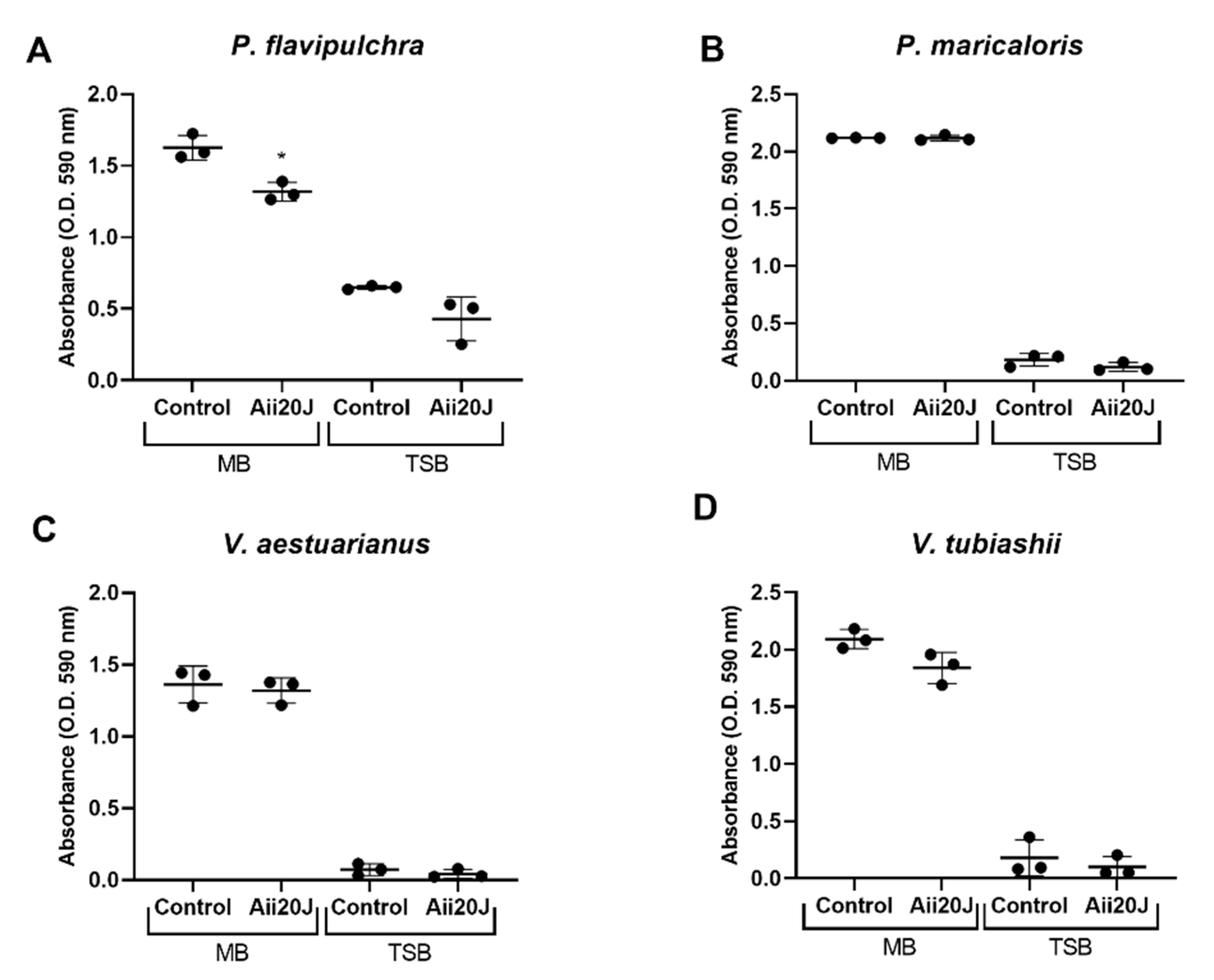
2.5. Effect of the AHL-lactonase Aii20J on Marine Biofilm Formation in the Attachment Model
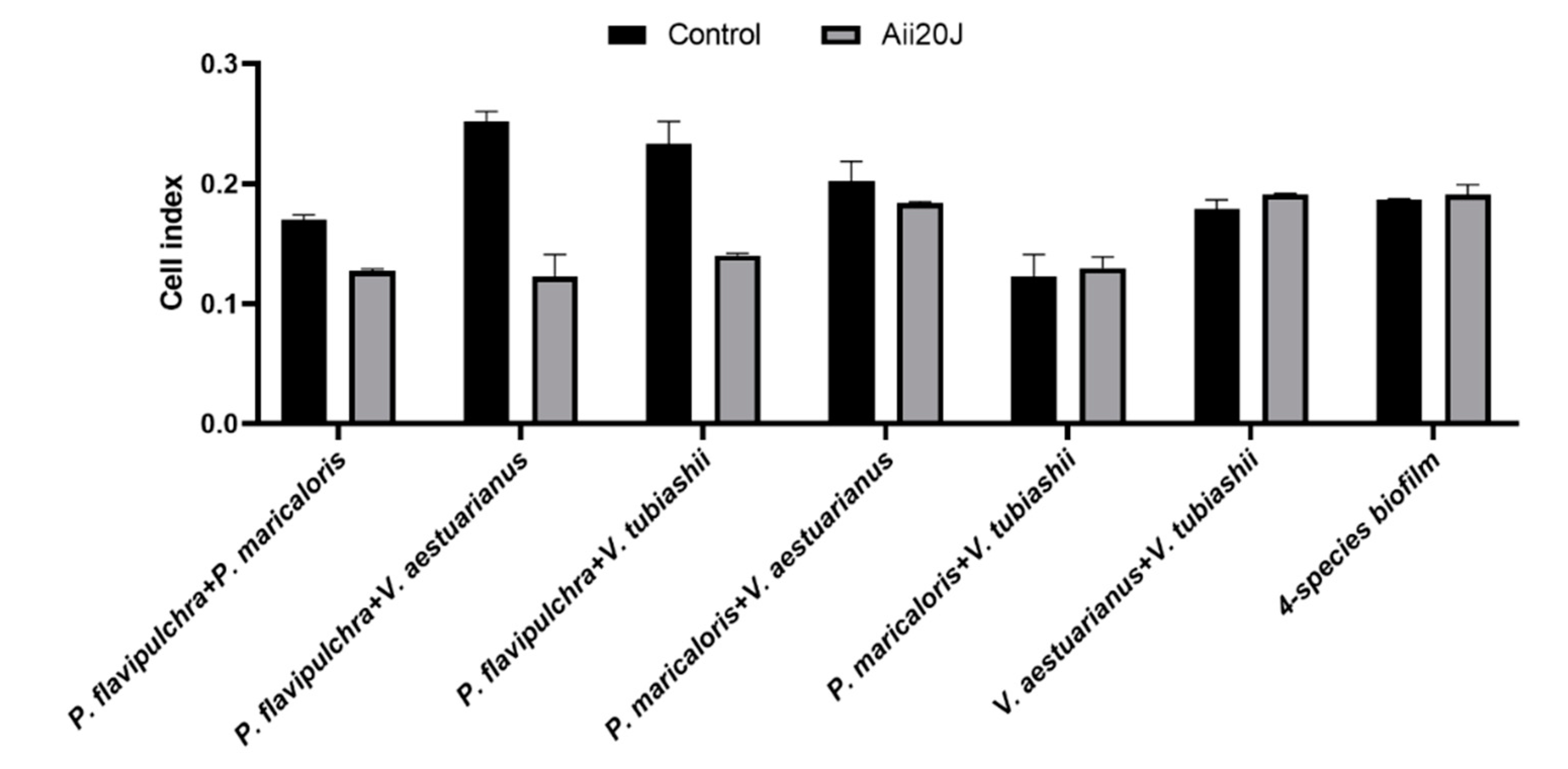
2.6. Effect of the AHL-lactonase Aii20J on Mixed-Species Biofilm Models
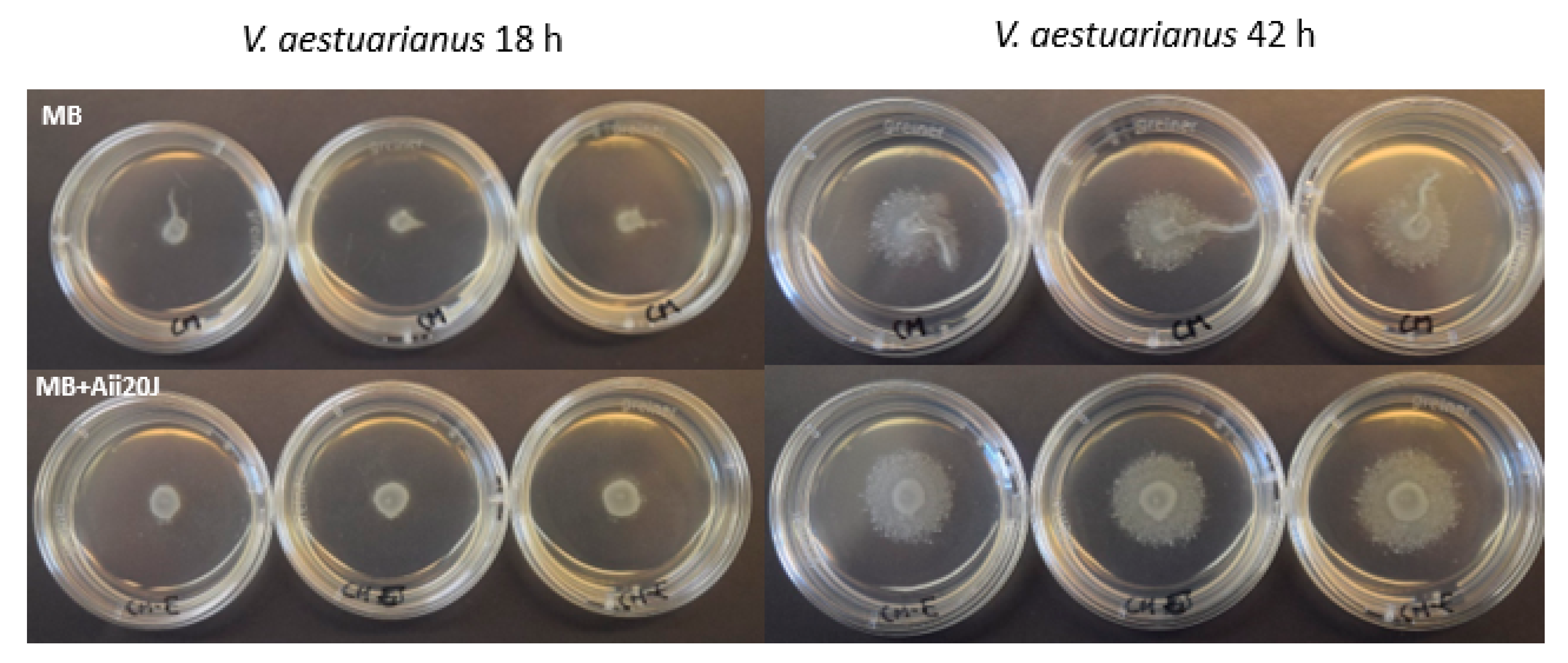
2.7. Effect of AHL-lactonase Aii20J on Motility
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Bioassay for Detection of AHL Production in Marine Cultures
4.3. Extraction and Identification of AHLs by HPLC-MS
4.4. Quorum Quenching Activity Assay
4.5. Quorum Sensing Inhibitors
4.6. Biofilm Quantification
4.7. Motility Assays
4.8. Statistical Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Chapman, J.; Hellio, C.; Sullivan, T.; Brown, R.; Russell, S.; Kiterringham, E.; Le Nor, L.; Regan, F. Bioinspired Synthetic Macroalgae: Examples from Nature for Antifouling Applications. Int. Biodeterior. Biodegrad. 2014, 86, 6–13. [Google Scholar] [CrossRef]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling Technology-Past, Present and Future Steps towards Efficient and Environmentally Friendly Antifouling Coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Caruso, G. Microbial Colonization in Marine Environments: Overview of Current Knowledge and Emerging Research Topics. J. Mar. Sci. Eng. 2020, 8, 78. [Google Scholar] [CrossRef]
- Fuqua, W.C.; Winans, S.C.; Greenberg, E.P. Quorum Sensing in Bacteria: The LuxR-LuxI Family of Cell Density-Responsive Transcriptional Regulators. J. Bacteriol. 1994, 176, 269–275. [Google Scholar] [CrossRef]
- Muras, A.; Otero, A. Breaking bad: Understanding how bacterial communication regulates biofilm-related oral diseases. In Trends in Quorum Sensing and Quorum Quenching: New Perspectives and Applications; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2020. [Google Scholar]
- Williams, P.; Winzer, K.; Chan, W.C.; Cámara, M. Look who’s Talking: Communication and Quorum Sensing in the Bacterial World. Philos. Trans. R Soc. Lond. B. Biol. Sci. 2007, 362, 1119–1134. [Google Scholar] [CrossRef]
- Wheeler, G.L.; Tait, K.; Taylor, A.; Brownlee, C.; Joint, I. Acyl-Homoserine Lactones Modulate the Settlement Rate of Zoospores of the Marine Alga Ulva intestinalis via a Novel Chemokinetic Mechanism. Plant. Cell Environ. 2005, 29, 608–618. [Google Scholar] [CrossRef]
- Tait, K.; Havenhand, J. Investigating a Possible Role for the Bacterial Signal Molecules N-Acylhomoserine Lactones in Balanus improvisus Cyprid Settlement. Mol. Ecol. 2013, 22, 2588–2602. [Google Scholar] [CrossRef]
- Yang, J.-L.; Li, Y.-F.; Guo, X.-P.; Liang, X.; Xu, Y.-F.; Ding, D.-W.; Bao, W.-Y.; Dobretsov, S. The Effect of Carbon Nanotubes and Titanium Dioxide Incorporated in PDMS on Biofilm Community Composition and Subsequent Mussel Plantigrade Settlement. Biofouling 2016, 32, 763–777. [Google Scholar] [CrossRef]
- Lee, S.; Park, S.K.; Kwon, H.; Lee, S.H.; Lee, K.; Nahm, C.H.; Jo, S.J.; Oh, H.S.; Park, P.K.; Choo, K.H.; et al. Crossing the Border between Laboratory and Field: Bacterial Quorum Quenching for Anti-Biofouling Strategy in an MBR. Environ. Sci. Technol. 2016, 50, 1788–1795. [Google Scholar] [CrossRef]
- Mayer, C.; Muras, A.; Parga, A.; Romero, M.; Rumbo-Feal, S.; Poza, M.; Ramos-Vivas, J.; Otero, A. Quorum Sensing as a Target for Controlling Surface Associated Motility and Biofilm Formation in Acinetobacter baumannii ATCC® 17978TM. Front. Microbiol. 2020, 11, 565548. [Google Scholar] [CrossRef]
- Muras, A.; Mayer, C.; Romero, M.; Camino, T.; Ferrer, M.D.; Mira, A.; Otero, A. Inhibition of Streptococcus mutans Biofilm Formation by Extracts of Tenacibaculum sp. 20J, a Bacterium with Wide-Spectrum Quorum Quenching Activity. J. Oral. Microbiol. 2018, 10, 1429788. [Google Scholar] [CrossRef]
- Muras, A.; Otero-Casal, P.; Blanc, V.; Otero, A. Acyl Homoserine Lactone-Mediated Quorum Sensing in the Oral Cavity: A Paradigm Revisited. Sci. Rep. 2020, 10, 9800. [Google Scholar] [CrossRef]
- Soler, A.; Arregui, L.; Arroyo, M.; Mendoza, J.A.; Muras, A.; Álvarez, C.; García-Vera, C.; Marquina, D.; Santos, A.; Serrano, S. Quorum Sensing versus Quenching Bacterial Isolates Obtained from MBR Plants Treating Leachates from Municipal Solid Waste. Int. J. Environ. Res. Public Health 2018, 15, 1019. [Google Scholar] [CrossRef]
- Dong, Y.-H.; Zhang, L.-H. Quorum Sensing and Quorum-Quenching Enzymes. J. Microbiol. 2005, 43, 101–109. [Google Scholar]
- Grandclément, C.; Tannières, M.; Moréra, S.; Dessaux, Y.; Faure, D. Quorum Quenching: Role in Nature and Applied Developments. FEMS Microbiol. Rev. 2016, 40, 86–116. [Google Scholar] [CrossRef]
- Camps, J.; Pujol, I.; Ballester, F.; Joven, J.; Simó, J.M. Paraoxonases as Potential Antibiofilm Agents: Their Relationship with Quorum-Sensing Signals in Gram-Negative Bacteria. Antimicrob. Agents Chemother. 2011, 55, 1325. [Google Scholar] [CrossRef]
- Gao, M.; Teplitski, M.; Robinson, J.B.; Bauer, W.D. Production of Substances by Medicago truncatula That Affect Bacterial Quorum Sensing. Mol. Plant. Microbe Interact. 2003, 16, 827–834. [Google Scholar] [CrossRef]
- Givskov, M.; de Nys, R.; Manefield, M.; Gram, L.; Maximilien, R.; Eberl, L.; Molin, S.; Steinberg, P.D.; Kjelleberg, S. Eukaryotic Interference with Homoserine Lactone-Mediated Prokaryotic Signalling. J. Bacteriol. 1996, 178, 6618–6622. [Google Scholar] [CrossRef]
- Mayer, C.; Romero, M.; Muras, A.; Otero, A. Aii20J, a Wide-Spectrum Thermostable N-Acylhomoserine Lactonase from the Marine Bacterium Tenacibaculum sp. 20J, Can Quench AHL-Mediated Acid Resistance in Escherichia coli. Appl. Microbiol. Biotechnol. 2015, 99, 9523–9539. [Google Scholar] [CrossRef]
- Mayer, C.; Muras, A.; Romero, M.; López, M.; Tomás, M.; Otero, A. Multiple Quorum Quenching Enzymes Are Active in the Nosocomial Pathogen Acinetobacter baumannii ATCC17978. Front. Cell Infect. Microbiol. 2018, 8, 9523–9539. [Google Scholar] [CrossRef]
- Muras, A.; López-Pérez, M.; Mayer, C.; Parga, A.; Amaro-Blanco, J.; Otero, A. High Prevalence of Quorum-Sensing and Quorum-Quenching Activity among Cultivable Bacteria and Metagenomic Sequences in the Mediterranean Sea. Genes 2018, 9, 100. [Google Scholar] [CrossRef]
- Romero, M.; Mayer, C.; Muras, A.; Otero, A. Silencing Bacterial Communication through Enzymatic Quorum-Sensing Inhibition. In Quorum Sensing vs Quorum Quenching: A Battle with No End in Sight; Kalia, V.C., Ed.; Springer: New Delhi, India, 2015; pp. 219–236. ISBN 978-81-322-1981-1. [Google Scholar]
- Dobretsov, S.; Dahms, H.-U.; YiLi, H.; Wahl, M.; Qian, P.-Y. The Effect of Quorum-Sensing Blockers on the Formation of Marine Microbial Communities and Larval Attachment: Antifouling Activity of QS Blockers. FEMS Microbiol. Ecol. 2007, 60, 177–188. [Google Scholar] [CrossRef]
- Dobretsov, S.; Teplitski, M.; Paul, V. Mini-Review: Quorum Sensing in the Marine Environment and Its Relationship to Biofouling. Biofouling 2009, 25, 413–427. [Google Scholar] [CrossRef]
- Dobretsov, S.; Teplitski, M.; Bayer, M.; Gunasekera, S.; Proksch, P.; Paul, V.J. Inhibition of Marine Biofouling by Bacterial Quorum Sensing Inhibitors. Biofouling 2011, 27, 893–905. [Google Scholar] [CrossRef]
- Huang, S.; Bergonzi, C.; Schwab, M.; Elias, M.; Hicks, R.E. Evaluation of Biological and Enzymatic Quorum Quencher Coating Additives to Reduce Biocorrosion of Steel. PLoS ONE 2019, 14, e0217059. [Google Scholar] [CrossRef]
- Jo, S.J.; Kwon, H.; Jeong, S.-Y.; Lee, S.H.; Oh, H.-S.; Yi, T.; Lee, C.-H.; Kim, T.G. Effects of Quorum Quenching on the Microbial Community of Biofilm in an Anoxic/Oxic MBR for Wastewater Treatment. J. Microbiol. Biotechnol. 2016, 26, 1593–1604. [Google Scholar] [CrossRef]
- Schwab, M.; Bergonzi, C.; Sakkos, J.; Staley, C.; Zhang, Q.; Sadowsky, M.J.; Aksan, A.; Elias, M. Signal Disruption Leads to Changes in Bacterial Community Population. Front. Microbiol. 2019, 10, 611. [Google Scholar] [CrossRef]
- García-Contreras, R.; Maeda, T.; Wood, T.K. Can Resistance against Quorum-Sensing Interference Be Selected? ISME J. 2016, 10, 4–10. [Google Scholar] [CrossRef]
- Guendouze, A.; Plener, L.; Bzdrenga, J.; Jacquet, P.; Rémy, B.; Elias, M.; Lavigne, J.-P.; Daudé, D.; Chabrière, E. Effect of Quorum Quenching Lactonase in Clinical Isolates of Pseudomonas aeruginosa and Comparison with Quorum Sensing Inhibitors. Front. Microbiol. 2017, 8, 227. [Google Scholar] [CrossRef]
- Wang, R.; Ding, W.; Long, L.; Lan, Y.; Tong, H.; Saha, S.; Wong, Y.H.; Sun, J.; Li, Y.; Zhang, W.; et al. Exploring the Influence of Signal Molecules on Marine Biofilms Development. Front. Microbiol. 2020, 11, 571400. [Google Scholar] [CrossRef]
- Scarascia, G.; Lehmann, R.; Machuca, L.L.; Morris, C.; Cheng, K.Y.; Kaksonen, A.; Hong, P.-Y. Effect of Quorum Sensing on the Ability of Desulfovibrio vulgaris To Form Biofilms and To Biocorrode Carbon Steel in Saline Conditions. Appl. Environ. Microbiol. 2019, 86, e01664-19. [Google Scholar] [CrossRef]
- Kwong, T.F.N.; Miao, L.; Li, X.; Qian, P.Y. Novel Antifouling and Antimicrobial Compound from a Marine-Derived Fungus Ampelomyces sp. Mar. Biotechnol. 2006, 8, 634–640. [Google Scholar] [CrossRef]
- Leroy, C.; Delbarre-Ladrat, C.; Ghillebaert, F.; Rochet, M.J.; Compère, C.; Combes, D. A Marine Bacterial Adhesion Microplate Test Using the DAPI Fluorescent Dye: A New Method to Screen Antifouling Agents. Lett. Appl. Microbiol. 2007, 44, 372–378. [Google Scholar] [CrossRef]
- Wilsanand, V.; Wagh, A.B.; Bapuji, M. Antibacterial Activities of Anthozoan Corals on Some Marine Microfoulers. Microbios 1999, 99, 137–145. [Google Scholar]
- Huang, Z.; Meric, G.; Liu, Z.; Ma, R.; Tang, Z.; Lejeune, P. LuxS-Based Quorum-Sensing Signaling Affects Biofilm Formation in Streptococcus mutans. J. Mol. Microbiol. Biotechnol. 2009, 17, 12–19. [Google Scholar] [CrossRef]
- Jellali, R.; Campistron, I.; Pasetto, P.; Laguerre, A.; Gohier, F.; Hellio, C.; Pilard, J.-F.; Mouget, J.-L. Antifouling Activity of Novel Polyisoprene-Based Coatings Made from Photocurable Natural Rubber Derived Oligomers. Prog. Org. Coat. 2013, 76, 1203–1214. [Google Scholar] [CrossRef]
- Ortega-Morales, B.O.; Chan-Bacab, M.J.; Miranda-Tello, E.; Fardeau, M.-L.; Carrero, J.C.; Stein, T. Antifouling Activity of Sessile Bacilli Derived from Marine Surfaces. J. Ind. Microbiol. Biotechnol. 2008, 35, 9–15. [Google Scholar] [CrossRef]
- Plouguerné, E.; Hellio, C.; Cesconetto, C.; Thabard, M.; Mason, K.; Véron, B.; Pereira, R.C.; da Gama, B.A.P. Antifouling Activity as a Function of Population Variation in Sargassum vulgare from the Littoral of Rio de Janeiro (Brazil). J. Appl. Phycol. 2010, 22, 717–724. [Google Scholar] [CrossRef]
- Plouguerné, E.; Ioannou, E.; Georgantea, P.; Vagias, C.; Roussis, V.; Hellio, C.; Kraffe, E.; Stiger-Pouvreau, V. Anti-Microfouling Activity of Lipidic Metabolites from the Invasive Brown Alga Sargassum muticum (Yendo) Fensholt. Mar. Biotechnol. 2010, 12, 52–61. [Google Scholar] [CrossRef]
- Franklin, M.J.; Chang, C.; Akiyama, T.; Bothner, B. New Technologies for Studying Biofilms. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef]
- Allkja, J.; Bjarnsholt, T.; Coenye, T.; Cos, P.; Fallarero, A.; Harrison, J.; Lopes, S.; Oliver, A.; Pereira, M.; Ramage, G.; et al. Minimum Information Guideline for Spectrophotometric and Fluorometric Methods to Assess Biofilm Formation in Microplates. Biofilm 2019, 2, 100010. [Google Scholar] [CrossRef]
- Ferrer, M.D.; Rodriguez, J.C.; Álvarez, L.; Artacho, A.; Royo, G.; Mira, A. Effect of Antibiotics on Biofilm Inhibition and Induction Measured by Real-Time Cell Analysis. J. Appl. Microbiol. 2017, 122, 640–650. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Hidalgo-Cantabrana, C.; Rodríguez, A.; García, P.; Ruas-Madiedo, P. Monitoring in Real Time the Formation and Removal of Biofilms from Clinical Related Pathogens Using an Impedance-Based Technology. PLoS ONE 2016, 11, e0163966. [Google Scholar] [CrossRef]
- Exterkate, R.A.M.; Crielaard, W.; Ten Cate, J.M. Different Response to Amine Fluoride by Streptococcus mutans and Polymicrobial Biofilms in a Novel High-Throughput Active Attachment Model. Caries. Res. 2010, 44, 372–379. [Google Scholar] [CrossRef]
- Muras, A.; Mayer, C.; Otero-Casal, P.; Exterkate, R.A.M.; Brandt, B.W.; Crielaard, W.; Otero, A.; Krom, B.P. Short Chain N-Acylhomoserine Lactone Quorum Sensing Molecules Promote Periodontal Pathogens in in vitro Oral Biofilms. Appl. Environ. Microbiol. 2020, e01941-19. [Google Scholar] [CrossRef]
- Buchholtz, C.; Nielsen, K.F.; Milton, D.L.; Larsen, J.L.; Gram, L. Profiling of Acylated Homoserine Lactones of Vibrio anguillarum in vitro and in vivo: Influence of Growth Conditions and Serotype. Syst. Appl. Microbiol. 2006, 29, 433–445. [Google Scholar] [CrossRef]
- Cicirelli, E.M.; Williamson, H.; Tait, K.; Fuqua, C. Acylated Homoserine Lactone Signaling in Marine Bacterial Systems. In Chemical Communication among Bacteria; Winans, S.C., Bassler, B.L., Eds.; ASM Press: Washington, DC, USA, 2014; pp. 251–272. ISBN 978-1-68367-149-7. [Google Scholar]
- Izzati Mohamad, N.; Yin, W.-F.; Chan, K.-G. Whole-Genome Sequence of Quorum-Sensing Vibrio tubiashii Strain T33. Genome Announc. 2015, 3, e01362-14. [Google Scholar] [CrossRef]
- Rai, N.; Rai, R.; Venkatesh, K.V. Quorum Sensing Biosensors. In Quorum Sensing vs Quorum Quenching: A Battle with No End in Sight; Kalia, V.C., Ed.; Springer: New Delhi, India, 2015; pp. 173–183. ISBN 978-81-322-1982-8. [Google Scholar]
- D’Souza, F.; Bruin, A.; Biersteker, R.; Donnelly, G.; Klijnstra, J.; Rentrop, C.; Willemsen, P. Bacterial Assay for the Rapid Assessment of Antifouling and Fouling Release Properties of Coatings and Materials. J. Ind. Microbiol. Biotechnol. 2010, 37, 363–370. [Google Scholar] [CrossRef]
- Santopolo, L.; Marchi, E.; Frediani, L.; Decorosi, F.; Viti, C.; Giovannetti, L. A Novel Approach Combining the Calgary Biofilm Device and Phenotype MicroArray for the Characterization of the Chemical Sensitivity of Bacterial Biofilms. Biofouling 2012, 28, 1023–1032. [Google Scholar] [CrossRef]
- Shakeri, S.; Kermanshahi, R.K.; Moghaddam, M.M.; Emtiazi, G. Assessment of Biofilm Cell Removal and Killing and Biocide Efficacy Using the Microtiter Plate Test. Biofouling 2007, 23, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Martín-Rodríguez, A.J.; González-Orive, A.; Hernández-Creus, A.; Morales, A.; Dorta-Guerra, R.; Norte, M.; Martín, V.S.; Fernández, J.J. On the Influence of the Culture Conditions in Bacterial Antifouling Bioassays and Biofilm Properties: Shewanella algae, a Case Study. BMC Microbiol. 2014, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Poleunis, C.; Rubio, C.; Compère, C.; Bertrand, P. Role of Salts on the BSA Adsorption on Stainless Steel in Aqueous Solutions. II. ToF-SIMS Spectral and Chemical Mapping Study. Surf. Interface Anal. 2002, 34, 55–58. [Google Scholar] [CrossRef]
- Kierek, K.; Watnick, P.I. The Vibrio cholerae O139 O-Antigen Polysaccharide Is Essential for Ca2+-Dependent Biofilm Development in Sea Water. PNAS 2003, 100, 14357–14362. [Google Scholar] [CrossRef] [PubMed]
- Patrauchan, M.A.; Sarkisova, S.; Sauer, K.; Franklin, M.J. Calcium Influences Cellular and Extracellular Product Formation during Biofilm-Associated Growth of a Marine Pseudoalteromonas sp. Microbiology 2005, 151, 2885–2897. [Google Scholar] [CrossRef]
- Zeraik, A.E.; Nitschke, M. Influence of Growth Media and Temperature on Bacterial Adhesion to Polystyrene Surfaces. Braz. Arch. Biol. Technol. 2012, 55, 569–576. [Google Scholar] [CrossRef]
- Milton, D.L. Quorum Sensing in Vibrios: Complexity for Diversification. Int. J. Med. Microbiol. 2006, 296, 61–71. [Google Scholar] [CrossRef]
- Solano, C.; Echeverz, M.; Lasa, I. Biofilm Dispersion and Quorum Sensing. Curr. Opin. Microbiol. 2014, 18, 96–104. [Google Scholar] [CrossRef]
- Tait, K.; Hutchison, Z.; Thompson, F.L.; Munn, C.B. Quorum Sensing Signal Production and Inhibition by Coral-Associated Vibrios. Environ. Microbiol. Rep. 2010, 2, 145–150. [Google Scholar] [CrossRef]
- Huang, W.; Lin, Y.; Yi, S.; Liu, P.; Shen, J.; Shao, Z.; Liu, Z. QsdH, a Novel AHL Lactonase in the RND-Type Inner Membrane of Marine Pseudoalteromonas byunsanensis Strain 1A01261. PLoS ONE 2012, 7, e46587. [Google Scholar] [CrossRef]
- Huang, Y.; Ki, J.; Case, R.; Qian, P. Diversity and Acyl-Homoserine Lactone Production among Subtidal Biofilm-Forming Bacteria. Aquat. Microb. Ecol. 2008, 52, 185–193. [Google Scholar] [CrossRef]
- Wang, Y.; Ikawa, A.; Okaue, S.; Taniguchi, S.; Osaka, I.; Yoshimoto, A.; Kishida, Y.; Arakawa, R.; Enomoto, K. Quorum Sensing Signaling Molecules Involved in the Production of Violacein by Pseudoalteromonas. Biosci. Biotechnol. Biochem. 2008, 72, 1958–1961. [Google Scholar] [CrossRef]
- Yu, Z.; Yu, D.; Mao, Y.; Zhang, M.; Ding, M.; Zhang, J.; Wu, S.; Qiu, J.; Yin, J. Identification and Characterization of a LuxI/R-Type Quorum Sensing System in Pseudoalteromonas. Res. Microbiol. 2019, 170, 243–255. [Google Scholar] [CrossRef]
- Liu, N.; Yu, M.; Zhao, Y.; Cheng, J.; An, K.; Zhang, X.-H. PfmA, a Novel Quorum-Quenching N-Acylhomoserine Lactone Acylase from Pseudoalteromonas flavipulchra. Microbiology 2017, 163, 1389–1398. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Y.; Yan, X.; Liu, C.; Wu, B.; He, X.; Liang, Y. Quorum Quenching Enzyme APTM01, an Acylhomoserine-Lactone Acylase from Marine Bacterium of Pseudoalteromonas tetraodonis Strain MQS005. Curr. Microbiol. 2019, 76, 1387–1397. [Google Scholar] [CrossRef]
- Wang, T.-N.; Guan, Q.-T.; Pain, A.; Kaksonen, A.H.; Hong, P.-Y. Discovering, Characterizing, and Applying Acyl Homoserine Lactone-Quenching Enzymes to Mitigate Microbe-Associated Problems under Saline Conditions. Front. Microbiol. 2019, 10, 823. [Google Scholar] [CrossRef]
- Romero, M.; Muras, A.; Mayer, C.; Buján, N.; Magariños, B.; Otero, A. In vitro Quenching of Fish Pathogen Edwardsiella tarda AHL Production Using Marine Bacterium Tenacibaculum sp. Strain 20J Cell Extracts. Dis Aquat. Organ. 2014, 108, 217–225. [Google Scholar] [CrossRef]
- Echazarreta, M.A.; Klose, K.E. Vibrio Flagellar Synthesis. Front. Cell Infect. Microbiol. 2019, 9, 131. [Google Scholar] [CrossRef]
- Kuehl, R.; Al-Bataineh, S.; Gordon, O.; Luginbuehl, R.; Otto, M.; Textor, M.; Landmann, R. Furanone at Subinhibitory Concentrations Enhances Staphylococcal Biofilm Formation by LuxS Repression. Antimicrob. Agents Chemother. 2009, 53, 4159–4166. [Google Scholar] [CrossRef]
- Manefield, M.; Rasmussen, T.B.; Henzter, M.; Andersen, J.B.; Steinberg, P.; Kjelleberg, S.; Givskov, M. Halogenated Furanones Inhibit Quorum Sensing through Accelerated LuxR Turnover. Microbiology 2002, 148, 1119–1127. [Google Scholar] [CrossRef]
- Hentzer, M.; Wu, H.; Andersen, J.B.; Riedel, K.; Rasmussen, T.B.; Bagge, N.; Kumar, N.; Schembri, M.A.; Song, Z.; Kristoffersen, P.; et al. Attenuation of Pseudomonas aeruginosa Virulence by Quorum Sensing Inhibitors. EMBO J. 2003, 22, 3803–3815. [Google Scholar] [CrossRef]
- Martinelli, D.; Grossmann, G.; Séquin, U.; Brandl, H.; Bachofen, R. Effects of Natural and Chemically Synthesized Furanones on Quorum Sensing in Chromobacterium violaceum. BMC Microbiol. 2004, 4, 25. [Google Scholar] [CrossRef]
- Maximilien, R.; de Nys, R.; Holmström, C.; Gram, L.; Givskov, M.C.; Crass, K.; Kjelleberg, S.; Steinberg, P. Chemical Mediation of Bacterial Surface Colonisation by Secondary Metabolites from the Red Alga Delisea pulchra. Aquat. Microb. Ecol. 1998, 15, 233–246. [Google Scholar] [CrossRef]
- Rasch, M.; Buch, C.; Austin, B.; Slierendrecht, W.J.; Ekmann, K.S.; Larsen, J.L.; Johansen, C.; Riedel, K.; Eberl, L.; Givskov, M.; et al. An Inhibitor of Bacterial Quorum Sensing Reduces Mortalities Caused by Vibriosis in Rainbow Trout (Oncorhynchus mykiss, Walbaum). Syst. Appl. Microbiol. 2004, 27, 350–359. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, Y.-G.; Zeng, L.-Y.; Pan, Y.; Huang, X.-Y.; Bian, L.-Q.; Zhu, Y.-J.; Zhang, R.-R.; Zhang, J. Evaluation of Antibacterial and Anti-Biofilm Properties of Kojic Acid against Five Food-Related Bacteria and Related Subcellular Mechanisms of Bacterial Inactivation. Food Sci. Technol. Int. 2019, 25, 3–15. [Google Scholar] [CrossRef]
- Bala, A.; Kumar, R.; Harjai, K. Inhibition of Quorum Sensing in Pseudomonas aeruginosa by Azithromycin and Its Effectiveness in Urinary Tract Infections. J. Med. Microbiol. 2011, 60, 300–306. [Google Scholar] [CrossRef]
- Tateda, K.; Comte, R.; Pechere, J.C.; Köhler, T.; Yamaguchi, K.; Van Delden, C. Azithromycin Inhibits Quorum Sensing in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2001, 45, 1930–1933. [Google Scholar] [CrossRef]
- Romero, M.; Martín-Cuadrado, A.B.; Roca-Rivada, A.; Cabello, A.M.; Otero, A. Quorum Quenching in Cultivable Bacteria from Dense Marine Coastal Microbial Communities: Quorum Quenching in Marine Bacteria. FEMS Microbiol. Ecol. 2011, 75, 205–217. [Google Scholar] [CrossRef]
- McClean, K.H.; Winson, M.K.; Fish, L.; Taylor, A.; Chhabra, S.R.; Cámara, M.; Daykin, M.; Lamb, J.H.; Swift, S.; Bycroft, B.W.; et al. Quorum Sensing and Chromobacterium Violaceum: Exploitation of Violacein Production and Inhibition for the Detection of N-Acylhomoserine Lactones. Microbiology 1997, 143, 3703–3711. [Google Scholar] [CrossRef]
- Morohoshi, T.; Kato, M.; Fukamachi, K.; Kato, N.; Ikeda, T. N-Acylhomoserine Lactone Regulates Violacein Production in Chromobacterium violaceum Type Strain ATCC 12472. FEMS Microbiol. Lett. 2008, 279, 124–130. [Google Scholar] [CrossRef]
- Romero, M.; Avendaño-Herrera, R.; Magariños, B.; Cámara, M.; Otero, A. Acylhomoserine Lactone Production and Degradation by the Fish Pathogen Tenacibaculum maritimum, a Member of the Cytophaga-Flavobacterium-Bacteroides (CFB) Group. FEMS Microbiol. Lett. 2010, 304, 131–139. [Google Scholar] [CrossRef]
- Yates, E.A.; Philipp, B.; Buckley, C.; Atkinson, S.; Chhabra, S.R.; Sockett, R.E.; Goldner, M.; Dessaux, Y.; Cámara, M.; Smith, H.; et al. N-Acylhomoserine Lactones Undergo Lactonolysis in a PH-, Temperature-, and Acyl Chain Length-Dependent Manner during Growth of Yersinia pseudotuberculosis and Pseudomonas aeruginosa. Infect. Immun. 2002, 70, 5635–5646. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.; Diggle, S.P.; Heeb, S.; Cámara, M.; Otero, A. Quorum Quenching Activity in Anabaena sp. PCC 7120: Identification of AiiC, a Novel AHL-Acylase. FEMS Microbiol. Lett. 2008, 280, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of Multiple Methods for Quantification of Microbial Biofilms Grown in Microtiter Plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar] [CrossRef] [PubMed]


| P. flavipulchra | P. maricaloris | V. aestuarianus CECT625 | V. tubiashii CECT631 | |
|---|---|---|---|---|
| C4-HSL | 374 | 347 | 626 | 503 |
| OC6-HSL | 2.31 | 0.49 | 0.8 | - |
| C7-HSL | 1.06 | - | - | - |
| C8-HSL | 0.74 | 0.66 | 39.2 | - |
| OC8-HSL | - | 0.46 | 15 | - |
| C10-HSL | 0.68 | - | 34.3 | - |
| OC10-HSL | 5.79 | 1.13 | 2958 | - |
| HC10-HSL | - | - | 26.47 | - |
| C12-HSL | - | - | 2.97 | - |
| OC12-HSL | - | - | 41.66 | - |
| HC12-HSL | - | - | 3.32 | - |
| C16-HSL | - | - | 2.2 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muras, A.; Parga, A.; Mayer, C.; Otero, A. Use of Quorum Sensing Inhibition Strategies to Control Microfouling. Mar. Drugs 2021, 19, 74. https://doi.org/10.3390/md19020074
Muras A, Parga A, Mayer C, Otero A. Use of Quorum Sensing Inhibition Strategies to Control Microfouling. Marine Drugs. 2021; 19(2):74. https://doi.org/10.3390/md19020074
Chicago/Turabian StyleMuras, Andrea, Ana Parga, Celia Mayer, and Ana Otero. 2021. "Use of Quorum Sensing Inhibition Strategies to Control Microfouling" Marine Drugs 19, no. 2: 74. https://doi.org/10.3390/md19020074
APA StyleMuras, A., Parga, A., Mayer, C., & Otero, A. (2021). Use of Quorum Sensing Inhibition Strategies to Control Microfouling. Marine Drugs, 19(2), 74. https://doi.org/10.3390/md19020074







