DSP Toxin Distribution across Organs in Mice after Acute Oral Administration
Abstract
1. Introduction
2. Results
2.1. Lethality and Symptoms
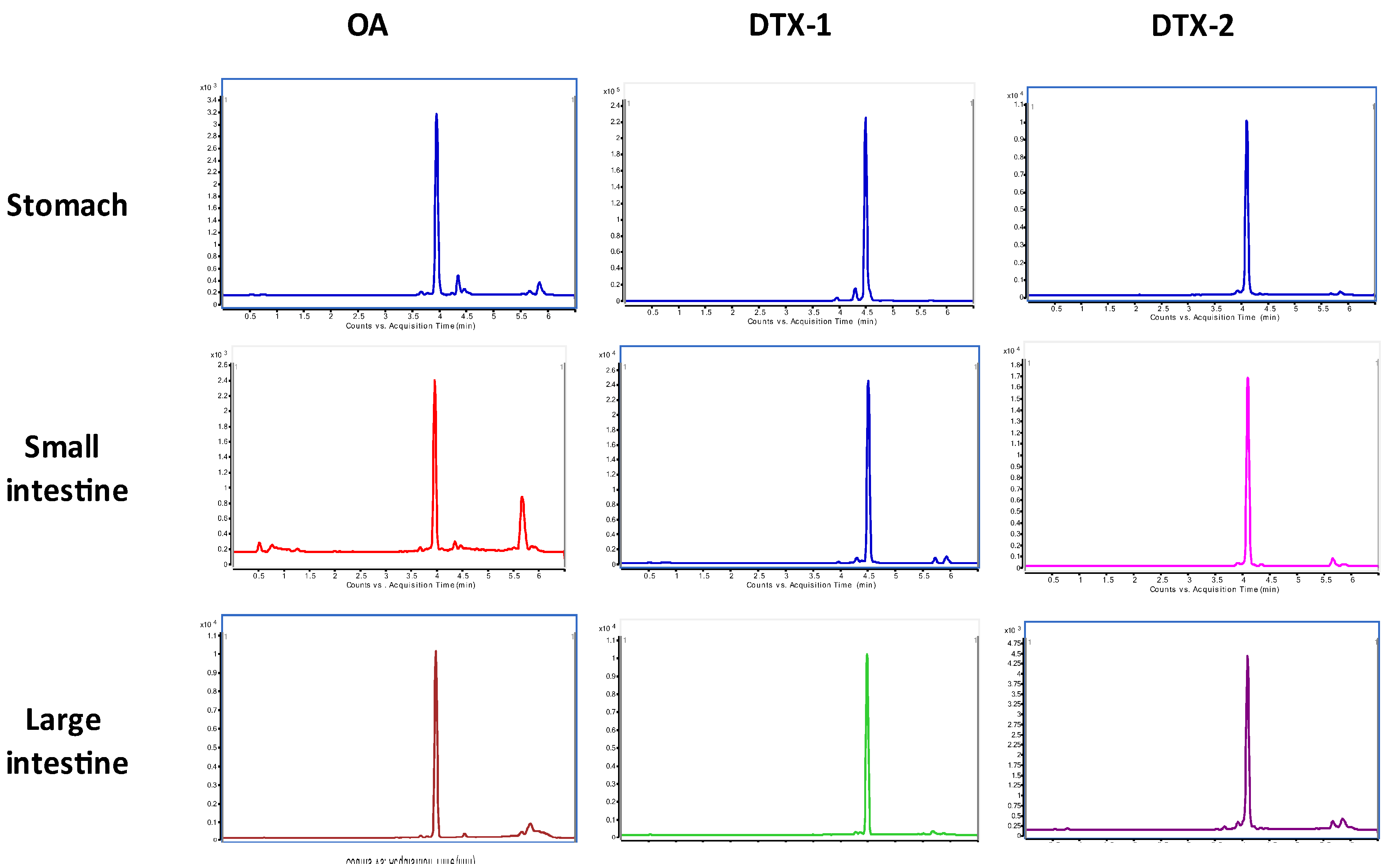
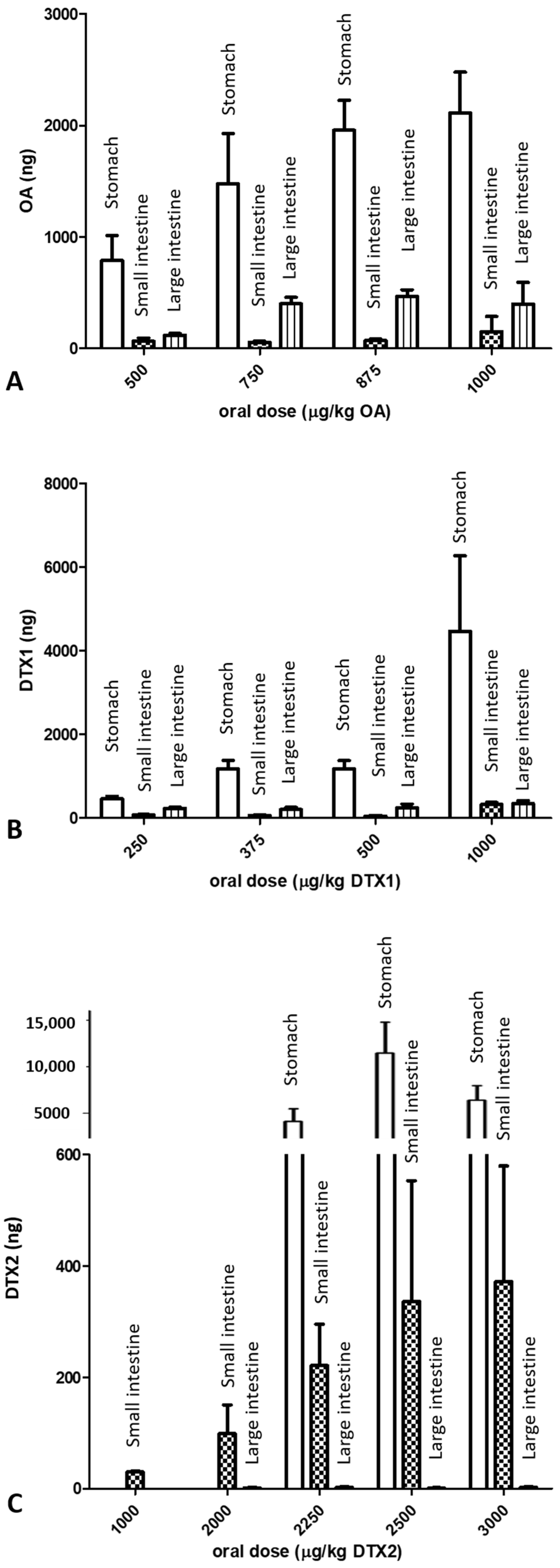
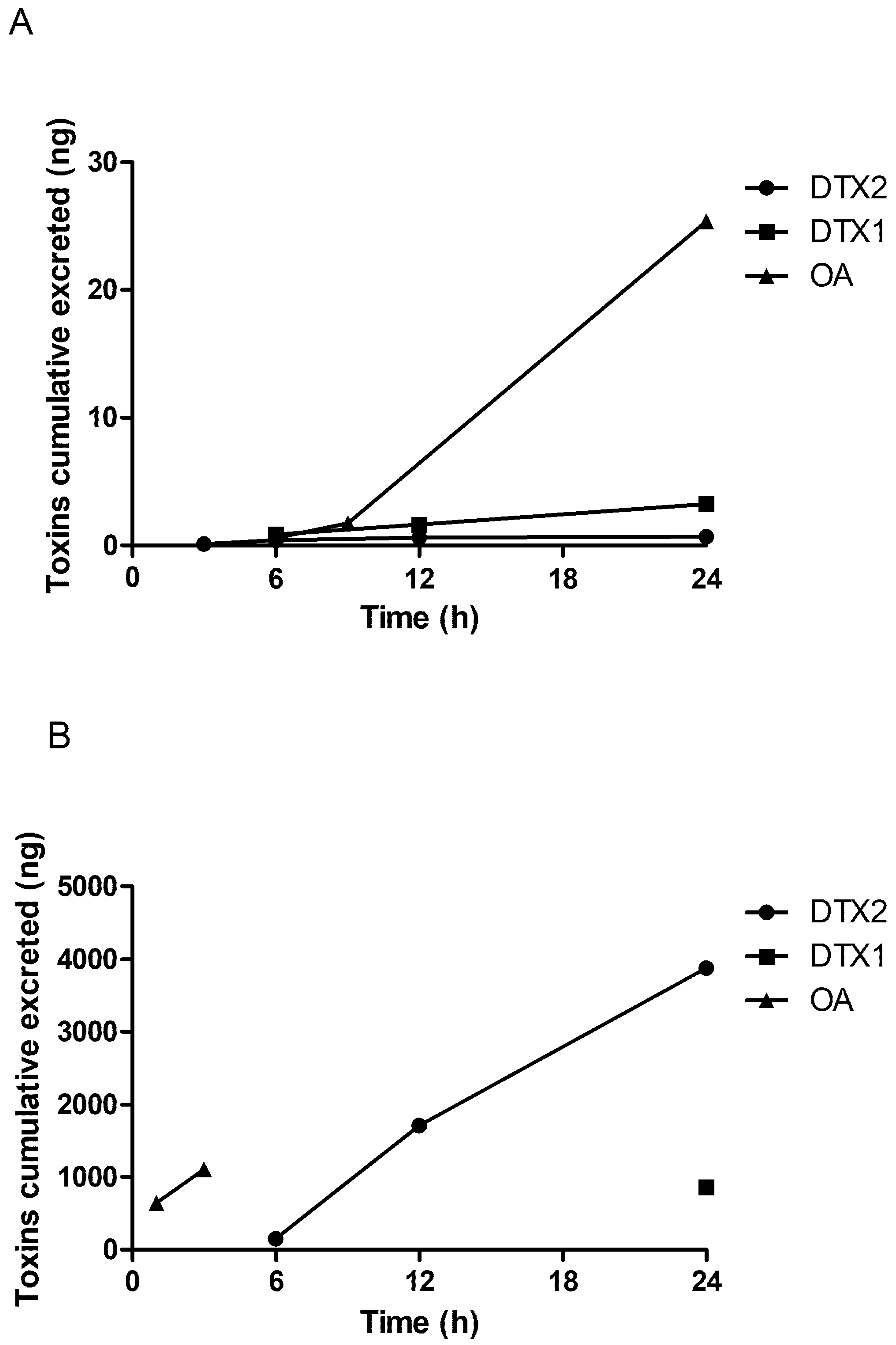
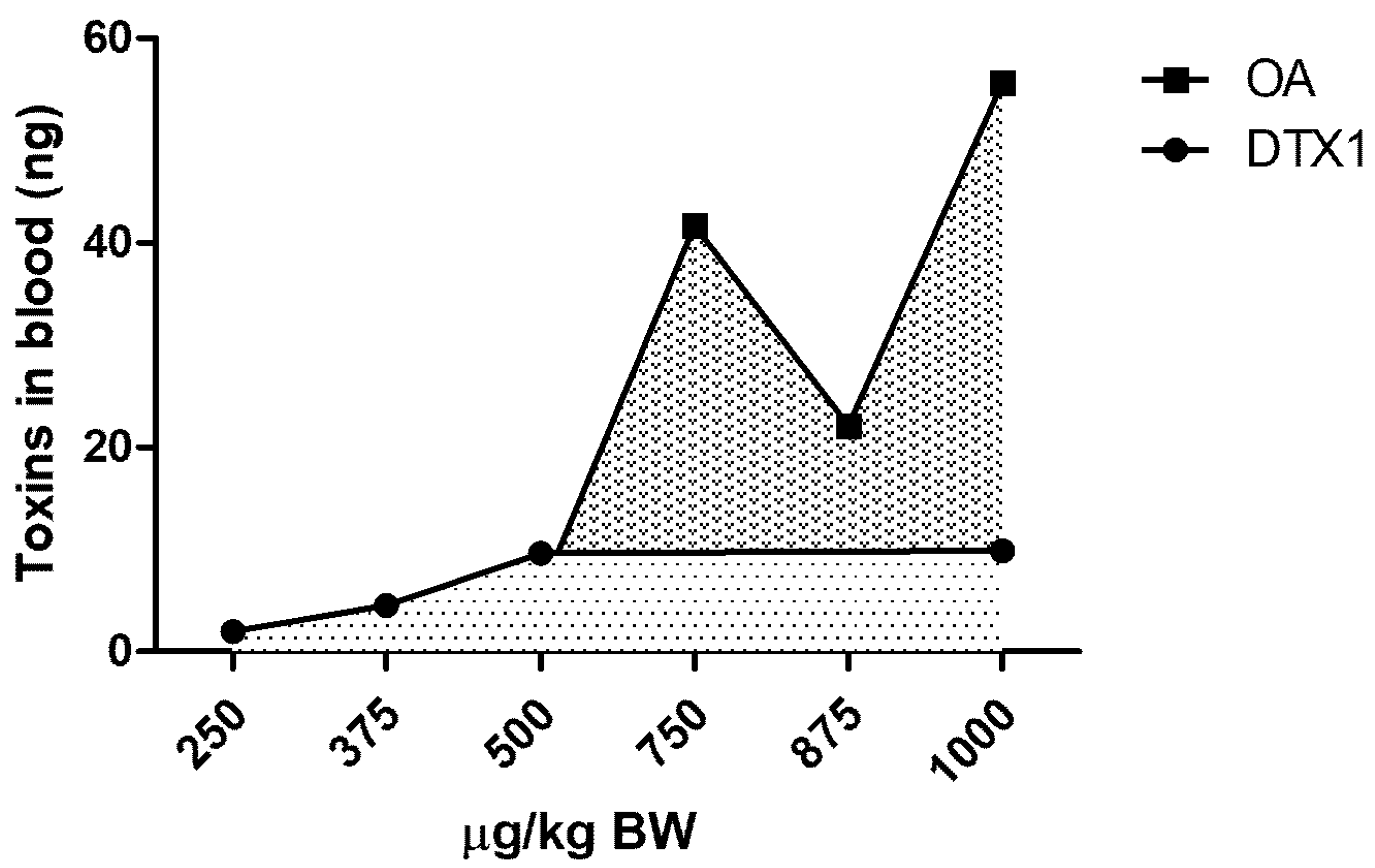
2.2. LC/MS/MS Analysis
3. Discussion
4. Materials and Methods
4.1. In Vivo Assays and Animal Conditions
4.2. LC/MS/MS Analysis of Mice Organs
LC/MS/MS Conditions
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yasumoto, T.; Oshima, Y.; Sugawara, W.; Fukuyo, Y.; Oguri, H.; Igarashi, T.; Fujita, N. Identification of Dinophysis fortii as the causative organism of diarrhetic shellfish poisoning. Nippon Suisan Gakkaishi 1980, 46, 1405–1411. [Google Scholar] [CrossRef]
- Van Dolah, F.M. Marine algal toxins: Origins, health effects, and their increased occurrence. Environ. Health Perspect. 2000, 108, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Reguera, B.; Riobó, P.; Rodríguez, F.; Díaz, P.A.; Pizarro, G.; Paz, B.; Franco, J.M.; Blanco, J. Dinophysis Toxins: Causative Organisms, Distribution and Fate in Shellfish. Mar. Drugs 2014, 12, 394–461. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.; Freitas, M.; De Almeida, A.M.; Martins, J.C.; Domínguez-Pérez, D.; Osório, H.; Vasconcelos, V.; Costa, P.R. OMICs Approaches in Diarrhetic Shellfish Toxins Research. Toxins 2020, 12, 493. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Doyle, J.; Jackson, D.; Marr, J.; Nixon, E.; Pleasance, S.; Quilliam, M.A.; Walter, J.A.; Wright, J.L.C. Isolation of a new diarrhetic shellfish poison from Irish mussels. J. Chem. Soc., Chem. Commun. 1992, 10, 39–41. [Google Scholar] [CrossRef]
- Twiner, M.J.; Doucette, G.J.; Pang, Y.; Fang, C.; Forsyth, C.J.; Miles, C.O. Structure–Activity Relationship Studies Using Natural and Synthetic Okadaic Acid/Dinophysistoxin Toxins. Mar. Drugs 2016, 14, 207. [Google Scholar] [CrossRef] [PubMed]
- Vilariño, N.; Louzao, M.C.; Abal, P.; Cagide, E.; Carrera, C.; Vieytes, M.R.; Botana, L.M. Human Poisoning from Marine Toxins: Unknowns for Optimal Consumer Protection. Toxins 2018, 10, 324. [Google Scholar] [CrossRef]
- Trainer, V.L.; Moore, L.; Bill, B.D.; Adams, N.G.; Harrington, N.; Borchert, J.; Da Silva, D.A.M.; Eberhart, B.-T.L. Diarrhetic Shellfish Toxins and Other Lipophilic Toxins of Human Health Concern in Washington State. Mar. Drugs 2013, 11, 1815–1835. [Google Scholar] [CrossRef]
- Carmody, E.P.; James, K.J.; Kelly, S.S. Dinophysistoxin-2: The predominant diarrhoetic shellfish toxin in Ireland. Toxicon 1996, 34, 351–359. [Google Scholar] [CrossRef]
- Salas, R.; Clarke, D. Review of DSP Toxicity in Ireland: Long-Term Trend Impacts, Biodiversity and Toxin Profiles from a Monitoring Perspective. Toxins 2019, 11, 61. [Google Scholar] [CrossRef]
- Blanco, J. Accumulation of Dinophysis Toxins in Bivalve Molluscs. Toxins 2018, 10, 453. [Google Scholar] [CrossRef]
- Visciano, P.; Schirone, M.; Berti, M.; Milandri, A.; Tofalo, R.; Suzzi, G. Marine Biotoxins: Occurrence, Toxicity, Regulatory Limits and Reference Methods. Front. Microbiol. 2016, 7, 1051. [Google Scholar] [CrossRef]
- Takai, A.; Murata, M.; Torigoe, K.; Isobe, M.; Mieskes, G.; Yasumoto, T. Inhibitory effect of okadaic acid derivatives on protein phosphatases. A study on structure-affinity relationship. Biochem. J. 1992, 284, 539–544. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Technical Paper on Toxicity Equivalency Factors for Marine Biotoxins Associated with Bivalve Molluscs; FAO/WHO: Rome, Italy, 2016; p. 108. [Google Scholar]
- Abal, P.; Louzao, M.C.; Suzuki, T.; Watanabe, R.; Vilariño, N.; Carrera, C.; Botana, A.M.; Vieytes, M.R.; Botana, L.M. Toxic Action Reevaluation of Okadaic Acid, Dinophysistoxin-1 and Dinophysistoxin-2: Toxicity Equivalency Factors Based on the Oral Toxicity Study. Cell. Physiol. Biochem. 2018, 49, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.F.; Holmes, C.F.; John, F.D. Molecular mechanisms underlying inhibition of protein phosphatases by marine toxins. Front. Biosci. 1999, 4, d646–d658. [Google Scholar] [CrossRef] [PubMed]
- Valdiglesias, V.; Prego-Faraldo, M.V.; Pásaro, E.; Méndez, J.; Laffon, B. Okadaic Acid: More than a Diarrheic Toxin. Mar. Drugs 2013, 11, 4328–4349. [Google Scholar] [CrossRef]
- Munday, R. Is Protein Phosphatase Inhibition Responsible for the Toxic Effects of Okadaic Acid in Animals? Toxins 2013, 5, 267–285. [Google Scholar] [CrossRef]
- Ito, E.; Terao, K. Injury and recovery process of intestine caused by okadaic acid and related compounds. Nat. Toxins 1994, 2, 371–377. [Google Scholar]
- Ito, E.; Yasumoto, T.; Takai, A.; Imanishi, S.Y.; Harada, K. Investigation of the distribution and excretion of okadaic acid in mice using immunostaining method. Toxicon 2002, 40, 159–165. [Google Scholar] [CrossRef]
- Franchinia, A.; Marchesini, E.; Poletti, R.; Ottaviani, E. Swiss mice CD1 fed on mussels contaminated by okadaic acid and yessotoxins: Effects on thymus and spleen. Eur. J. Histochem. 2005, 49, 179–188. [Google Scholar]
- Fu, L.-L.; Zhao, X.-Y.; Ji, L.-D.; Xu, J. Okadaic acid (OA): Toxicity, detection and detoxification. Toxicon 2019, 160, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.-Y.; Lin, L.; Gao, Y.; Hong, H.-S.; Wang, D.-Z. Quantitative proteomic analysis of okadaic acid treated mouse small intestines reveals differentially expressed proteins involved in diarrhetic shellfish poisoning. J. Proteom. 2012, 75, 2038–2052. [Google Scholar] [CrossRef] [PubMed]
- Louzao, M.C.; Fernández, D.A.; Abal, P.; Fraga-Corral, M.; Vilariño, N.; Vieytes, M.R.; Botana, L.M. Diarrhetic effect of okadaic acid could be related with its neuronal action: Changes in neuropeptide Y. Toxicol. Lett. 2015, 237, 151–160. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Opinion of the Scientific Panel on Contaminants in the Food chain on a request from the European Commission Marine biotoxins in shellfish-okadaic acid and analogues. EFSA J. 2008, 589, 1–62. [Google Scholar]
- Tubaro, A.; Sosa, S.; Carbonatto, M.; Altinier, G.; Vita, F.; Melato, M.; Satake, M.; Yasumoto, T. Oral and intraperitoneal acute toxicity studies of yessotoxin and homoyessotoxins in mice. Toxicon 2003, 41, 783–792. [Google Scholar] [CrossRef]
- Abal, P.; Louzao, M.C.; Cifuentes, J.M.; Vilariño, N.; Rodríguez, I.; Alfonso, A.; Vieytes, M.R.; Botana, L.M. Characterization of the dinophysistoxin-2 acute oral toxicity in mice to define the Toxicity Equivalency Factor. Food Chem. Toxicol. 2017, 102, 166–175. [Google Scholar] [CrossRef]
- EU-RL-MB. EU-RL: EU-Harmonised Standard Operating Procedure for Determination of Lipophilic Marine Biotoxins in Molluscs by LC–MS/MS; Version 5; EU-RL-MB: Vigo, Spain, 2015. [Google Scholar]
- Uchida, H.; Watanabe, R.; Matsushima, R.; Oikawa, H.; Nagai, S.; Kamiyama, T.; Baba, K.; Miyazono, A.; Kosaka, Y.; Kaga, S.; et al. Toxin Profiles of Okadaic Acid Analogues and Other Lipophilic Toxins in Dinophysis from Japanese Coastal Waters. Toxins 2018, 10, 457. [Google Scholar] [CrossRef]
- Alves, T.; Mafra, L.L. Diel Variations in Cell Abundance and Trophic Transfer of Diarrheic Toxins during a Massive Dinophysis Bloom in Southern Brazil. Toxins 2018, 10, 232. [Google Scholar] [CrossRef]
- Aune, T.; Larsen, S.; Aasen, J.A.; Rehmann, N.; Satake, M.; Hess, P. Relative toxicity of dinophysistoxin-2 (DTX-2) compared with okadaic acid, based on acute intraperitoneal toxicity in mice. Toxicon 2007, 49, 1–7. [Google Scholar] [CrossRef]
- Aune, T.; Espenes, A.; Aasen, J.A.B.; Quilliam, M.A.; Hess, P.; Larsen, S. Study of possible combined toxic effects of azaspiracid-1 and okadaic acid in mice via the oral route. Toxicon 2012, 60, 895–906. [Google Scholar] [CrossRef]
- Dietrich, J.; Grass, I.; Günzel, D.; Herek, S.; Braeuning, A.; Lampen, A.; Hessel-Pras, S. The marine biotoxin okadaic acid affects intestinal tight junction proteins in human intestinal cells. Toxicol. Vitr. 2019, 58, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Matias, W.G.; Traore, A.; Creppy, E. Variations in the distribution of okadaic acid in organs and biological fluids of mice related to diarrhoeic syndrome. Hum. Exp. Toxicol. 1999, 18, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Chernoff, N.; Hill, D.; Lang, J.R.; Schmid, J.; Le, T.; Farthing, A.; Huang, H. The Comparative Toxicity of 10 Microcystin Congeners Administered Orally to Mice: Clinical Effects and Organ Toxicity. Toxins 2020, 12, 403. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, T.; Murata, M.; Oshima, Y.; Sano, M.; Matsumoto, G.; Clardy, J. Diarrhetic shellfish toxins. Tetrahedron 1985, 41, 1019–1025. [Google Scholar] [CrossRef]
- Larsen, K.; Petersen, D.; Wilkins, A.L.; Samdal, I.A.; Sandvik, M.; Rundberget, T.; Goldstone, D.C.; Arcus, V.; Hovgaard, P.; Rise, F.; et al. Clarification of the C-35 Stereochemistries of Dinophysistoxin-1 and Dinophysistoxin-2 and Its Consequences for Binding to Protein Phosphatase. Chem. Res. Toxicol. 2007, 20, 868–875. [Google Scholar] [CrossRef]
- Sosa, S.; Ardizzone, M.; Beltramo, D.; Vita, F.; Dell’Ovo, V.; Barreras, A.; Yasumoto, T.; Tubaro, A. Repeated oral co-exposure to yessotoxin and okadaic acid: A short term toxicity study in mice. Toxicon 2013, 76, 94–102. [Google Scholar] [CrossRef]
- Tubaro, A.; Sosa, S.; Altinier, G.; Soranzo, M.; Satake, M.; Della Loggia, R.; Yasumoto, T. Short-term oral toxicity of homoyessotoxins, yessotoxin and okadaic acid in mice. Toxicon 2004, 43, 439–445. [Google Scholar] [CrossRef]
- Suzuki, H.; Okada, Y. Comparative toxicity of dinophysistoxin-1 and okadaic acid in mice. J. Veter Med Sci. 2018, 80, 616–619. [Google Scholar] [CrossRef]
- Reale, O.; Huguet, A.; Fessard, V. Novel Insights on the Toxicity of Phycotoxins on the Gut through the Targeting of Enteric Glial Cells. Mar. Drugs 2019, 17, 429. [Google Scholar] [CrossRef]
- Fernández, D.A.; Louzao, M.C.; Fraga-Corral, M.; Vilariño, N.; Vieytes, M.R.; Botana, L.M. Experimental Basis for the High Oral Toxicity of Dinophysistoxin 1: A Comparative Study of DSP. Toxins 2014, 6, 211–228. [Google Scholar] [CrossRef]
- Nielsen, L.T.; Hansen, P.J.; Krock, B.; Vismann, B. Accumulation, transformation and breakdown of DSP toxins from the toxic dinoflagellate Dinophysis acuta in blue mussels, Mytilus edulis. Toxicon 2016, 117, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, R.; Uchida, H.; Watanabe, R.; Oikawa, H.; Kosaka, Y.; Tanabe, T.; Suzuki, T. Distribution of Diarrhetic Shellfish Toxins in Mussels, Scallops, and Ascidian. Food Saf. 2018, 6, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, J.; Peng, J.; Zhong, Y.; Zheng, G.; Guo, M.; Tan, Z.-J.; Zhai, Y.; Lu, S. Nontarget Screening and Toxicity Evaluation of Diol Esters of Okadaic Acid and Dinophysistoxins Reveal Intraspecies Difference of Prorocentrum lima. Environ. Sci. Technol. 2020, 54, 12366–12375. [Google Scholar] [CrossRef] [PubMed]
- Kolrep, F.; Rein, K.S.; Lampen, A.; Hessel-Pras, S. Metabolism of okadaic acid by NADPH-dependent enzymes present in human or rat liver S9 fractions results in different toxic effects. Toxicol. Vitr. 2017, 42, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Huhn, J.; Jeffrey, P.D.; Larsen, K.; Rundberget, T.; Rise, F.; Cox, N.R.; Arcus, V.; Shi, Y.; Miles, C.O. A Structural Basis for the Reduced Toxicity of Dinophysistoxin-2. Chem. Res. Toxicol. 2009, 22, 1782–1786. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Watanabe, R.; Yoshino, A.; Oikawa, H.; Uchida, H.; Matsushima, R.; Nagai, S.; Kamiyama, T.; Yamazaki, T.; Kawaguchi, M.; et al. Preparation of diarrheic shellfish toxins (DSTs) and paralytic shellfish toxins (PSTs) by large algal culture and chemical conversion. In Proceedings of the 16th International Conference on Harmful Algae and International Society for the Study of Harmful Algae, Cawthron Institute, Wellington, New Zealand, 27–31 October 2014; pp. 34–39. [Google Scholar]
- Watanabe, R.; Sugai, C.; Yamazaki, T.; Matsushima, R.; Uchida, H.; Matsumiya, M.; Takatsu, A.; Suzuki, T. Quantitative Nuclear Magnetic Resonance Spectroscopy Based on PULCON Methodology: Application to Quantification of Invaluable Marine Toxin, Okadaic Acid. Toxins 2016, 8, 294. [Google Scholar] [CrossRef] [PubMed]
- OECD/OCDE. OECD Guidelines for the Testing of Chemicals 425. Acute Oral Toxicity-Up and Down Procedure; Organisation for Economic Co-operation and Development: Paris, France, 2008; pp. 1–27. [Google Scholar]
- Guada, M.; Imbuluzqueta, E.; De Mendoza, A.E.-H.; Lana, H.; Dios-Viéitez, M.; Blanco-Prieto, M. Ultra high performance liquid chromatography–tandem mass spectrometry method for cyclosporine a quantification in biological samples and lipid nanosystems. J. Chromatogr. B 2013, 927, 164–172. [Google Scholar] [CrossRef]


| Toxin | Dose (µg/kg bw) | Lethality (%) | Number Mice |
|---|---|---|---|
| OA | 1000 | 67 | 3 |
| 875 | 67 | 9 | |
| 750 | 43 | 7 | |
| 500 | 40 | 5 | |
| DTX1 | 1000 | 67 | 3 |
| 500 | 60 | 5 | |
| 375 | 0 | 9 | |
| 250 | 0 | 7 | |
| DTX2 | 3000 | 100 | 5 |
| 2500 | 86 | 7 | |
| 2250 | 44 | 9 | |
| 2000 | 33 | 3 | |
| 1000 | 0 | 3 |
| Appearance of Symptoms (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OA (µg/kg bw) | DTX1 (µg/kg bw) | DTX2 (µg/kg bw) | |||||||||||
| Symptom | 1000 | 875 | 750 | 500 | 1000 | 500 | 375 | 250 | 3000 | 2500 | 2250 | 2000 | 1000 |
| diarrhea | 66.67 | 100 | 57.14 | 100 | 66.67 | 80 | 66.67 | 71.43 | 100 | 100 | 33.33 | 66.67 | 66.67 |
| apathy | 100 | 88.89 | 100 | 100 | 100 | 100 | 88.89 | 85.71 | 100 | 100 | 44.44 | 66.67 | 66.67 |
| piloerection | 100 | 55.56 | 42.86 | 20 | 33.33 | 40 | 44.44 | 28.57 | 40 | 14.29 | 11.11 | 33.33 | 66.67 |
| squint-eyes | 100 | 77.78 | 57.14 | 60 | 100 | 80 | 77.78 | 57.14 | 20 | 0 | 0 | 0 | 33.33 |
| spasms | 33.33 | 22.22 | 28.57 | 0 | 33.33 | 20 | 22.22 | 28.57 | 0 | 0 | 11.11 | 33.33 | 33.33 |
| cyanosis | 66.67 | 88.89 | 57.14 | 0 | 66.67 | 80 | 66.67 | 0 | 60 | 85.71 | 33.33 | 66.67 | 0 |
| on hind legs | 0 | 44.44 | 14.29 | 60 | 0 | 20 | 22.22 | 14.29 | 0 | 14.29 | 0 | 0 | 33.33 |
| dyspnea | 0 | 0 | 14.29 | 0 | 33.33 | 0 | 0 | 0 | 40 | 14.29 | 0 | 0 | 0 |
| Liver | Stomach | Small Intestine | Large Intestine | |
|---|---|---|---|---|
| OA | 96 ± 23 | 4540 ± 1326 | 408 ± 271 | 2340 ± 706 |
| DTX1 | 93 ± 6 | 3006 ± 782 | 182 ± 27 | 1009 ± 83 |
| DTX2 | 21 ± 11 * | 142 ± 105 * | 75 ± 38 | 176 ± 120 * |
| Stomach Content | Small Intestine Content | Large Intestine Content | |
|---|---|---|---|
| OA | 2112 ± 365 | 151 ± 135 | 397 ± 193 |
| DTX1 | 4468 ± 1802 | 321 ± 50 | 341 ± 72 |
| DTX2 | - | 30 ± 1.3 | 0.19 ± 0.092 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Louzao, M.C.; Abal, P.; Costas, C.; Suzuki, T.; Watanabe, R.; Vilariño, N.; Botana, A.M.; R. Vieytes, M.; Botana, L.M. DSP Toxin Distribution across Organs in Mice after Acute Oral Administration. Mar. Drugs 2021, 19, 23. https://doi.org/10.3390/md19010023
Louzao MC, Abal P, Costas C, Suzuki T, Watanabe R, Vilariño N, Botana AM, R. Vieytes M, Botana LM. DSP Toxin Distribution across Organs in Mice after Acute Oral Administration. Marine Drugs. 2021; 19(1):23. https://doi.org/10.3390/md19010023
Chicago/Turabian StyleLouzao, M. Carmen, Paula Abal, Celia Costas, Toshiyuki Suzuki, Ryuichi Watanabe, Natalia Vilariño, Ana M. Botana, Mercedes R. Vieytes, and Luis M. Botana. 2021. "DSP Toxin Distribution across Organs in Mice after Acute Oral Administration" Marine Drugs 19, no. 1: 23. https://doi.org/10.3390/md19010023
APA StyleLouzao, M. C., Abal, P., Costas, C., Suzuki, T., Watanabe, R., Vilariño, N., Botana, A. M., R. Vieytes, M., & Botana, L. M. (2021). DSP Toxin Distribution across Organs in Mice after Acute Oral Administration. Marine Drugs, 19(1), 23. https://doi.org/10.3390/md19010023







