Phosphatidylserine from Portunus trituberculatus Eggs Alleviates Insulin Resistance and Alters the Gut Microbiota in High-Fat-Diet-Fed Mice
Abstract
1. Introduction
2. Results
2.1. Fatty Acid Composition of Pt-PS
2.2. Pt-PS Protects Mice from HFD-Induced Obesity
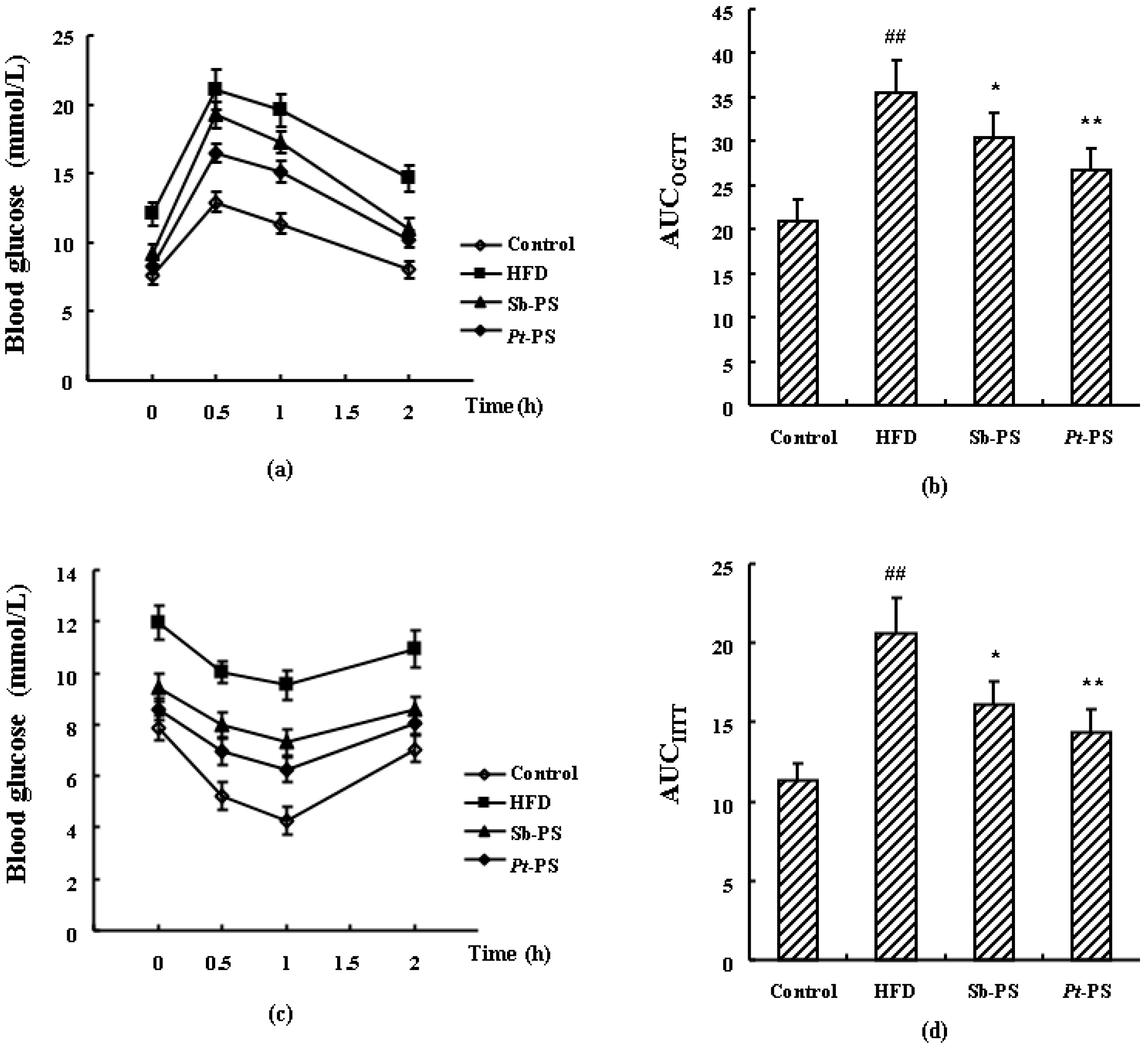
2.3. Pt-PS Improves Insulin Resistance
2.4. Pt-PS Regulates Serum Adipokines
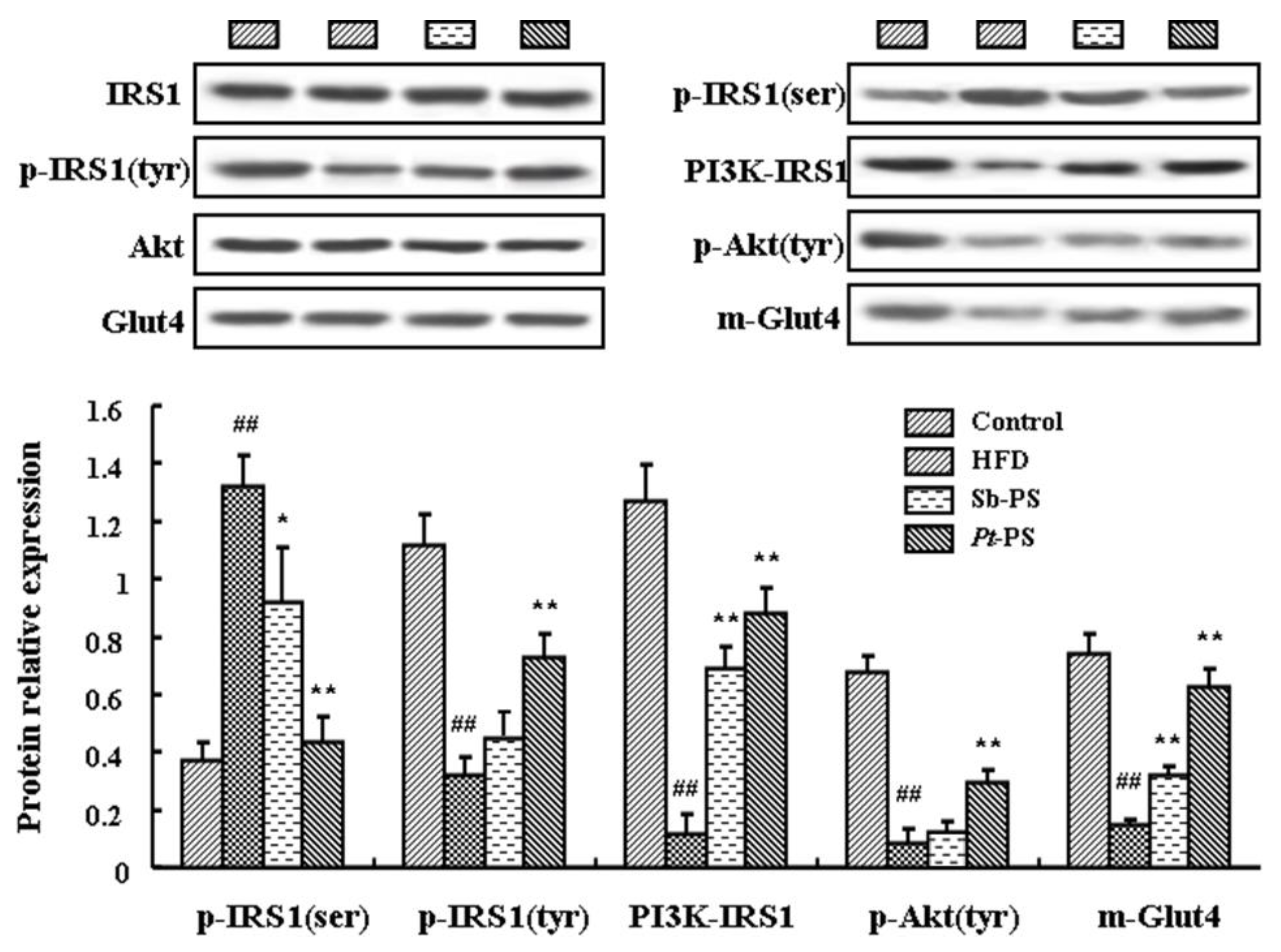
2.5. Pt-PS Activates the PI3K/Akt Pathway in Skeletal Muscle
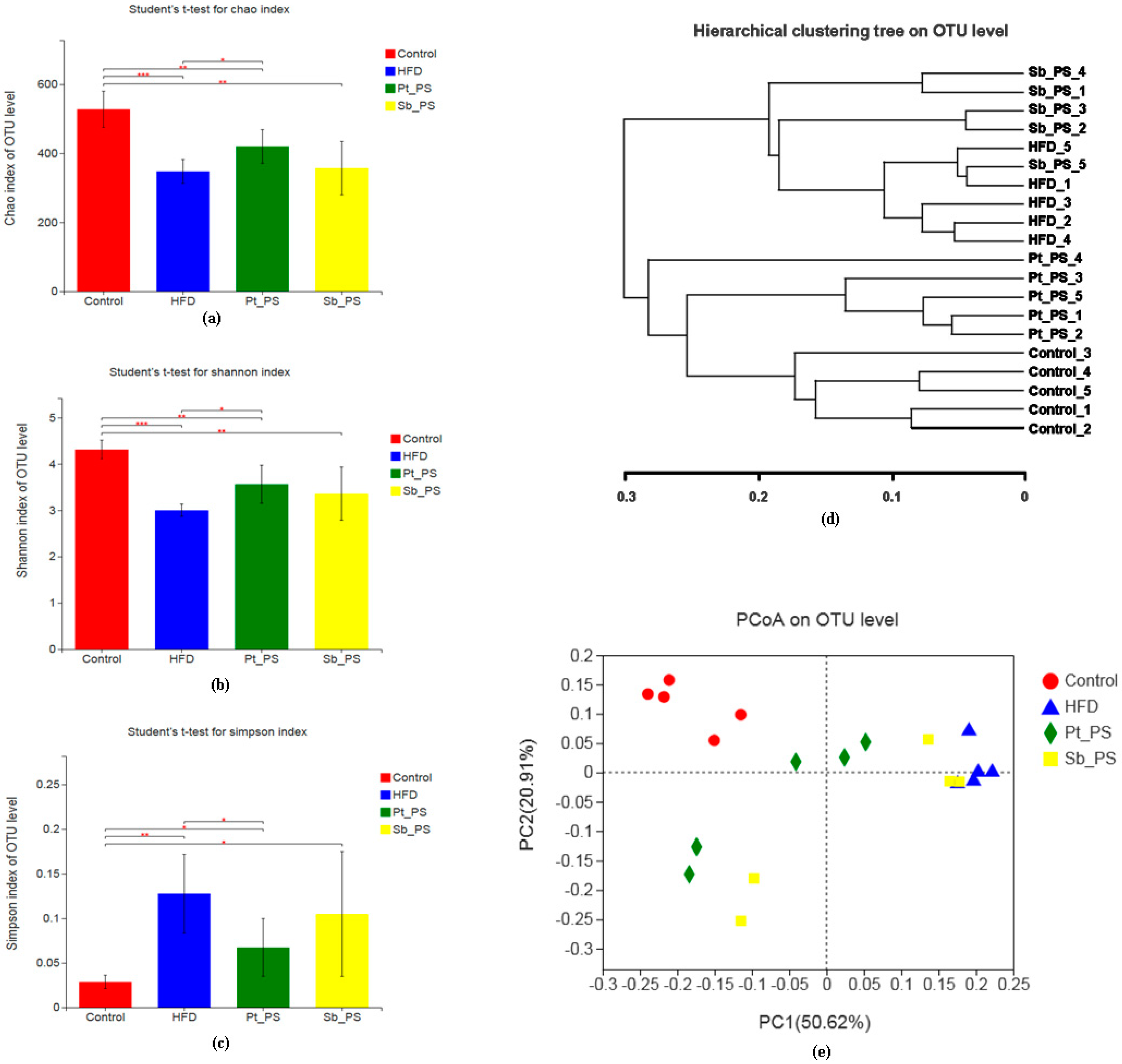
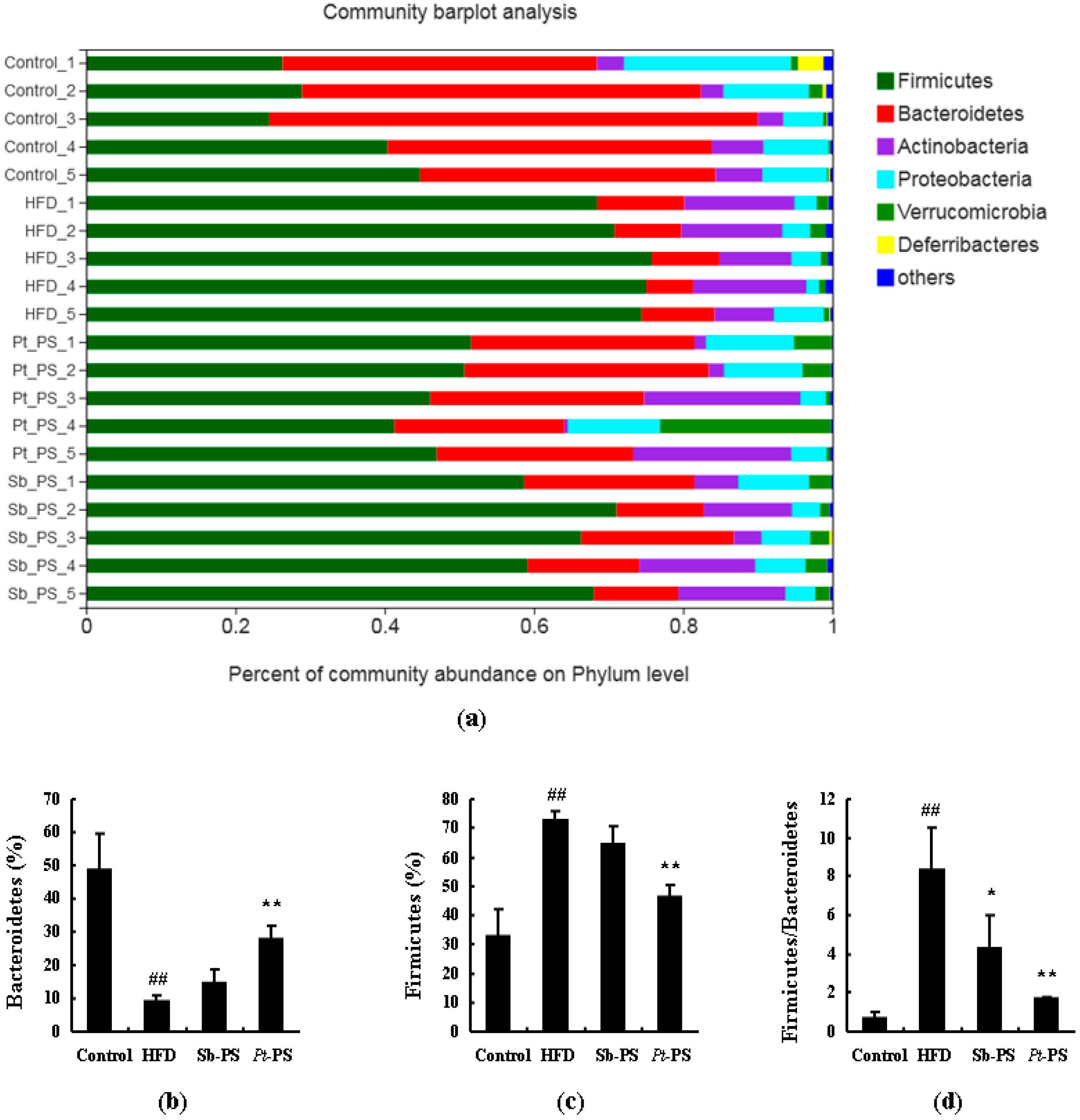
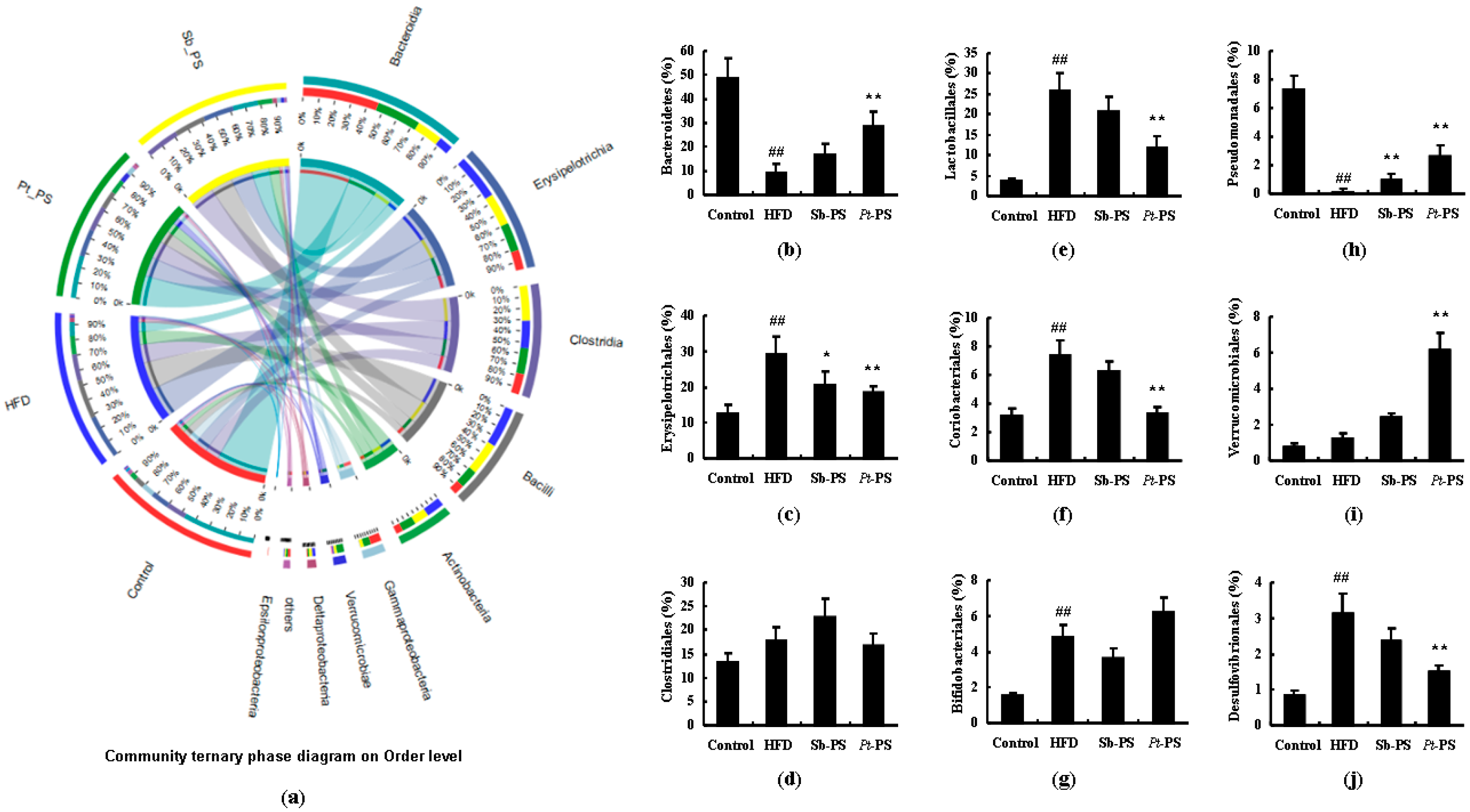
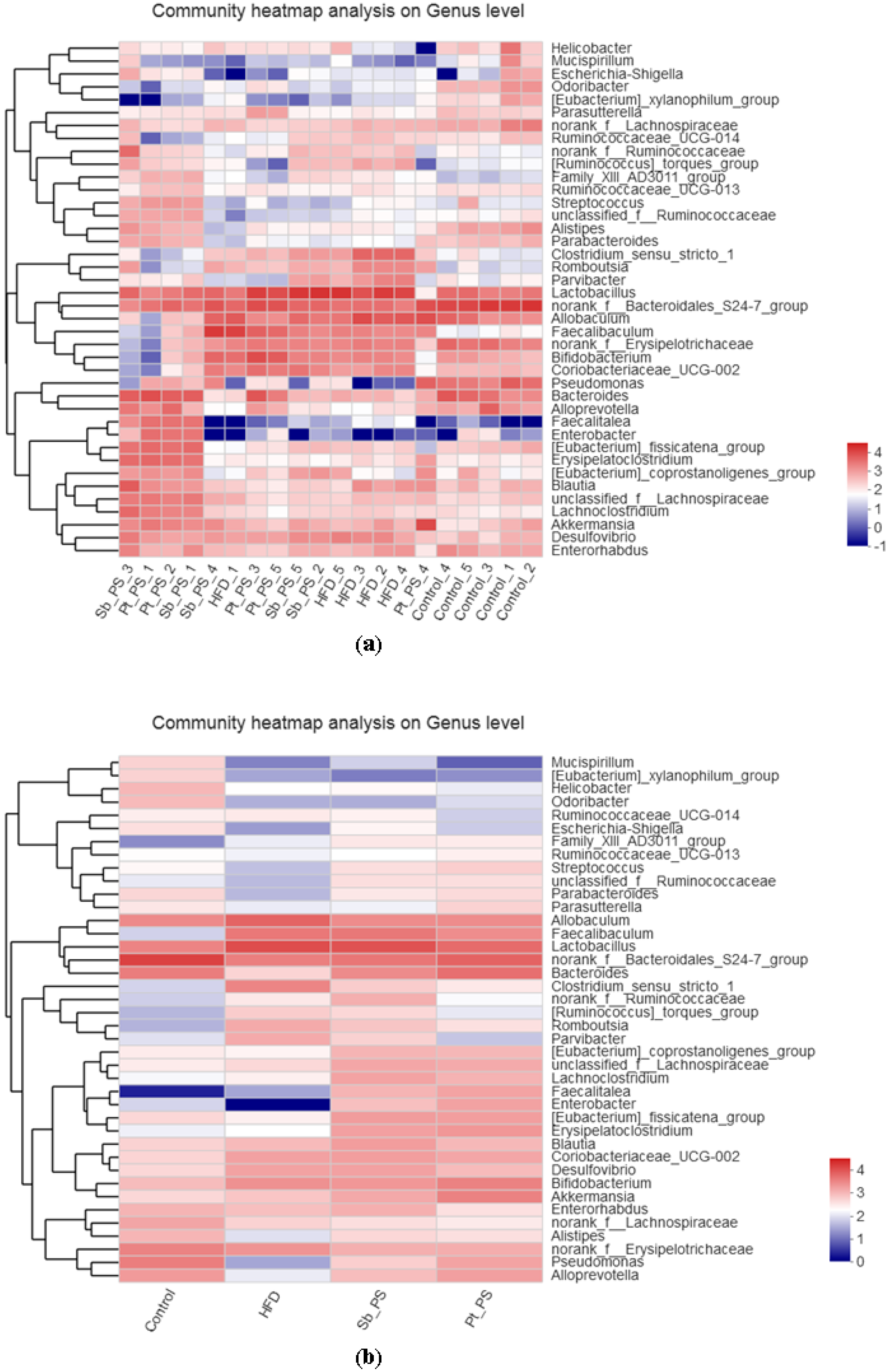
2.6. Pt-PS Restores Gut Microbiota Dysbiosis
2.7. Pt-PS Decreases LPS Concentration and Increases SCFAs Concentrations
3. Discussion
4. Materials and Methods
4.1. Preparation of Pt-PS
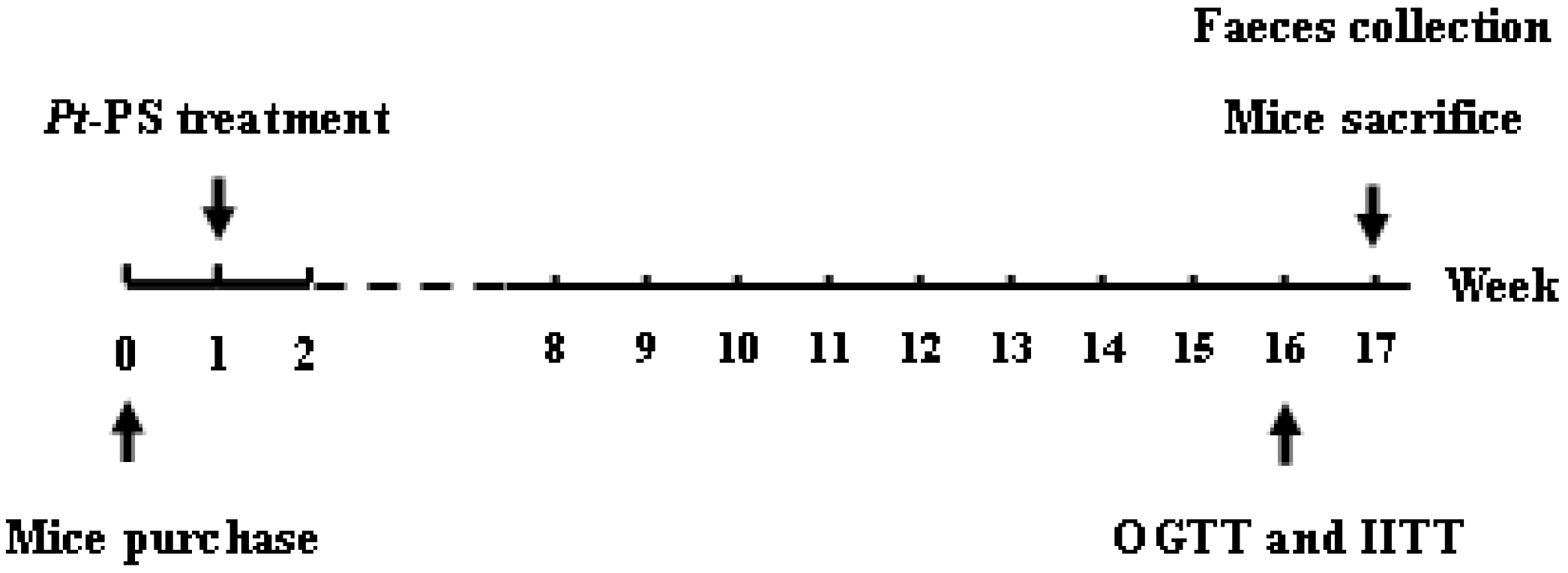
4.2. Animal Experiments
4.3. OGTT and IITT
4.4. Insulin Stimulation and Plasma Membrane Preparation
4.5. Analysis of Insulin Resistance-Related Parameters
4.6. Adipokines Measurement
4.7. Immunoprecipitation and Western Blotting
4.8. Fecal LPS and SCFAs Determination
4.9. Fecal DNA Extraction and Microbiota Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brown, A.E.; Walker, M. Genetics of Insulin Resistance and the Metabolic Syndrome. Curr. Cardiol. Rep. 2016, 18, 75. [Google Scholar] [CrossRef]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef]
- Saltiel, A.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Mutt, S.J.; Raza, G.S.; Mäkinen, M.J.; Keinänen-Kiukaanniemi, S.; Järvelin, M.; Herzig, K.-H. Vitamin D Deficiency Induces Insulin Resistance and Re-Supplementation Attenuates Hepatic Glucose Output via the PI3K-AKT-FOXO1 Mediated Pathway. Mol. Nutr. Food Res. 2020, 64, e1900728. [Google Scholar] [CrossRef]
- Jeong, O.; Kim, H.S. Dietary chokeberry and dried jujube fruit attenuates high-fat and high-fructose diet-induced dyslipidemia and insulin resistance via activation of the IRS-1/PI3K/Akt pathway in C57BL/6 J mice. Nutr. Metab. 2019, 16, 38. [Google Scholar] [CrossRef]
- Cui, J.-F.; Ye, H.; Zhu, Y.-J.; Li, Y.-P.; Wang, J.; Wang, P. Characterization and Hypoglycemic Activity of a Rhamnan-Type Sulfated Polysaccharide Derivative. Mar. Drugs 2019, 17, 21. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, Y.; Yu, D.; Jin, L.; Gong, X.; Zhang, B. Perilla oil regulates intestinal microbiota and alleviates insulin resistance through the PI3K/AKT signaling pathway in type-2 diabetic KKAy mice. Food Chem. Toxicol. 2020, 135, 110965. [Google Scholar] [CrossRef]
- Esteves, J.V.; Enguita, F.J.; Machado, U.F. MicroRNAs-Mediated Regulation of Skeletal Muscle GLUT4 Expression and Translocation in Insulin Resistance. J. Diabetes Res. 2017, 2017, 7267910. [Google Scholar] [CrossRef]
- Saad, M.; Santos, A.; Prada, P.O. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology 2016, 31, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Dabke, K.; Hendrick, G.; Devkota, S. The gut microbiome and metabolic syndrome. J. Clin. Investig. 2019, 129, 4050–4057. [Google Scholar] [CrossRef]
- Jiao, N.; Baker, S.S.; Nugent, C.A.; Tsompana, M.; Cai, L.; Wang, Y.; Buck, M.J.; Genco, R.J.; Baker, R.D.; Zhu, R.; et al. Gut microbiome may contribute to insulin resistance and systemic inflammation in obese rodents: A meta-analysis. Physiol. Genom. 2018, 50, 244–254. [Google Scholar] [CrossRef]
- Mazidi, M.; Rezaie, P.; Kengne, A.P.; Avan, A.; Ferns, G.A. Gut microbiome and metabolic syndrome. Diabetes Metab. Syndr. Clin. Res. Rev. 2016, 10, S150–S157. [Google Scholar] [CrossRef]
- Greenhill, C. Gut microbiota: Firmicutes and Bacteroidetes involved in insulin resistance by mediating levels of glucagon-like peptide 1. Nat. Rev. Endocrinol. 2015, 11, 254. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, T.P.; Dallinga-Thie, G.M.; De Vos, W.M.; Nieuwdorp, M.; Van Raalte, D.H. Causality of small and large intestinal microbiota in weight regulation and insulin resistance. Mol. Metab. 2016, 5, 759–770. [Google Scholar] [CrossRef]
- Wang, Y.; Dilidaxi, D.; Wu, Y.; Sailike, J.; Sun, X.; Nabi, X.-H. Composite probiotics alleviate type 2 diabetes by regulating intestinal microbiota and inducing GLP-1 secretion in db/db mice. Biomed. Pharmacother. 2020, 125, 109914. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Gallagher, E.J.; Gallagher, E.J. Diabetes, Obesity, and Breast Cancer. Endocrinology 2018, 159, 3801–3812. [Google Scholar] [CrossRef]
- Lv, J.; Gao, B.; Liu, P.; Li, J.; Meng, X. Linkage mapping aided by de novo genome and transcriptome assembly in Portunus trituberculatus: Applications in growth-related QTL and gene identification. Sci. Rep. 2017, 7, 7874. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, L.; Liu, P.; Li, J. Transcriptomic variation of eyestalk reveals the genes and biological processes associated with molting in Portunus trituberculatus. PLoS ONE 2017, 12, e0175315. [Google Scholar] [CrossRef]
- Ng’Ambi, J.W.; Li, R.; Mu, C.; Song, W.; Liu, L.; Wang, C. Dietary administration of saponin stimulates growth of the swimming crab Portunus trituberculatus and enhances its resistance against Vibrio alginolyticus infection. Fish Shellfish. Immunol. 2016, 59, 305–311. [Google Scholar] [CrossRef]
- Pan, L.; Hu, D.; Liu, M.; Hu, Y.; Liu, S. Molecular cloning and sequence analysis of two carbonic anhydrase in the swimming crab Portunus trituberculatus and its expression in response to salinity and pH stress. Gene 2016, 576, 347–357. [Google Scholar] [CrossRef]
- Hu, S.; Wang, J.; Yan, X.; Yang, H.; Li, S.; Jiang, W.; Liu, Y. Egg oil from Portunus trituberculatus alleviates insulin resistance through activation of insulin signaling in mice. Appl. Physiol. Nutr. Metab. 2019, 44, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Yang, H.; Gao, X.; Li, S.; Jiang, W.; Liu, Y. Egg oil from Portunus trituberculatus alleviated obesity and regulated gut microbiota in mice. Sci. Rep. 2020, 10, 8454. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of Animal and Marine Origin: Structure, Function, and Anti-Inflammatory Properties. Molecules 2017, 22, 1964. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Okajima, H.; Okada, Y.; Watanabe, H. Studies on Furan Fatty Acids of Salmon Roe Phospholipids. J. Biochem. 1988, 103, 836–839. [Google Scholar] [CrossRef]
- Liang, P.; Li, R.; Sun, H.; Zhang, M.; Cheng, W.; Chen, L.; Cheng, X.; Akoh, C.C. Phospholipids composition and molecular species of large yellow croaker (Pseudosciaena crocea) roe. Food Chem. 2018, 245, 806–811. [Google Scholar] [CrossRef]
- Gallagher, M.L.; Paramore, L.; Alves, D.; Rulifson, R.A. Comparison of phospholipid and fatty acid composition of wild and cultured striped bass eggs. J. Fish Biol. 1998, 52, 1218–1228. [Google Scholar] [CrossRef]
- Zhang, T.-T.; Xu, J.; Wang, Y.; Xue, C. Health benefits of dietary marine DHA/EPA-enriched glycerophospholipids. Prog. Lipid Res. 2019, 75, 100997. [Google Scholar] [CrossRef]
- Zhou, M.-M.; Che, H.-X.; Huang, J.-Q.; Zhang, T.; Xu, J.; Xue, C.; Wang, Y.-M. Comparative Study of Different Polar Groups of EPA-Enriched Phospholipids on Ameliorating Memory Loss and Cognitive Deficiency in Aged SAMP8 Mice. Mol. Nutr. Food Res. 2018, 62, 1700637. [Google Scholar] [CrossRef]
- Wang, C.; Wang, D.; Xu, J.; Yanagita, T.; Xue, C.; Zhang, T.; Wang, Y. DHA enriched phospholipids with different polar groups (PC and PS) had different improvements on MPTP-induced mice with Parkinson’s disease. J. Funct. Foods 2018, 45, 417–426. [Google Scholar] [CrossRef]
- Wen, M.; Ding, L.; Zhang, L.; Zhou, M.; Xue, J.; Wang, J.; Wang, Y.; Xue, C. DHA-PC and DHA-PS improved A beta 1–40 induced cognitive deficiency uncoupled with an increase in brain DHA in rats. J. Funct. Foods 2016, 22, 417–430. [Google Scholar] [CrossRef]
- Lou, Q.-M.; Wang, Y.-M.; Liu, X.; Xue, C. Lipid profile and fatty acid compositions in body wall of Apostichopus japonicus (selenka). J. Food Biochem. 2011, 36, 317–321. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Stalin, A.; Balakrishna, K.; Ignacimuthu, S.; Paulraj, M.G.; Vishal, R. Insulin sensitization via partial agonism of PPARγ and glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway by embelin in type 2 diabetic rats. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 2243–2255. [Google Scholar] [CrossRef]
- Kim, S.; Jwa, H.; Yanagawa, Y.; Park, T. Extract from Dioscorea batatas Ameliorates Insulin Resistance in Mice Fed a High-Fat Diet. J. Med. Food 2012, 15, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, Y.; Liu, Y.; Zhu, D.; Yu, J.; Li, G.; Sun, Z.; Wang, W.; Jiang, H.; Hong, Z. MiR-27a promotes insulin resistance and mediates glucose metabolism by targeting PPAR-γ-mediated PI3K/AKT signaling. Aging 2019, 11, 7510–7524. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, F.; He, Y.; Zhang, Q.; Zhang, Y.; Zhou, G.; Yang, H.; Zhou, P. A novel PTP1B inhibitor extracted from Ganoderma lucidum ameliorates insulin resistance by regulating IRS1-GLUT4 cascades in the insulin signaling pathway. Food Funct. 2018, 9, 397–406. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, M.; Roy, A.; Dinda, S. Dietary plant flavonoids in prevention of obesity and diabetes. Adv. Protein Chem. Struct. Biol. 2019, 120, 159–235. [Google Scholar] [CrossRef]
- Hatem-Vaquero, M.; Griera, M.; García-Jerez, A.; Luengo, A.; Álvarez, J.; Rubio, J.A.; Calleros, L.; Rodríguez-Puyol, D.; Rodríguez-Puyol, M.; De Frutos, S. Peripheral insulin resistance in ILK-depleted mice by reduction of GLUT4 expression. J. Endocrinol. 2017, 234, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Garabadu, D.; Krishnamurthy, S. Metformin attenuates hepatic insulin resistance in type-2 diabetic rats through PI3K/Akt/GLUT-4 signalling independent to bicuculline-sensitive GABAA receptor stimulation. Pharm. Biol. 2017, 55, 722–728. [Google Scholar] [CrossRef]
- Caselli, C. Role of adiponectin system in insulin resistance. Mol. Genet. Metab. 2014, 113, 155–160. [Google Scholar] [CrossRef]
- Yadav, A.; Kataria, M.A.; Saini, V.; Yadav, A. Role of leptin and adiponectin in insulin resistance. Clin. Chim. Acta 2013, 417, 80–84. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 2018, 234, 8152–8161. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.M.; Miner, J.L. Role of resistin in insulin sensitivity in rodents and humans. Curr. Protein Pept. Sci. 2009, 10, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Obin, M.S.; Zhao, L. The gut microbiota, obesity and insulin resistance. Mol. Asp. Med. 2013, 34, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liu, D.; Zhan, J.; Luo, M.; Han, J.; Wang, P.; Zhou, Z. New insight into the mechanism of POP-induced obesity: Evidence from DDE-altered microbiota. Chemosphere 2020, 244, 125123. [Google Scholar] [CrossRef]
- Yue, S.; Zhao, D.; Peng, C.-X.; Tan, C.; Wang, Q.; Gong, J.S. Effects of theabrownin on serum metabolites and gut microbiome in rats with a high-sugar diet. Food Funct. 2019, 10, 7063–7080. [Google Scholar] [CrossRef]
- Monk, J.M.; Liddle, D.M.; Hutchinson, A.L.; Wu, W.; Lepp, D.; Ma, D.W.; Robinson, L.E.; Power, K.A. Fish oil supplementation to a high-fat diet improves both intestinal health and the systemic obese phenotype. J. Nutr. Biochem. 2019, 72, 108216. [Google Scholar] [CrossRef]
- Liu, J.; Yue, S.; Yang, Z.; Feng, W.; Meng, X.; Wang, A.; Peng, C.; Wang, C.; Yan, D.; Yan, Z. Oral hydroxysafflor yellow A reduces obesity in mice by modulating the gut microbiota and serum metabolism. Pharmacol. Res. 2018, 134, 40–50. [Google Scholar] [CrossRef]
- Hu, S.; Xu, Y.; Gao, X.; Li, S.; Jiang, W.; Liu, Y.; Su, L.; Yang, H. Long-Chain Bases from Sea Cucumber Alleviate Obesity by Modulating Gut Microbiota. Mar. Drugs 2019, 17, 455. [Google Scholar] [CrossRef]
- Le Sage, F.; Meilhac, O.; Gonthier, M.-P. Porphyromonas gingivalis lipopolysaccharide induces pro-inflammatory adipokine secretion and oxidative stress by regulating Toll-like receptor-mediated signaling pathways and redox enzymes in adipocytes. Mol. Cell. Endocrinol. 2017, 446, 102–110. [Google Scholar] [CrossRef]
- Mei, X.; Zhang, X.; Wang, Z.; Gao, Z.; Liu, G.; Hu, H.; Zou, L.; Li, X. Insulin Sensitivity-Enhancing Activity of Phlorizin Is Associated with Lipopolysaccharide Decrease and Gut Microbiota Changes in Obese and Type 2 Diabetes (db/db) Mice. J. Agric. Food Chem. 2016, 64, 7502–7511. [Google Scholar] [CrossRef]
- Tan, J.K.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
| Fatty Acids | Proportion (%) | Fatty Acids | Proportion (%) |
|---|---|---|---|
| C15:0 | 0.59 ± 0.08 | C20:3 | 2.49 ± 0.27 |
| C16:0 | 10.95 ± 1.57 | C20:4 | 1.60 ± 0.49 |
| C16:1 | 0.83 ± 0.01 | C20:5 | 18.70 ± 3.32 |
| C17:0 | 5.18 ± 0.84 | C22:2 | 2.70 ± 0.56 |
| C17:1 | 7.32 ± 0.86 | C22:6 | 30.43 ± 2.08 |
| C17:3 | 0.12 ± 0.01 | C23:1 | 0.60 ± 0.11 |
| C18:0 | 3.45 ± 0.61 | Others | 8.52 ± 1.40 |
| C18:1 | 1.87 ± 0.30 | ∑SFA | 22.19 ± 3.15 |
| C18:2 | 0.59 ± 0.01 | ∑UFA | 69.29 ± 5.20 |
| C18:3 | 0.50 ± 0.02 | ∑MUFA | 12.16 ± 2.24 |
| C20:0 | 2.01 ± 0.54 | ∑PUFA | 57.13 ± 5.63 |
| C20:1 | 1.56 ± 0.39 | ∑(EPA + DHA) | 50.13 ± 2.81 |
| Control | HFD | Sb-PS | Pt-PS | |
|---|---|---|---|---|
| Body weight gain (g) | 12.21 ± 1.33 | 25.07 ± 2.67 ## | 20.52 ± 1.86 * | 16.61 ± 1.44 ** |
| Food intake (g/w) | 30.86 ± 2.75 | 25.95 ± 2.04 # | 25.67 ± 2.23 | 26.20 ± 2.49 |
| Calorie intake (kcal/w) | 126.38 ± 9.94 | 170.27 ± 11.36 # | 168.78 ± 12.47 | 171.44 ± 16.35 |
| Perirenal fat weight (g) | 0.20 ± 0.01 | 0.64 ± 0.07 ## | 0.41 ± 0.03 ** | 0.31 ± 0.02 ** |
| Epididymal fat weight (g) | 0.58 ± 0.06 | 2.28 ± 0.30 ## | 1.84 ± 0.21 * | 1.17 ± 0.16 ** |
| Abdominal subcutaneous fat weight (g) | 0.19 ± 0.01 | 0.92 ± 0.09 ## | 0.65 ± 0.07 * | 0.45 ± 0.08 ** |
| Hepatic weight (g) | 1.13 ± 0.12 | 1.95 ± 0.14 ## | 1.50 ± 0.20 * | 1.36 ± 0.15 ** |
| Fasting blood glucose (mmol/L) | 7.73 ± 0.57 | 12.01 ± 0.94 ## | 9.30 ± 0.87 * | 8.48 ± 0.90 ** |
| Serum insulin (mU/L) | 9.26 ± 0.85 | 14.04 ± 1.32## | 11.36 ± 0.89 * | 10.42 ± 1.01** |
| HOMA-IR | 3.20 ± 0.36 | 7.59 ± 0.84 ## | 4.63 ± 0.52 ** | 3.96 ± 0.27 ** |
| QUICKI | 0.54 ± 0.02 | 0.45 ± 0.02 ## | 0.49 ± 0.03 | 0.51 ± 0.01 * |
| Serum TNF-α (pg/mL) | 63.58 ± 38.92 | 126.47 ± 47.61 ## | 101.15 ± 40.03 | 86.34 ± 31.48 * |
| Serum adiponectin (ng/mL) | 173.45 ± 15.21 | 82.67 ± 6.89 ## | 144.73 ± 11.40 ** | 162.06 ± 9.74 ** |
| Serum leptin (ng/mL) | 0.18 ± 0.02 | 0.46 ± 0.04 ## | 0.39 ± 0.05 | 0.24 ± 0.03 ** |
| Serum resistin (ng/mL) | 4.27 ± 0.30 | 11.26 ± 0.87 ## | 7.85 ± 0.69 ** | 5.94 ± 0.82 ** |
| Parameters | Control | HFD | Sb-PS | Pt-PS |
|---|---|---|---|---|
| Fecal LPS (µg/g feces) | 5.23 ± 0.64 | 13.47 ± 1.09 ## | 11.67 ± 1.10 | 7.53 ± 0.77 ** |
| Fecal acetate (mmol/L) | 19.21 ± 2.84 | 8.80 ± 0.85 ## | 11.36 ± 1.04 * | 14.05 ± 1.37 ** |
| Fecal propionate (mmol/L) | 6.62 ± 0.60 | 3.27 ± 0.39 ## | 3.76 ± 0.44 | 4.68 ± 0.41 * |
| Fecal butyrate (mmol/L) | 1.82 ± 0.13 | 0.67 ± 0.08 ## | 0.89 ± 0.10 | 1.43 ± 0.12 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.; Du, M.; Su, L.; Yang, H. Phosphatidylserine from Portunus trituberculatus Eggs Alleviates Insulin Resistance and Alters the Gut Microbiota in High-Fat-Diet-Fed Mice. Mar. Drugs 2020, 18, 483. https://doi.org/10.3390/md18090483
Hu S, Du M, Su L, Yang H. Phosphatidylserine from Portunus trituberculatus Eggs Alleviates Insulin Resistance and Alters the Gut Microbiota in High-Fat-Diet-Fed Mice. Marine Drugs. 2020; 18(9):483. https://doi.org/10.3390/md18090483
Chicago/Turabian StyleHu, Shiwei, Mengyu Du, Laijin Su, and Huicheng Yang. 2020. "Phosphatidylserine from Portunus trituberculatus Eggs Alleviates Insulin Resistance and Alters the Gut Microbiota in High-Fat-Diet-Fed Mice" Marine Drugs 18, no. 9: 483. https://doi.org/10.3390/md18090483
APA StyleHu, S., Du, M., Su, L., & Yang, H. (2020). Phosphatidylserine from Portunus trituberculatus Eggs Alleviates Insulin Resistance and Alters the Gut Microbiota in High-Fat-Diet-Fed Mice. Marine Drugs, 18(9), 483. https://doi.org/10.3390/md18090483







