Comparison of Physicochemical Characteristics and Macrophage Immunostimulatory Activities of Polysaccharides from Chlamys farreri
Abstract
1. Introduction
2. Results and Discussion
2.1. Isolation and Purification of CFPs
2.1.1. Optimization of Extraction Conditions
2.1.2. Removal of Proteins from Crude Polysaccharides
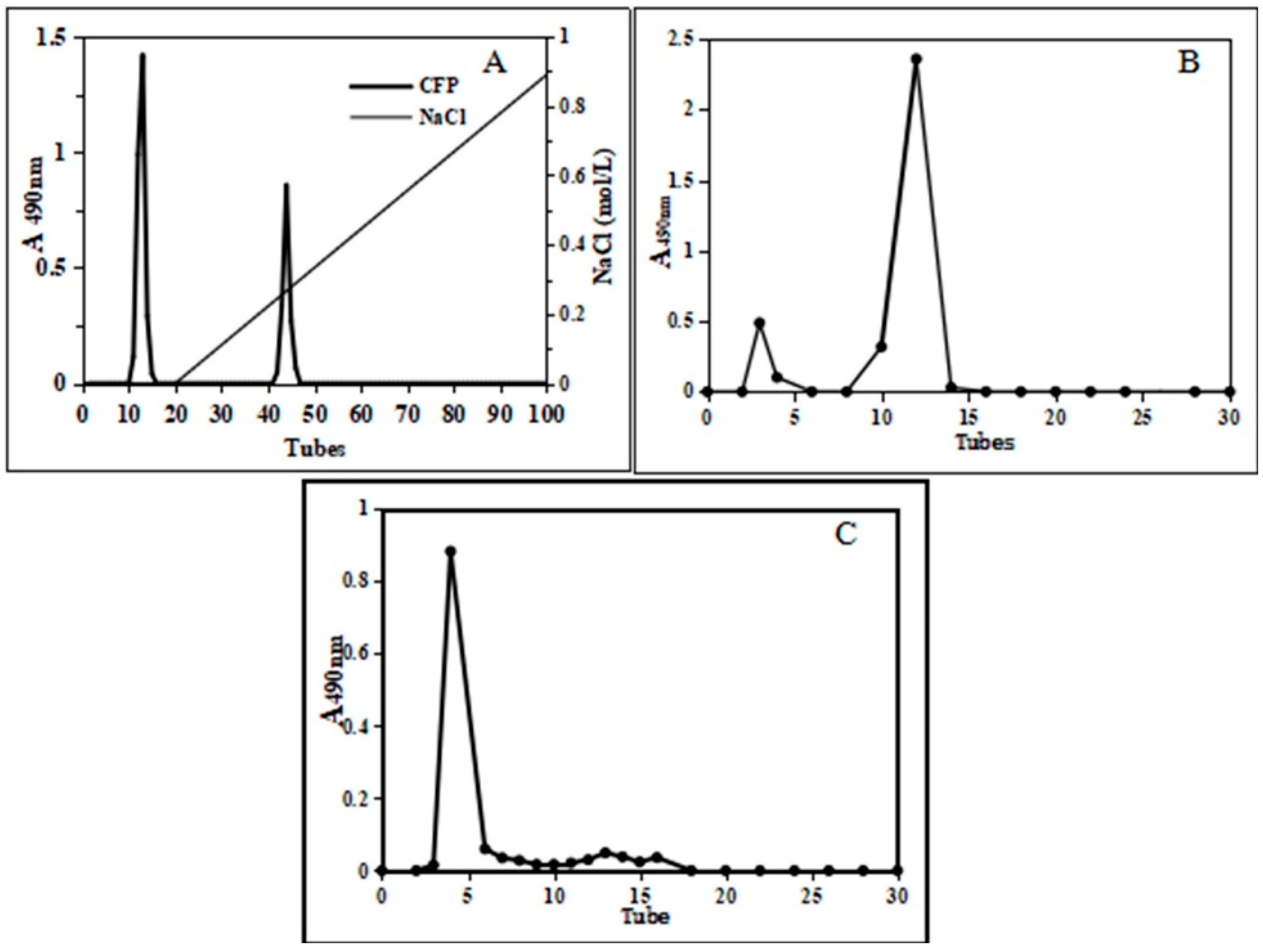
2.1.3. Separation of Deproteinized Polysaccharide
2.2. Chemical-Physical Properties of Polysaccharides
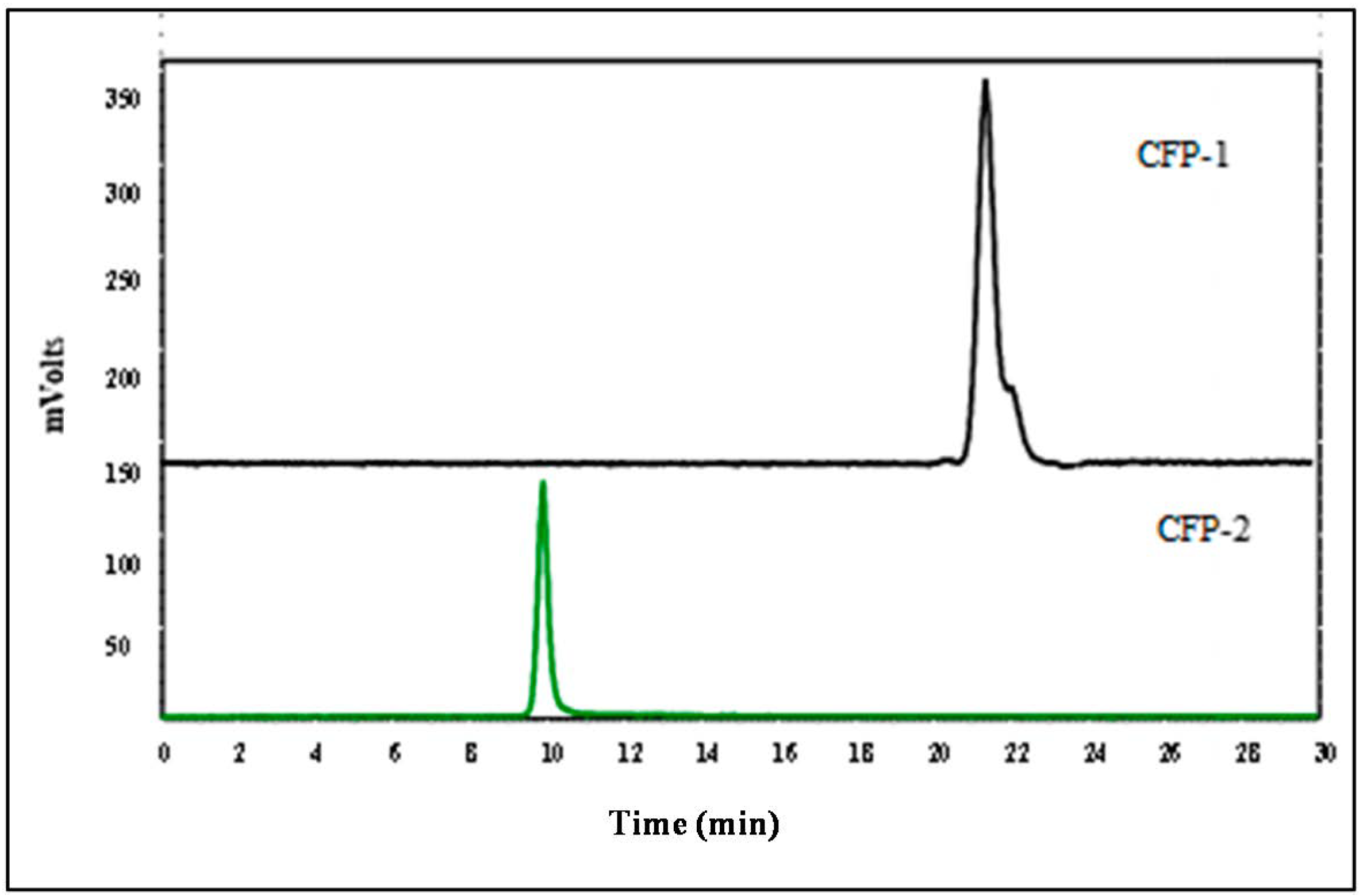
2.2.1. The Molecular Weight of CFP-1 and CFP-2
2.2.2. Chemical Composition Analysis
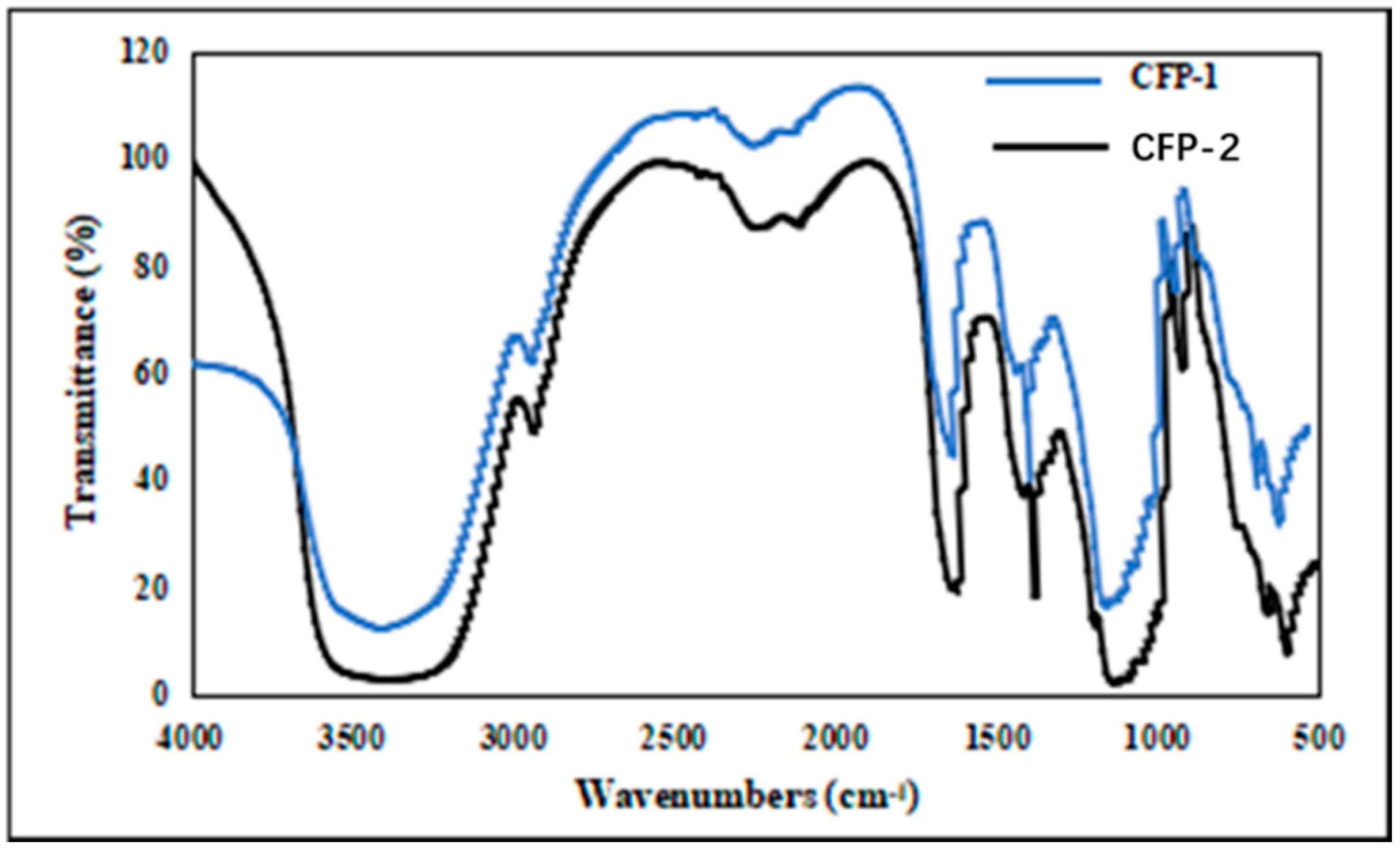
2.2.3. Fourier Transform Infrared (FT-IR) Spectrum and UV Scanning Spectrum of CFP-1 and CFP-2
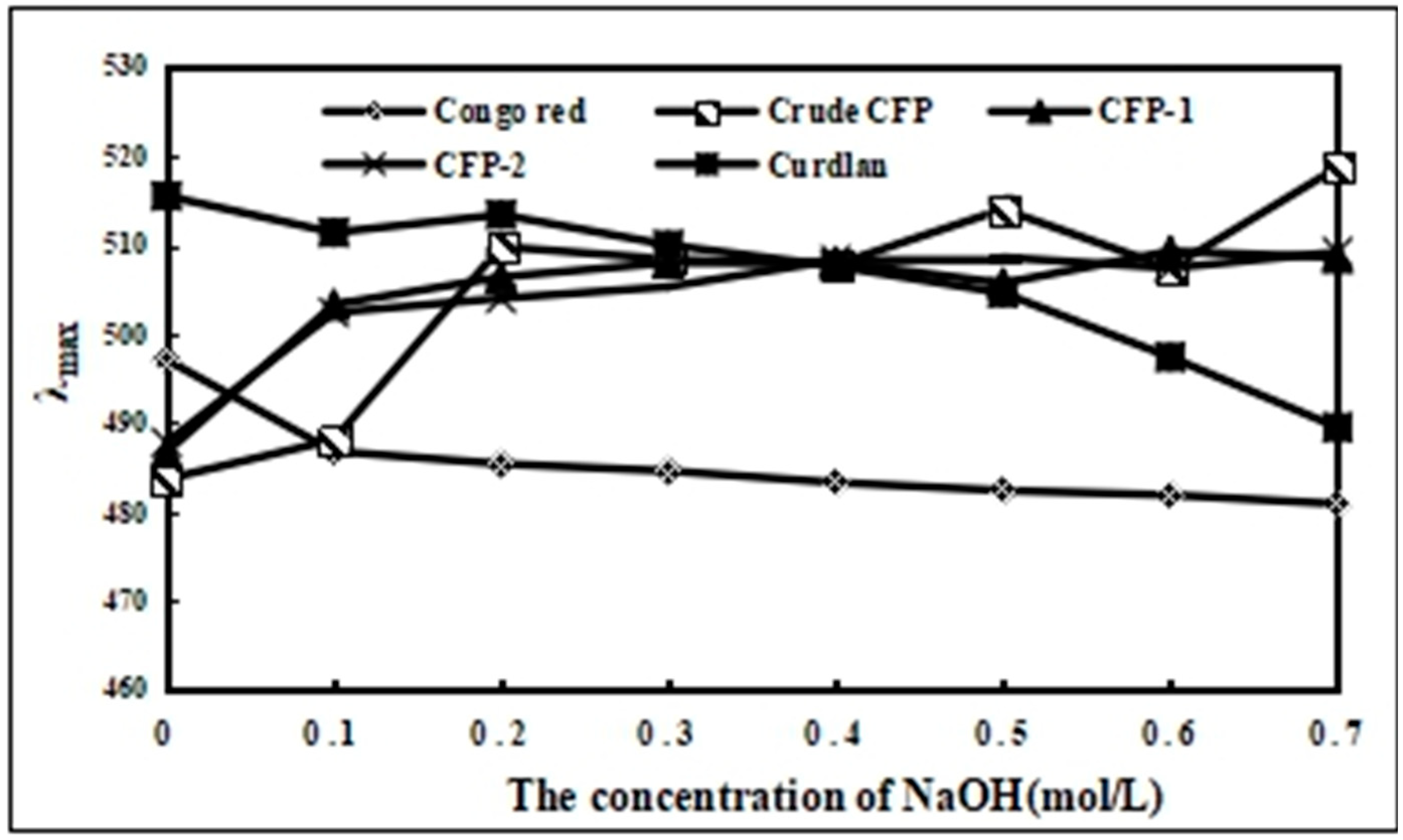
2.2.4. The Congo-Red Testing for Crude CFP, CFP-1 and CFP-2
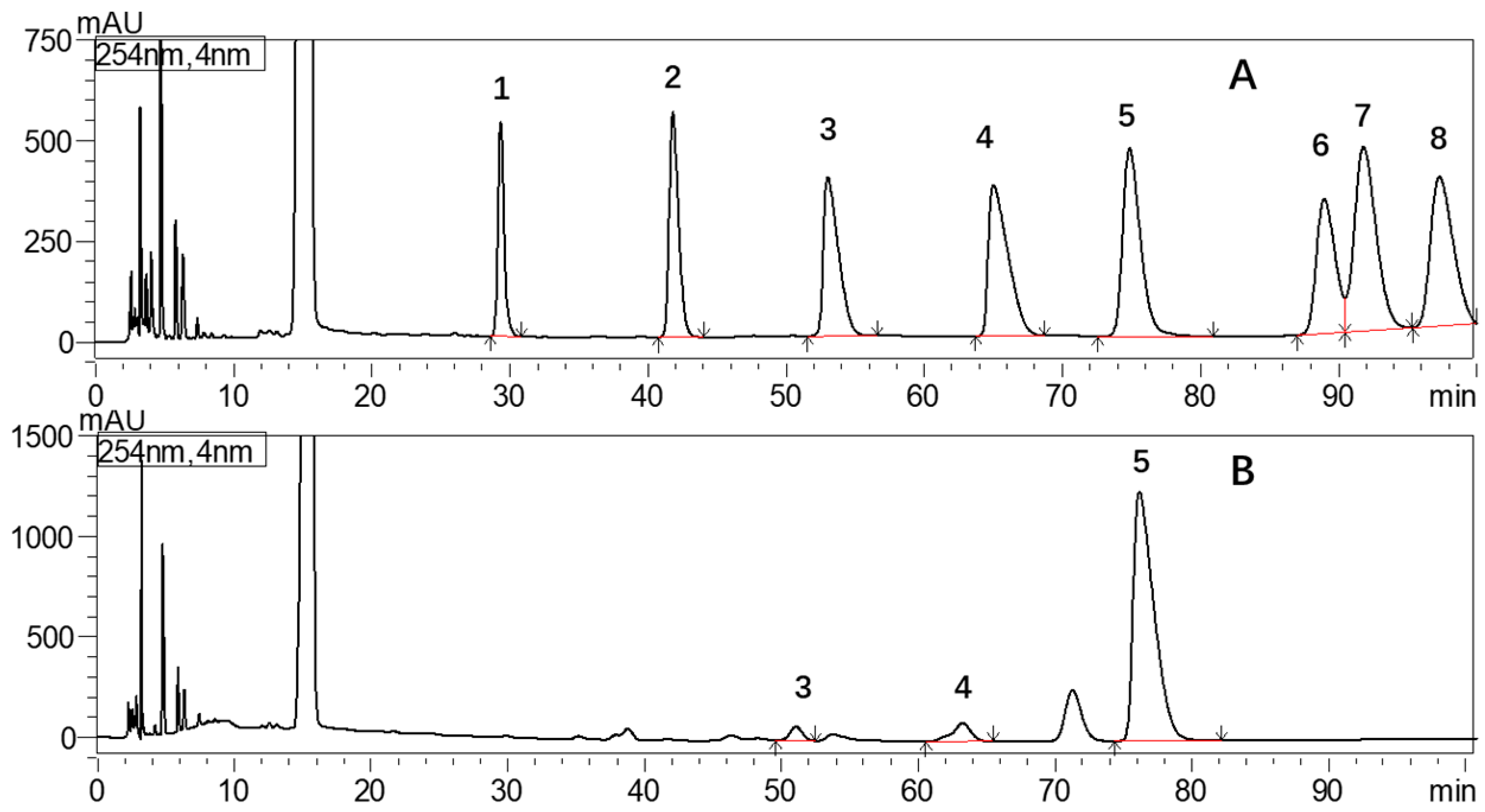
2.2.5. GC-MS of Alditol Acetate Derivatives from the Methylated Product of CFP-1 and CFP-2
2.3. Immunostimulatory Activity
2.3.1. Effects of Polysaccharides on RAW264.7 Cell Proliferation
2.3.2. Effects of Polysaccharides on Phagocytic Activity of RAW264.7 Cells
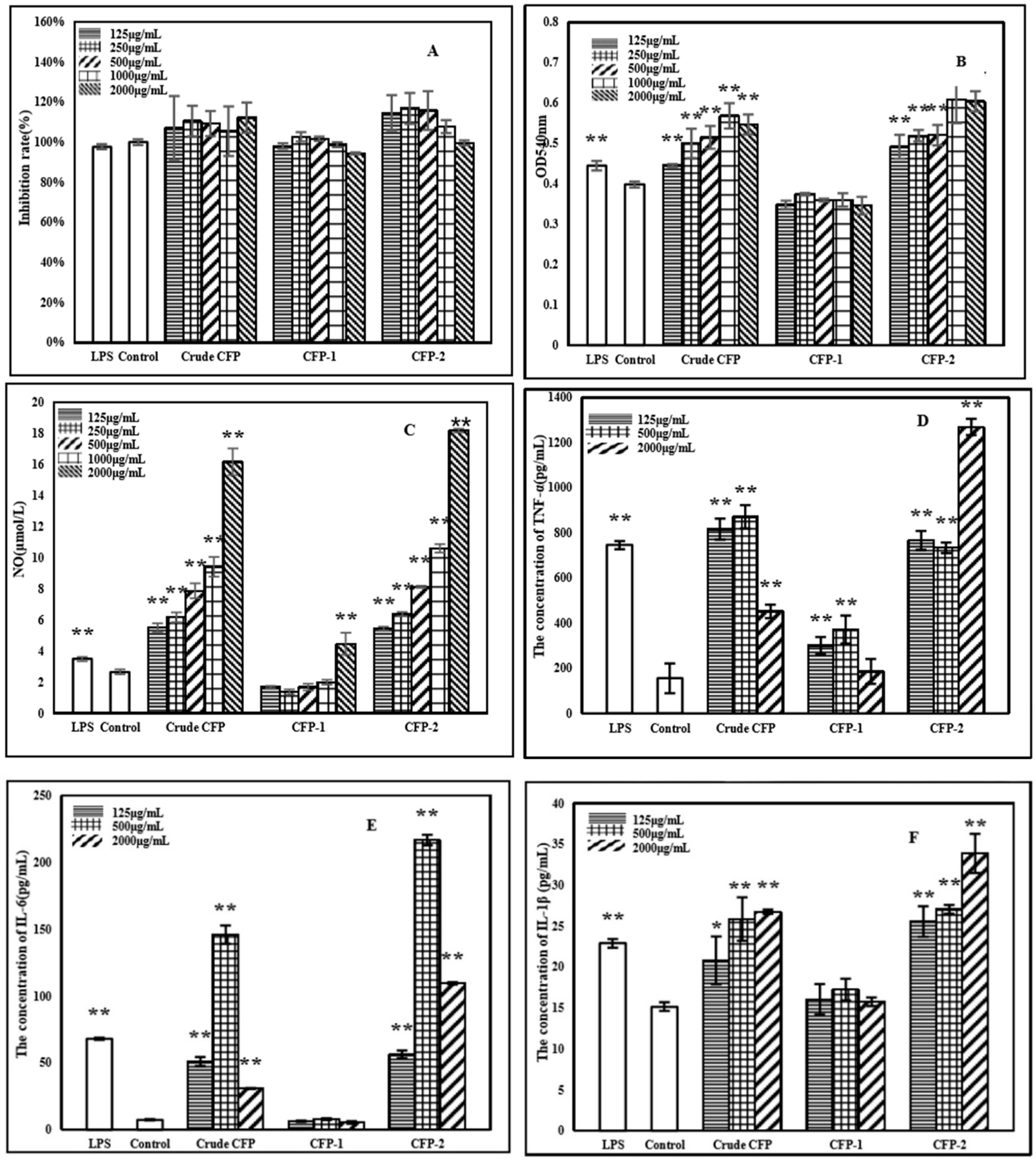
2.3.3. Effects of Polysaccharides on NO Production
2.3.4. Effects of Polysaccharides on the Cytokines Secretion by RAW264.7 Cells
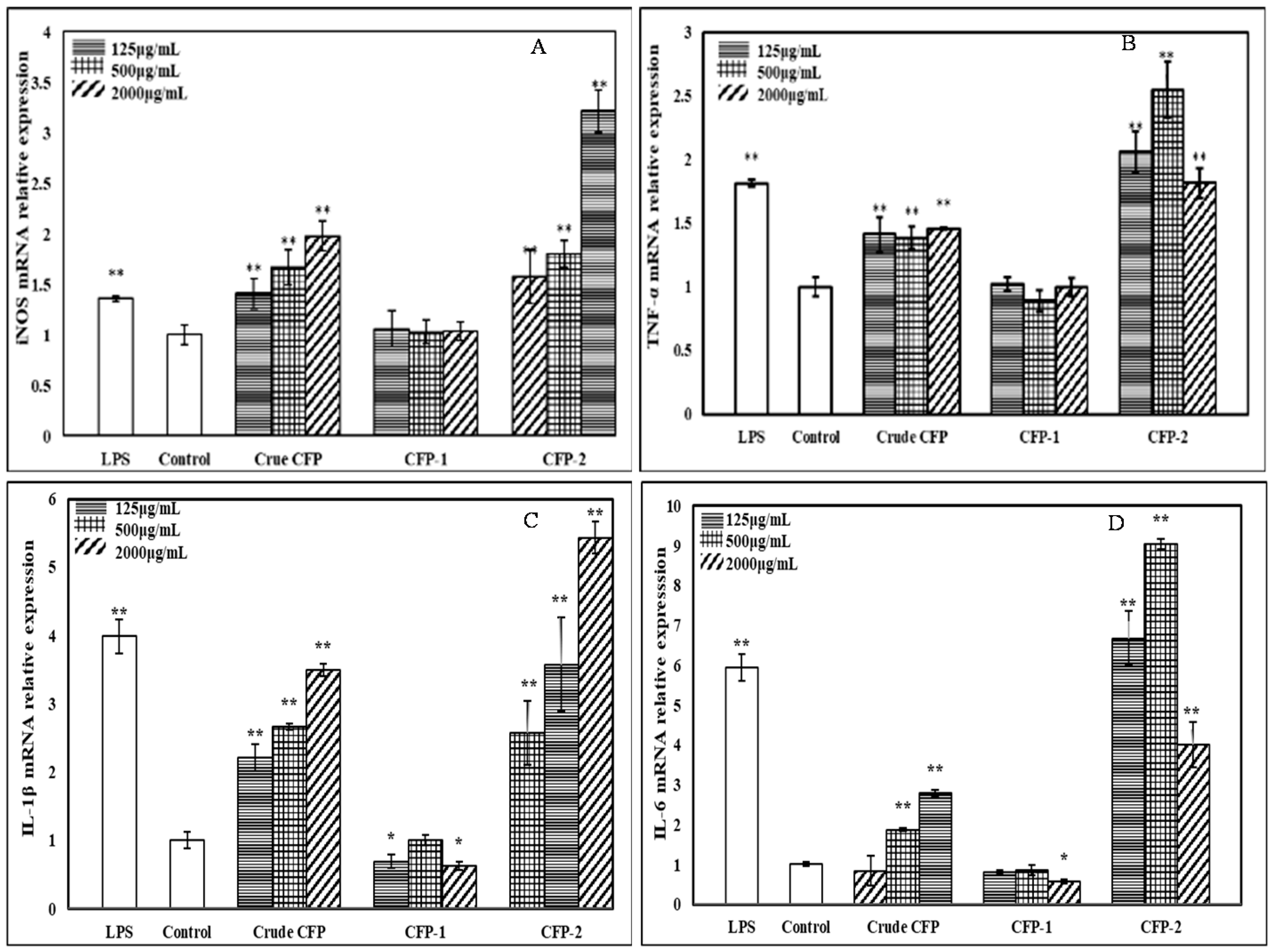
2.3.5. Effects of the Polysaccharides on mRNA Expression of iNOS and Cytokines
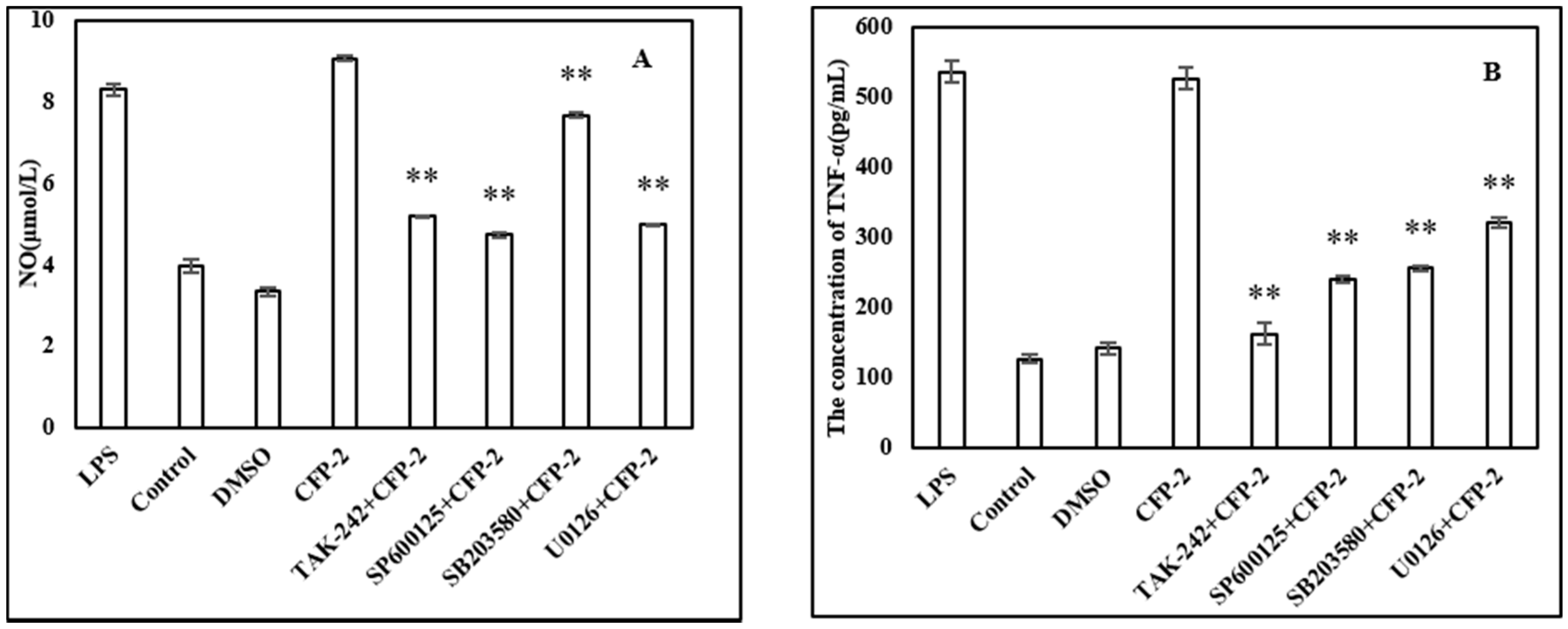
2.3.6. Effects of Inhibitors on the Cytokines Secretion by RAW264.7 Cells
3. Materials and Methods
3.1. Materials
3.2. Isolation and Purification of Polysaccharides from Chlamys farreri
3.2.1. Optimization of Extraction Conditions
3.2.2. Removal of Proteins from Crude Polysaccharides
Sevag Method
Ethanol-Ammonium Sulfate ATPS
3.2.3. The Separation of Polysaccharide from Chlamys farreri
DEAE Cellulose-52 Column Chromatography
Gel Permeation Chromatography on Sephadex G75
3.3. Chemical-Physical Properties of Polysaccharides
3.3.1. Determination of Glucuronic Acid Content
3.3.2. Determination of Homogeneity and Molecular Weight
3.3.3. UV and FT-IR Analyses
3.3.4. Congo Red Analysis
3.3.5. Analysis of Monosaccharide Composition
3.3.6. Methylation Analysis
3.4. Immunostimulatory Activity of CFPs
3.4.1. Cell Culture
3.4.2. Determination of Proliferation of RAW264.7 Cells
3.4.3. Macrophages Phagocytosis Assay
3.4.4. Measurement of NO Production
3.4.5. Measurement of TNF-α, IL-1β, IL-6 and IL-10
3.4.6. Quantification of Messenger RNA (mRNA) (Bejing Solarbio Science & Technology Co., LtD. Beijing, China)
3.4.7. Related Signal Path Experiment
3.5. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Q.; Wu, X.Y.; Shi, F.L.; Liu, Y. Comparison of antidiabetic effects of saponins and polysaccharides from Momordica charantia L. in STZ-induced type 2 diabetic mice. Biomed. Pharmacother. 2019, 109, 744–750. [Google Scholar] [PubMed]
- Li, Q.; Feng, Y.X.; He, W. Post-screening characterisation and in vivo evaluation of an anti-inflammatory polysaccharide fraction from Eucommia ulmoides. Carbohydr. Polym. 2017, 169, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Kim, K.N.; Lee, S.H. Anti-inflammatory activity of polysaccharide purified from AMG-assistant extract of Ecklonia cava in LPS-stimulated RAW 264.7 macrophages. Carbohydr. Polym. 2011, 85, 80–85. [Google Scholar] [CrossRef]
- Cheong, K.L.; Xia, L.X.; Liu, Y. Isolation and characterization of polysaccharides from oysters (Crassostrea gigas) with anti-tumor activities using an aqueous two-phase system. Mar. Drugs. 2017, 15, 338. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y.; Li, R.C.; Zhao, Y.J.; Liu, Y. Separation of polysaccharides from Spirulina platensis by HSCCC with ethanol-ammonium sulfate ATPS and their antioxidant activities. Carbohydr. Polym. 2017, 173, 465–472. [Google Scholar] [PubMed]
- Gao, J.; Zhang, T.; Jin, Z.Y.; Xu, X.M.; Wang, J.H.; Zhang, X.Q.; Chen, H.Q. Structural characterisation: Physicochemical properties and antioxidant activity of polysaccharide from Lilium lancifolium Thunb. Food Chem. 2015, 169, 430–438. [Google Scholar]
- Lim, J.; Kale, M.; Kim, D.H.; Kim, H.S.; Chon, J.W.; Seo, K.H.; Lee, G.H.; Yokoyama, W.; Kim, H. Anti-obesity effect of exopolysaccharides isolated from Kefir Grains. J. Agric. Food Chem. 2017, 65, 10011–10019. [Google Scholar] [CrossRef]
- Akhtar, M.; Tariq, A.F.; Awais, M.M. Studies on wheat bran Arabinoxylan for its immunostimulatory and protective effects against avian coccidiosis. Carbohydr. Polym. 2012, 90, 333–339. [Google Scholar]
- Ramzani, S.M.; Mehdi, T.; Sangguan, Y. Isolation and chemical characterization of a novel immunostimulating galactofucan from freshwater, Azolla filiculoides. Int. J. Biol. Macromol. 2018, 118, 2082–2091. [Google Scholar]
- Leung, M.Y.K.; Liu, C.; Koon, J.C.M. Polysaccharide biological response modifiers. Immunol. Lett. 2006, 105, 101–114. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Manuel, V.; Coimbra, M.A. Structure–function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Pan, W.J. Polysaccharide isolated from Sarcodon aspratus induces RAW264.7 activity via TLR4-mediated NF-κB and MAPK signaling pathways. Int. J. Biol. Macromol. 2018, 120, 1039–1047. [Google Scholar] [PubMed]
- Yang, Y.; Zhao, X.L.; Li, J.; Jiang, H.; Shan, X.D.; Wang, Y.; Ma, W.B.; Hao, J.J.; Yu, G.L. A β-glucan from Durvillaea Antarctica has immunomodulatory effects on RAW264.7 macrophages via Toll-like receptor 4. Carbohydr. Polym. 2018, 191, 255–265. [Google Scholar] [CrossRef]
- Wang, M.M.; Liu, Y.; Qiang, M.L.; Wang, J.H. Structural elucidation of a pectin-type polysaccharide from Hovenia dulcis peduncles and its proliferative activity on RAW264.7 cells. Int. J. Biol. Macromol. 2017, 104, 1246–1253. [Google Scholar]
- Ramesh, H.P.; Yamaki, K.; Tsushida, T. Effect of fenugreek (Trigonella foenum-graecum L.) galactomannan fractions on phagocytosisinrat macrophages and on proliferation and IgM secretion in HB4C5 cells. Carbohydr. Polym. 2002, 50, 79–83. [Google Scholar] [CrossRef]
- Khan, B.M.; Liu, Y. Marine mollusks: Food with benefits. Compr. Rev. Food Sci. Food Saf. 2019, 18, 548–564. [Google Scholar]
- Choresh, O.; Loya, Y.; Müller, W.E.; Wiedenmann, J.; Azem, A. The mitochondrial 60-kDa heat shock protein in marine invertebrates: Biochemical purification and molecular characterization. Cell Stress Chaperone 2004, 9, 38–48. [Google Scholar]
- Zhang, H.L.; Cui, S.H.; Zha, X.Q. Jellyfish skin polysaccharides: Extraction and inhibitory activity on macrophage-derived foam cell formation. Carbohydr. Polym. 2014, 106, 393–402. [Google Scholar] [CrossRef]
- Luo, L.; Wu, M.; Xu, L.; Lian, W.J.; Xiang, J.Y.; Lu, F.; Gao, N.Y.; Xiao, C.; Wang, S.M.; Zhao, J.H. Comparison of physicochemical characteristics and anticoagulant activities of polysaccharides from three sea cucumbers. Mar. Drugs. 2013, 11, 399–417. [Google Scholar]
- Yuan, Y.; Kanno, M.; Kijima, A. Genetic diversity of wild populations of Chlamys farreri in Japan and their genetic relationship with cultured stocks in China. Aquaculture 2012, 370–37, 109–122. [Google Scholar] [CrossRef]
- Liu, B.; Liu, H.M.; Ai, C.Q.; Zhu, Z.J.; Wen, C.G.; Song, S.; Zhu, B.W. Distribution of uronic acid-containing polysaccharides in 5 species of shellfishes. Carbohydr. Polym. 2017, 164, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.Y.; Hu, Y.D.; Qi, L.K.; Xu, T.T.; Yang, Y.S.; Xu, Z.C.; Lai, X.P.; Wang, X.L.; Zhang, D.Y.; Li, S.J. An effective and recyclable deproteinization method for polysaccharide from oyster by magnetic chitosan microspheres. Carbohydr. Polym. 2018, 195, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.L.; Yan, X.L.; Cheong, K.L.; Liu, Y. Extraction, purification, and characterization of polysaccharides from marine algae Gracilaria lemaneiformis with anti-tumor activity. Process Biochem. 2018, 73, 197–203. [Google Scholar] [CrossRef]
- Ren, Y.L.; Zheng, G.Q.; You, L.J.; Wen, L.G.; Li, C.; Fu, X.; Zhou, L. Structural characterization and macrophage immunomodulatory activity of a polysaccharide isolated from Gracilaria lemaneiformis. J. Funct. Foods. 2017, 33, 286–296. [Google Scholar] [CrossRef]
- Wu, M.Y.; Li, X.; Zhao, L.Y.; Xiao, C.; Gao, N.; Luo, L.; Yang, L.; Li, Z.; Chen, L.Y.; Zhao, J.H. Structural analysis and anticoagulant activities of the novel sulfated fucan possessing a regular well-defined repeating unit from sea cucumber. Mar. Drugs. 2015, 13, 2063–2084. [Google Scholar]
- Wang, W.; Zou, Y.; Li, Q.; Mao, R.W.; Shao, X.J.; Jin, D.; Zheng, D.H.; Zhao, T.; Zhu, H.F.; Zhang, L.; et al. Immunomodulatory effects of a polysaccharide purified from Lepidium meyenii Walp. on macrophages. Process Biochem. 2016, 51, 542–553. [Google Scholar] [CrossRef]
- Wang, L.J.; Yao, Y.; Sang, W.; Yang, X.S.; Ren, G.X. Structural features and immunostimulating effects of three acidic polysaccharides isolated from Panax quinquefolius. Int. J. Biol. Macromol. 2015, 80, 77–86. [Google Scholar] [CrossRef]
- Li, C.; Huang, Q.; Fu, X. Characterization, antioxidant and immunomodulatory activities of polysaccharides from Prunella vulgaris Linn. Int. J. Biol. Macromol. 2015, 75, 298–305. [Google Scholar]
- Falch, B.H.; Ryan, L.; Stokke, B.T. The cytokine stimulating activity of (1→ 3)-beta-D-glucans is dependent on the triple helix conformation. Carbohydr. Res. 2000, 329, 587–596. [Google Scholar] [CrossRef]
- Ohno, N.; Miura, N.N.; Chiba, N. Comparison of the immunopharmacological activities of triple and single-helical schizophyllan in mice. Biol. Pharm. Bull. 1995, 18, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.F.; Yan, H.D.; Zhang, X.W. Structure and immuno-stimulating activities of a new heteropolysaccharide from Lentinula edodes. J. Agr. Food Chem. 2012, 60, 11560–11566. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.P.; Zhang, L.N.; Duan, X.B. Novel highly branched water-soluble heteropolysaccharides as immunopotentiators to inhibit S-180 tumor cell growth in BALB/c mice. Carbohydr. Polym. 2012, 87, 427–434. [Google Scholar] [CrossRef]
- Deng, C.; Fu, H.T.; Teng, L.P.; Hu, Z.; Xu, X.; Chen, J. Antitumor activity of the regenerated triple-helical polysaccharide from Dictyophora indusiate. Int. J. Biol. Macromol. 2013, 61, 453–458. [Google Scholar]
- Satitmanwiwat, S.; Ratanakhanokchai, K.; Laohakunjit, N. Improved purity and immunostimulatory activity of β-(1→3) (1→6)-Glucan from Pleurotus sajor-caju using cell Wall-Degrading Enzymes. J. Agr. Food Chem. 2012, 60, 5423–5430. [Google Scholar] [CrossRef]
- Luo, Q.; Sun, Q.; Wu, L.S.; Yang, Z.R. Structural characterization of an immunoregulatory polysaccharide from the fruiting bodies of Lepista sordida. Carbohydr. Polym. 2012, 88, 820–824. [Google Scholar]
- Zhao, G.H.; Kan, J.Q.; Li, Z.X.; Chen, Z.D. Characterization and immunostimulatory activity of an (1→6)-a-d-glucan from the root of Ipomoea batatas. Int. Immunopharmacol. 2005, 5, 1436–1445. [Google Scholar] [CrossRef]
- Yan, J.K.; Wang, W.Q.; Li, L.; Wu, J.Y. Physiochemical properties and antitumor activities of two α-glucans isolated from hot water and alkaline extracts of Cordyceps (Cs-HK1) fungal mycelia. Carbohydr. Polym. 2011, 85, 753–758. [Google Scholar] [CrossRef]
- Feng, H.B.; Fan, J.; Qiu, H.; Wang, Z.H.; Yan, Z.H.; Yuan, L.H.; Guan, L.; Du, X.G.; Song, Z.H.; Han, X.F.; et al. Chuanminshen violaceum polysaccharides improve the immune responses of foot-and-mouth disease vaccine in mice. Int. J. Biol. Macromol. 2015, 78, 405–416. [Google Scholar] [CrossRef]
- Gordon, S. Phagocytosis: An Immunobiologic Process. Immunity 2016, 44, 463–475. [Google Scholar] [CrossRef]
- Wang, Y.F.; Tian, Y.Q.; Shao, J.J. Macrophage immunomodulatory activity of the polysaccharide isolated from Collybia radicata mushroom. Int. J. Biol. Macromol. 2018, 108, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Holderness, J.; Schepetkin, I.; Freedman, A.B. Polysaccharides isolated from Açaí Fruit induce innate immune responses. PLoS ONE 2011, 6, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Karnjanapratum, S.; Tabarsa, M.; Cho, M.L. Characterization and immunomodulatory activities of sulfated polysaccharides from Capsosiphon fulvescens. Int. J. Biol. Macromol. 2012, 51, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Maeda, R.; Ida, T.; Ihara, H.; Sakamoto, T. Immunostimulatory activity of polysaccharides isolated from Caulerpa lentillifera on macrophage cells. Biosci. Biotech. Bioch. 2012, 76, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.; Raptis, J.; Rice, P.J.; Kalbfleisch, J.H.; Stout, R.D.; Ensley, H.E.; Browder, W.; Willams, D.L. The influence of glucan polymer structure and solution conformation on binding to (1,3)-β-D-glucan receptors in a human monocyte-like cell line. Glycobiology 2000, 10, 339–346. [Google Scholar] [CrossRef]
- Wang, J.G.; Zhang, L.; Yu, Y.H. Enhancement of antitumor activities in sulfated and carboxymethylated polysaccharides of Ganoderma lucidum. J. Agr. Food Chem. 2009, 57, 10565–10572. [Google Scholar] [CrossRef]
- Li, G.Y.; Chen, S.G.; Wang, Y. A novel glycosaminoglycan-like polysaccharide from abalone Haliotis discus hannai Ino: Purification, structure identification and anticoagulant activity. Int. J. Biol. Macromol. 2011, 49, 1160–1166. [Google Scholar] [CrossRef]
- Tripathi, P.; Kashyap, L. The role of nitric oxide in inflammatory reactions. FEMS Immunol. Med. Mic. 2007, 51, 443–452. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, Y.J.; Lee, H.K.; Ryu, H.S.; Kim, J.S.; Yoon, M.J.; Hoon, J.T.; Kim, Y.S.; Han, S.B. Activation of macrophages by polysaccharide isolated from Paecilomyces cicadae through toll-like receptor 4. Food Chem. Toxicol. 2012, 50, 3190–3197. [Google Scholar] [CrossRef]
- Jones, E.; Adcock, I.M.; Ahmed, B.Y.; Punchard, N.A. Modulation of LPS stimulated NF-kappa B mediated Nitric Oxide production by PKCε and JAK2 in RAW macrophages. J. Inflam. 2007, 4, 23–27. [Google Scholar] [CrossRef]
- Wang, C.W.; Liu, J.T.; Huang, Y.C.; Zhang, X.H. In vitro polysaccharide extraction from Cipangopaludina cathayensis and its pharmacological potential. J. Environ. Biol. 2016, 37, 1069–1072. [Google Scholar] [PubMed]
- Mack, D.; Fischer, W.; Krokotsch, A. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: Purification and structural analysis. J. Bacteriol. 1996, 178, 175–183. [Google Scholar] [PubMed]
- Li, C.; Fu, X.; Huang, Q. Ultrasonic extraction and structural identification of polysaccharides from Prunella vulgaris and its antioxidant and antiproliferative activities. Eur. Food Res. Technol. 2015, 240, 49–60. [Google Scholar]
- Wu, J.J.; Chen, M.M.; Shi, S.S.; Wang, H.J.; Li, N.; Su, J.; Huang, Z.L.; Jin, H.; Ji, X.Q.; Wang, S.C. Hypoglycemic effect and mechanism of a pectic polysaccharide with hexenuronic acid from the fruits of Ficus pumila L. in C57BL/KsJ db/db mice. Carbohydr. Polym. 2017, 178, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Abdelnasser, S.M.; Yahya, S.M.M.; Mohamed, W.F. Antitumor exopolysaccharides derived from novel marine bacillus: Isolation, characterization aspect and biological activity. Asian Pac. J. Cancer Prev. 2017, 18, 1847–1854. [Google Scholar]


| Factors | Yield of Total Sugar (%) | ||||
|---|---|---|---|---|---|
| V/W ratio (65 °C, 3 h) | 30 | 40 | 50 | 60 | 70 |
| 26.31 ± 0.82 | 26.95 ± 0.60 | 28.27 ± 1.12 | 28.78 ± 0.64 | 28.50 ± 0.73 | |
| Temperature (°C) (50 V/W, 3 h) | 35 | 50 | 65 | 80 | 95 |
| 21.84 ± 0.47 | 22.93 ± 0.52 | 25.21 ± 0.70 | 25.64 ± 1.12 | 25.74 ± 0.33 | |
| Time (h) (50 V/W, 65 °C) | 1 | 2 | 3 | 4 | 5 |
| 24.04 ± 0.22 | 25.10 ± 0.34 | 25.47 ± 0.31 | 26.30 ± 0.11 | 26.45 ± 0.14 | |
| Method | Rps (%) a | Lps (%) a | Rpro (%) a | |
|---|---|---|---|---|
| ATPS | Top phase | 19.34 ± 0.62 | 14.83 ± 2.77 | 16.60 ± 0.80 |
| Bottom phase | 65.82 ± 2.16 | 8.04 ± 0.45 | ||
| Sevag | 81.72 ± 2.37 | 18.28 ± 2.37 | 39.81 ± 4.10 | |
| Retention Time (min) | Methylated Sugar | Linkage Types | Molar Ratio (%) |
|---|---|---|---|
| CFP-1 | |||
| 13.80 | 2,4,6-Me3-O-methyl-d-Glcp | 2-d-Glcp | 2.12 |
| 13.98 | 2,3,6-Me3-Omethyl-d-Glcp | 4-d-Glcp | 1.00 |
| 15.68 | 1,3,5,6-Me4-O-methyl-d-Glcp | 3,4,6-d-Glcp | 6.54 |
| CFP-2 | |||
| 12.92 | 2,3,4,6-Me4-O-methyl-d-Glcp | T-d-Glcp | 1.00 |
| 13.98 | 2,3,6-Me3-O-methyl-d-Glcp | 4-d-Glcp | 9.73 |
| 15.07 | 2,6-Me2-O-methyl-d-Glcp | 3,4-Glcp | 1.72 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, F.; Liu, Z.; Liu, Y.; Cheong, K.-L.; Teng, B.; Khan, B.M. Comparison of Physicochemical Characteristics and Macrophage Immunostimulatory Activities of Polysaccharides from Chlamys farreri. Mar. Drugs 2020, 18, 429. https://doi.org/10.3390/md18080429
Shi F, Liu Z, Liu Y, Cheong K-L, Teng B, Khan BM. Comparison of Physicochemical Characteristics and Macrophage Immunostimulatory Activities of Polysaccharides from Chlamys farreri. Marine Drugs. 2020; 18(8):429. https://doi.org/10.3390/md18080429
Chicago/Turabian StyleShi, Fulin, Zhicong Liu, Yang Liu, Kit-Leong Cheong, Bo Teng, and Bilal Muhammad Khan. 2020. "Comparison of Physicochemical Characteristics and Macrophage Immunostimulatory Activities of Polysaccharides from Chlamys farreri" Marine Drugs 18, no. 8: 429. https://doi.org/10.3390/md18080429
APA StyleShi, F., Liu, Z., Liu, Y., Cheong, K.-L., Teng, B., & Khan, B. M. (2020). Comparison of Physicochemical Characteristics and Macrophage Immunostimulatory Activities of Polysaccharides from Chlamys farreri. Marine Drugs, 18(8), 429. https://doi.org/10.3390/md18080429





