Briarenols Q–T: Briaranes from A Cultured Octocoral Briareum stechei (Kükenthal, 1908)
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Molecular Mechanics Calculations
3.5. In Vitro Anti-inflammatory Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bergman, W.; Feeney, R.J. The isolation of a new thymine pentoside from sponge. J. Am. Chem. Soc. 1950, 72, 2809–2810. [Google Scholar] [CrossRef]
- Rocha, J.; Peixe, L.; Gomes, N.C.M.; Calado, R. Cnidarians as a source of new marine bioactive compounds—an overview of the last decade and future steps for bioprospecting. Mar. Drugs 2011, 9, 1860–1886. [Google Scholar] [CrossRef] [PubMed]
- Ćetković, H.; Halasz, M.; Bosnar, M.H. Sponges: A reservoir of genes implicated in human cancer. Mar. Drugs 2018, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.-Y. Harvesting drugs from the seas and how Taiwan could contribute to this effort. Changhua J. Med. 2004, 9, 1–6. [Google Scholar]
- Samimi-Namin, K.; van Ofwegen, L.P. Overview of the genus Briareum (Cnidaria, Octocorallia, Briareidae) in the Indo-Pacific, with the description of a new species. Zookeys 2016, 557, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Allinger, N.L. Conformational analysis. 130. MM2. A hydrocarbon force field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc. 1977, 99, 8127–8134. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Chin, H.-K.; Peng, B.-R.; Chen, Y.-Y.; Hu, C.-C.; Zheng, L.-G.; Huynh, T.-H.; Su, T.-P.; Zhang, Y.-L.; Wen, Z.-H.; et al. Survey of briarane-type diterpenoids–Part VII. Heterocycles 2020, 100, 857–870, and previous review articles in this series. [Google Scholar]
- Sheu, J.-H.; Sung, P.-J.; Cheng, M.-C.; Liu, H.-Y.; Fang, L.-S.; Duh, C.-Y.; Chiang, M.Y. Novel cytotoxic diterpenes, excavatolides A–E, isolated from the Formosan gorgonian Briareum excavatum. J. Nat. Prod. 1998, 61, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.-H.; Sung, P.-J.; Su, J.-H.; Liu, H.-Y.; Duh, C.-Y.; Chiang, M.Y. Briaexcavatolides A–J, new diterpenes from the gorgonian Briareum excavatum. Tetrahedron 1999, 55, 14555–14564. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Kuo, L.-M.; Chen, L.-Y.; Lee, G.-H.; Peng, B.-R.; Chen, Y.-Y.; Chen, Y.-H.; Hwang, T.-L.; Sheu, J.-H.; Sung, P.-J. Identification and characterization of chlorine-containing briaranes from a cultured octocoral Briareum excavatum. Heterocycles, 2020; submitted. [Google Scholar]
- Iwasaki, J.; Ito, H.; Aoyagi, M.; Sato, Y.; Iguchi, K. Briarane-type diterpenoids from the Okinawan soft coral Pachyclavularia violacea. J. Nat. Prod. 2006, 69, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Groweiss, A.; Look, S.A.; Fenical, W. Solenolides, new antiinflammatory and antiviral diterpenoids from a marine octocoral of the genus Solenopodium. J. Org. Chem. 1988, 53, 2401–2406. [Google Scholar] [CrossRef]
- Patra, A.; Majumdar, A.; Mandal, K.K.; Ghosh, A.; Banerjee, D.; Haldar, B.P. Secondary metabolites from the cnidarian Cavernularia sp.: Structures of the new briaranes cavernulin A and B. J. Indian Chem. Soc. 2001, 78, 619–626. [Google Scholar] [CrossRef]
- Lin, C.-C.; Chen, W.-F.; Lee, G.-H.; Wen, Z.-H.; Fang, L.-S.; Kuo, Y.-H.; Lee, C.-Y.; Sung, P.-J. Fragilides M–O, new triacetoxybriaranes from the gorgonian coral Junceella fragilis (Ellisellidae). Heterocycles 2019, 98, 984–994. [Google Scholar]
- Bayer, F.M. Key to the genera of octocorallia exclusive of Pennatulacea (Coelenterata: Anthozoa), with diagnoses of new taxa. Proc. Biol. Soc. Wash. 1981, 94, 902–947. [Google Scholar]
- Benayahu, Y.; Jeng, M.-S.; Perkol-Finkel, S.; Dai, C.-F. Soft corals (Octocorallia: Alcyonacea) from Southern Taiwan. II. Species diversity and distributional patterns. Zool. Stud. 2004, 43, 548–560. [Google Scholar]
- Miyazaki, Y.; Reimer, J.D. Morphological and genetic diversity of Briareum (Anthozoa: Octocorallia) from the Ryukyu Archipelago, Japan. Zool. Sci. 2014, 31, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Chen, N.-F.; Feng, C.-W.; Cheng, S.-Y.; Hung, H.-C.; Tsui, K.-H.; Hsu, C.-H.; Sung, P.-J.; Chen, W.-F.; Wen, Z.-H. A coral-derived compound improves functional recovery after spinal cord injuruy through its antiapoptotic and anti-inflammatory effects. Mar. Drugs 2016, 14, 160. [Google Scholar] [CrossRef] [PubMed]
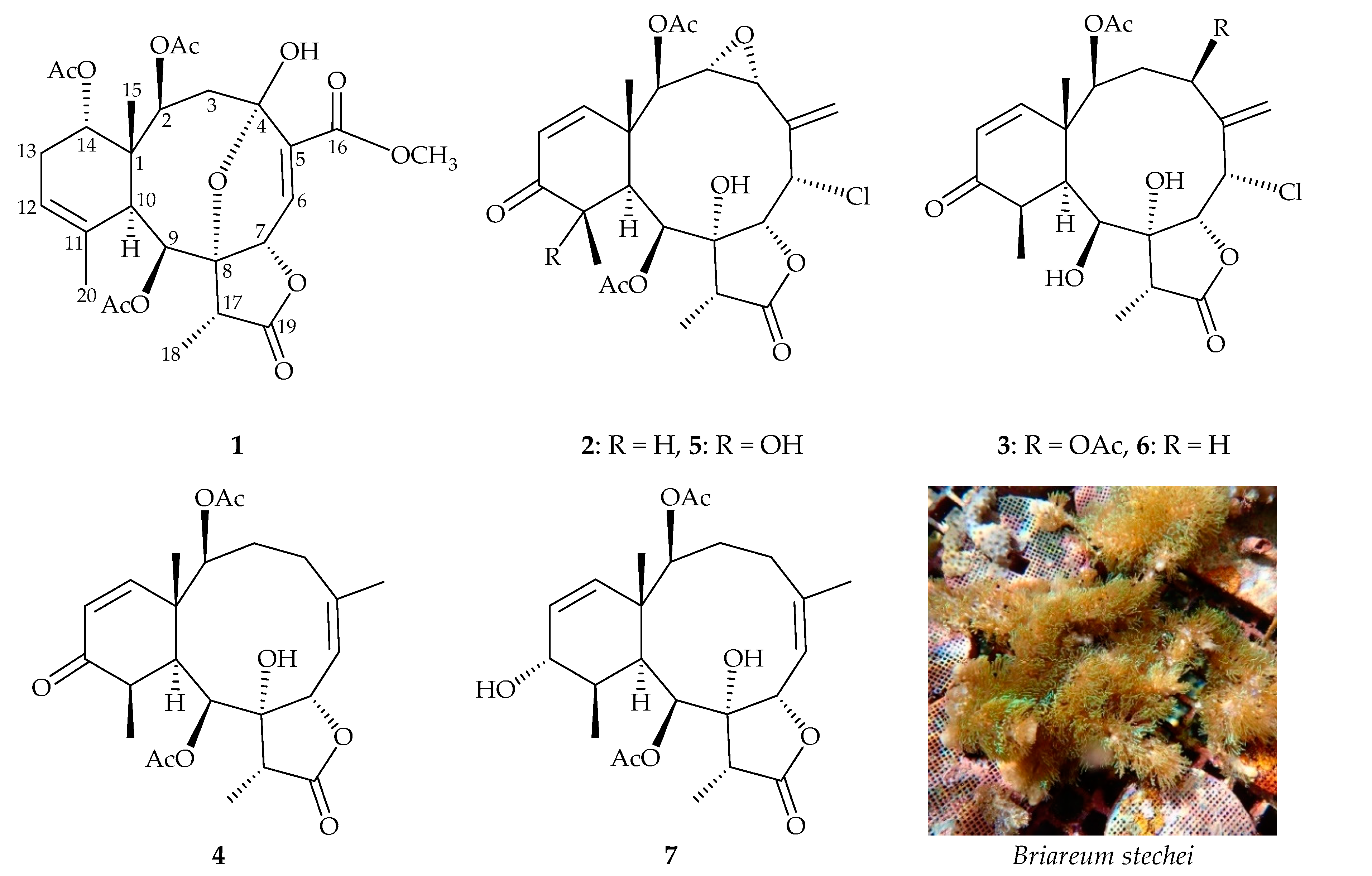
 ), heteronuclear multiple-bond correlation (HMBC;
), heteronuclear multiple-bond correlation (HMBC;  ), and nuclear Overhauser effect spectroscopy (NOESY;
), and nuclear Overhauser effect spectroscopy (NOESY;  ) correlations of 1.
) correlations of 1.
 ), heteronuclear multiple-bond correlation (HMBC;
), heteronuclear multiple-bond correlation (HMBC;  ), and nuclear Overhauser effect spectroscopy (NOESY;
), and nuclear Overhauser effect spectroscopy (NOESY;  ) correlations of 1.
) correlations of 1.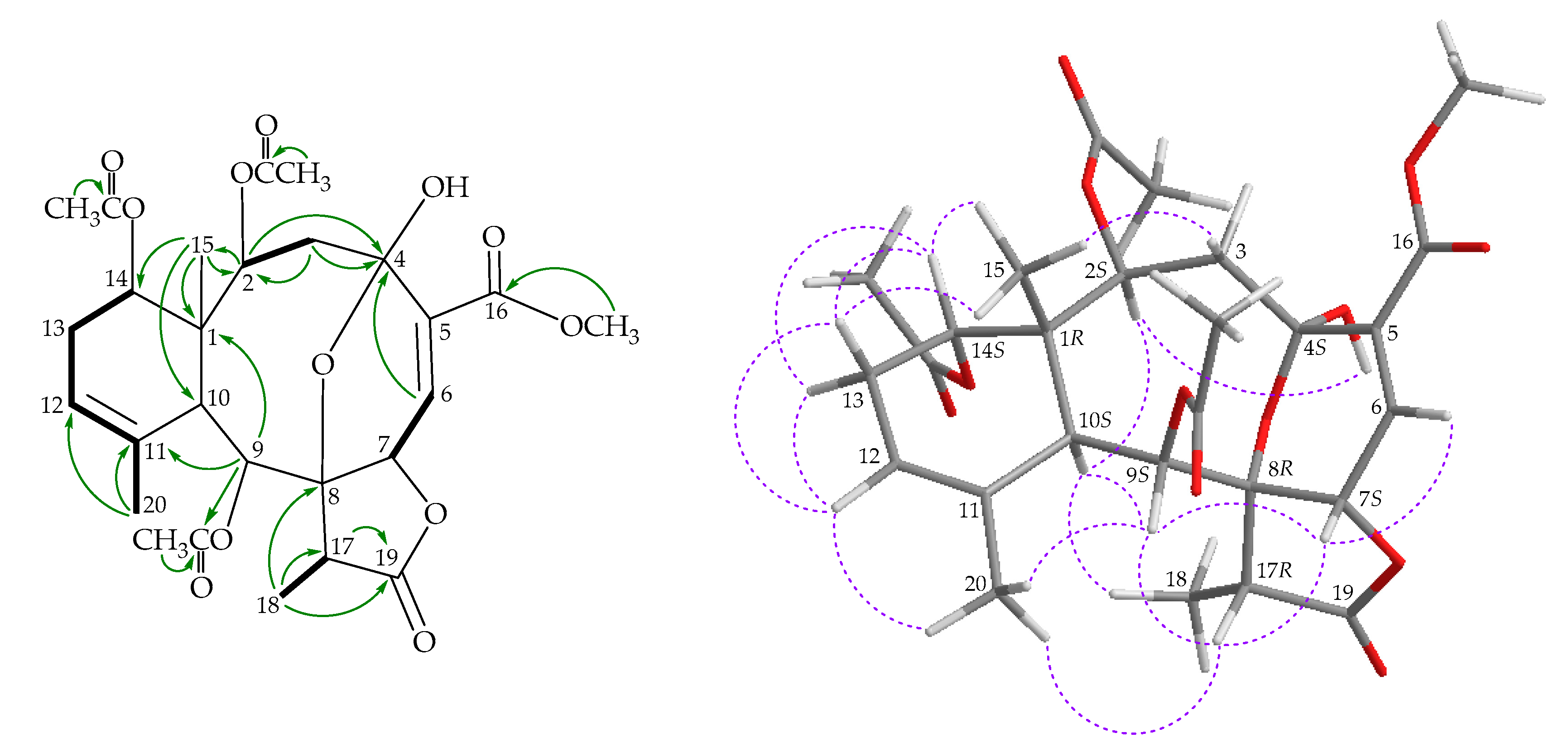
 ), HMBC (
), HMBC ( ), and NOESY (
), and NOESY ( ) correlations of 2.
) correlations of 2.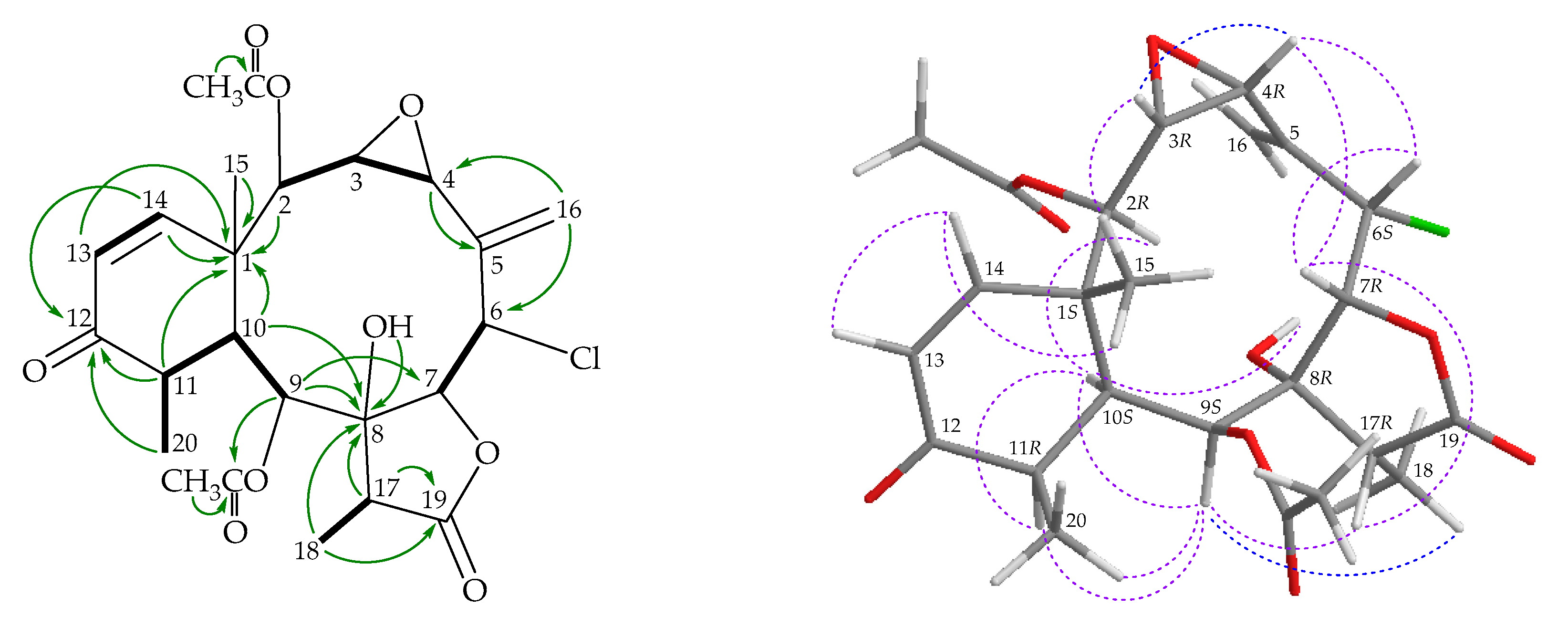
 ), HMBC (
), HMBC ( ), and NOESY (
), and NOESY ( ) correlations of 3.
) correlations of 3.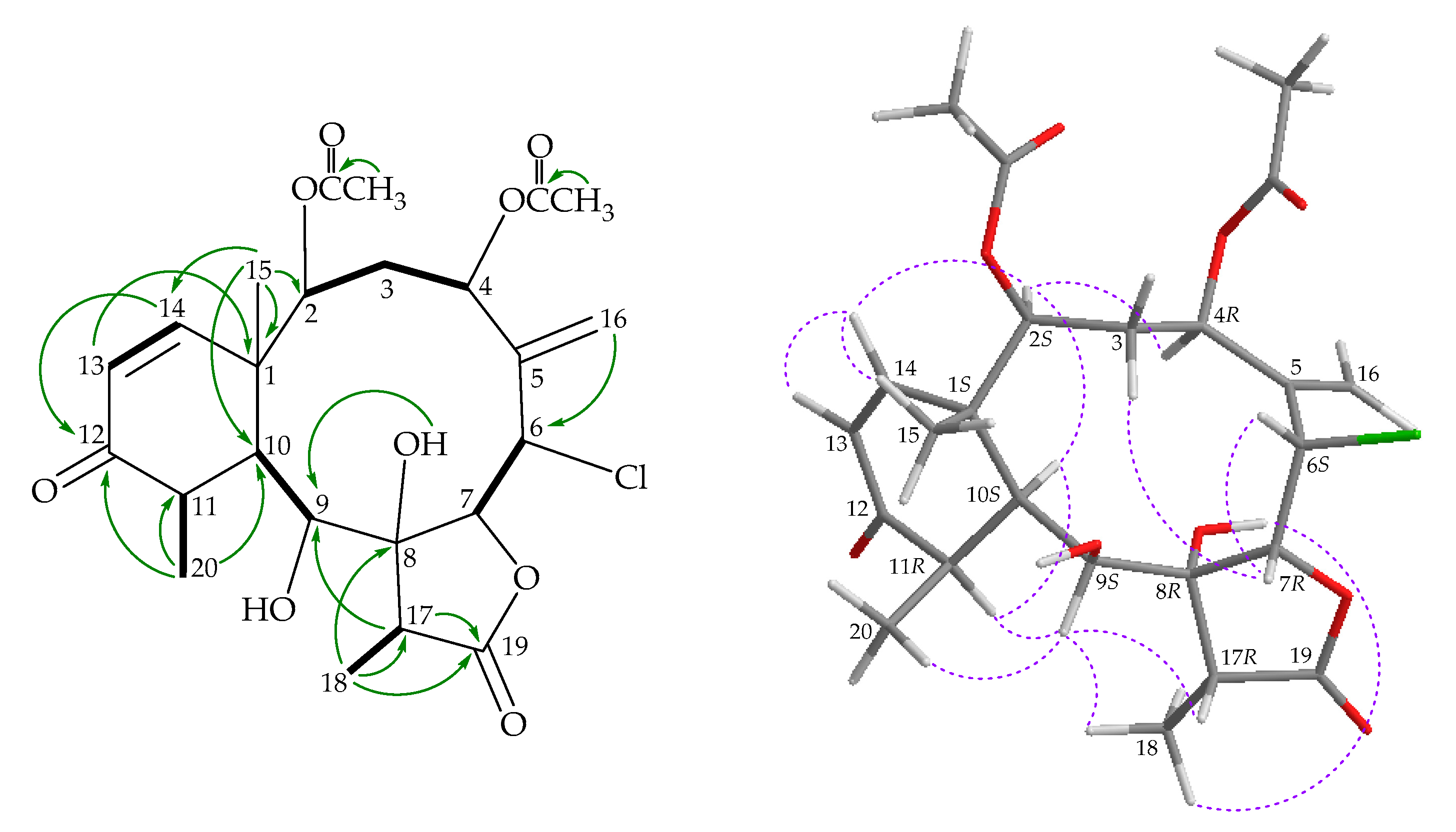
 ), HMBC (
), HMBC ( ), and NOESY (
), and NOESY ( ) correlations of 4.
) correlations of 4.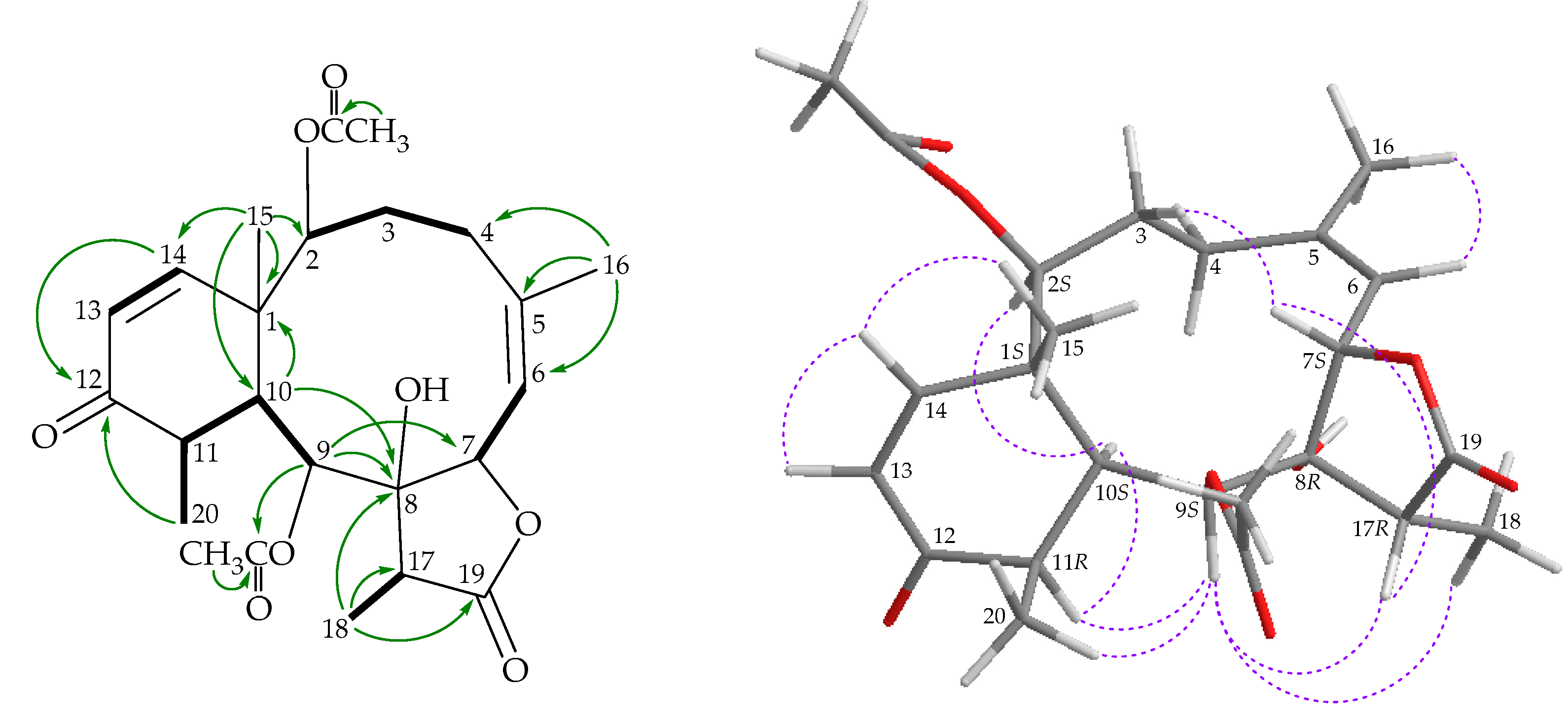
| Position | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1 | 44.3, C a | 41.1, C | 43.9, C | 44.1, C |
| 2 | 72.1, CH | 76.0, CH | 78.1, CH | 79.5, CH |
| 3 | 41.8, CH2 | 60.2, CH | 34.5, CH2 | 31.6, CH2 |
| 4 | 94.0, C | 57.4, CH | 74.2, CH | 28.9, CH2 |
| 5 | 138.4, C | 133.6, C | n. o. c | 147.2, C |
| 6 | 128.0, CH | 60.9, CH | 62.3, CH | 118.1, CH |
| 7 | 70.1, CH | 76.3, CH | 77.2, CH | 77.7, CH |
| 8 | 80.8, C | 84.2, C | 85.0, C | 82.2, C |
| 9 | 77.2, CH | 68.4, CH | 74.2, CH | 71.3, CH |
| 10 | 40.7, CH | 39.2, CH | 38.8, CH | 38.3, CH |
| 11 | 131.1, C | 44.7, CH | 47.3, CH | 48.5, CH |
| 12 | 122.0, CH | 201.6, C | 202.4, C | 202.6, C |
| 13 | 28.4, CH2 | 124.4, CH | 124.5, CH | 124.1, CH |
| 14 | 71.8, CH | 152.5, CH | 155.1, CH | 154.6, CH |
| 15 | 13.4, CH3 | 14.6, CH3 | 18.1, CH3 | 15.4, CH3 |
| 16 | 164.6, C | 120.3, CH2 | 122.8, CH2 | 28.4, CH3 |
| 17 | 48.0, CH | 45.5, CH | 45.4, CH | 42.6, CH |
| 18 | 8.7, CH3 | 6.2, CH3 | 7.7, CH3 | 6.8, CH3 |
| 19 | 174.9, C | 173.7, C | 175.6, C | 175.5, C |
| 20 | 24.2, CH3 | 15.6, CH3 | 15.3, CH3 | 15.0, CH3 |
| OAc-2 | 169.2, C b 21.3, CH3 | 169.6, C 20.9, CH3 | 169.8, C 21.0, CH3 | 168.9, C 21.7, CH3 |
| OAc-4 | 169.1, C 21.0, CH3 | |||
| OAc-9 | 169.7, C 21.0, CH3 | 169.3, C 21.8, CH3 | 170.2, C 21.1, CH3 | |
| OAc-14 | 173.2, C b 21.3, CH3 | |||
| OCH3-16 | 52.4, CH3 |
| Position | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 2 | 5.11 d (7.2) | 4.75 d (9.0) | 4.63 dd (3.6, 3.0) | 4.44 dd (6.6, 1.2) |
| 3α | 2.46 d (16.8) | 1.86 ddd (15.6, 3.6, 3.6) | 1.70 m | |
| β | 3.40 dd (16.8, 7.2) | 3.48 dd (9.0, 4.2) | 3.00 ddd (15.6, 12.0, 3.0) | 2.75 m |
| 4α | 5.81 dd (12.0, 3.6) | 2.06 ddd (14.4, 14.4, 4.8) | ||
| β | 3.67 d (4.2) | 2.54 m | ||
| 6 | 6.84 d (4.8) | 5.39 m | 5.29 br s | 5.44 br d (10.2) |
| 7 | 4.44 d (4.8) | 5.08 d (3.6) | 5.41 d (3.0) | 5.23 d (10.2) |
| 9 | 6.05 s | 5.57 d (8.4) | 3.90 dd (6.0, 6.0) | 5.30 d (4.8) |
| 10 | 3.10 br s | 2.51 dd (8.4, 4.2) | 2.67 br s | 2.69 dd (4.8, 4.2) |
| 11 | 2.87 qd (7.2, 4.2) | 2.45 m | 2.50 qd (7.2, 4.2) | |
| 12 | 5.56 br s | |||
| 13α/β | 2.02 m; 2.38 br d (18.0) | 5.88 dd (10.2, 0.6) | 5.89 d (10.2) | 5.85 d (10.2) |
| 14 | 5.18 d (4.2) | 6.37 d (10.2) | 6.47 d (10.2) | 6.39 d (10.2) |
| 15 | 1.04 s | 1.28 s | 1.46 s | 1.23 s |
| 16a/b | 5.79 d (3.0); 6.06 d (3.0) | 5.72 s; 5.89 s | 1.99 d (1.2) | |
| 17 | 2.79 q (7.2) | 2.50 q (7.2) | 3.05 q (7.8) | 2.44 q (7.2) |
| 18 | 1.46 d (7.2) | 1.25 d (7.2) | 1.19 d (7.8) | 1.21 d (7.2) |
| 20 | 1.96 br s | 1.30 d (7.2) | 1.25 d (7.2) | 1.32 d (7.2) |
| OH-4 | 6.11 s | |||
| OH-8 | 3.52 s | 3.42 s | n. o. b | |
| OAc-2 | 2.04 s a | 2.23 s | 2.09 s a | 2.24 s |
| OAc-4 | 2.17 s a | |||
| OAc-9 | 2.03 s | 2.27 s | 2.13 s | |
| OAc-14 | 2.11 s a | |||
| OCH3-16 | 3.81 s |
| Compound | iNOS | COX-2 |
|---|---|---|
| Expression (% of LPS) at 10 μM | ||
| Control | 1.28 ± 0.29 | 0.76 ± 0.13 |
| LPS | 100.00 ± 1.87 | 100.00 ± 3.26 |
| 1 | 98.27 ± 5.13 | 94.00 ± 3.47 |
| 2 | 84.53 ± 4.66 * | 112.96 ± 4.54 |
| 3 | 78.50 ± 3.45 * | 97.66 ± 4.60 |
| 4 | 79.95 ± 2.94 * | 104.66 ± 7.86 |
| Dexamethasone | 24.56 ± 1.85 * | 6.56 ± 1.18 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-L.; Chiang, C.-C.; Lee, Y.-T.; Wen, Z.-H.; Wu, Y.-C.; Wu, Y.-J.; Hwang, T.-L.; Wu, T.-Y.; Chang, C.-Y.; Sung, P.-J. Briarenols Q–T: Briaranes from A Cultured Octocoral Briareum stechei (Kükenthal, 1908). Mar. Drugs 2020, 18, 383. https://doi.org/10.3390/md18080383
Zhang Y-L, Chiang C-C, Lee Y-T, Wen Z-H, Wu Y-C, Wu Y-J, Hwang T-L, Wu T-Y, Chang C-Y, Sung P-J. Briarenols Q–T: Briaranes from A Cultured Octocoral Briareum stechei (Kükenthal, 1908). Marine Drugs. 2020; 18(8):383. https://doi.org/10.3390/md18080383
Chicago/Turabian StyleZhang, Yi-Lin, Chih-Chao Chiang, Yi-Ting Lee, Zhi-Hong Wen, Yang-Chang Wu, Yu-Jen Wu, Tsong-Long Hwang, Tung-Ying Wu, Chia-Yuan Chang, and Ping-Jyun Sung. 2020. "Briarenols Q–T: Briaranes from A Cultured Octocoral Briareum stechei (Kükenthal, 1908)" Marine Drugs 18, no. 8: 383. https://doi.org/10.3390/md18080383
APA StyleZhang, Y.-L., Chiang, C.-C., Lee, Y.-T., Wen, Z.-H., Wu, Y.-C., Wu, Y.-J., Hwang, T.-L., Wu, T.-Y., Chang, C.-Y., & Sung, P.-J. (2020). Briarenols Q–T: Briaranes from A Cultured Octocoral Briareum stechei (Kükenthal, 1908). Marine Drugs, 18(8), 383. https://doi.org/10.3390/md18080383







