Are Helicobacter pylori Infection and Fucoidan Consumption Associated with Fucoidan Absorption?
Abstract
1. Introduction
2. Results
2.1. Prevalence of H. Pylori Infection AccordingtTo the Frequency of Mozuku Consumption and Age
2.2. Urinary Fucoidan Values before and after Fucoidan Ingestion
2.3. Basal Levels (before Ingestion) of Fucoidan
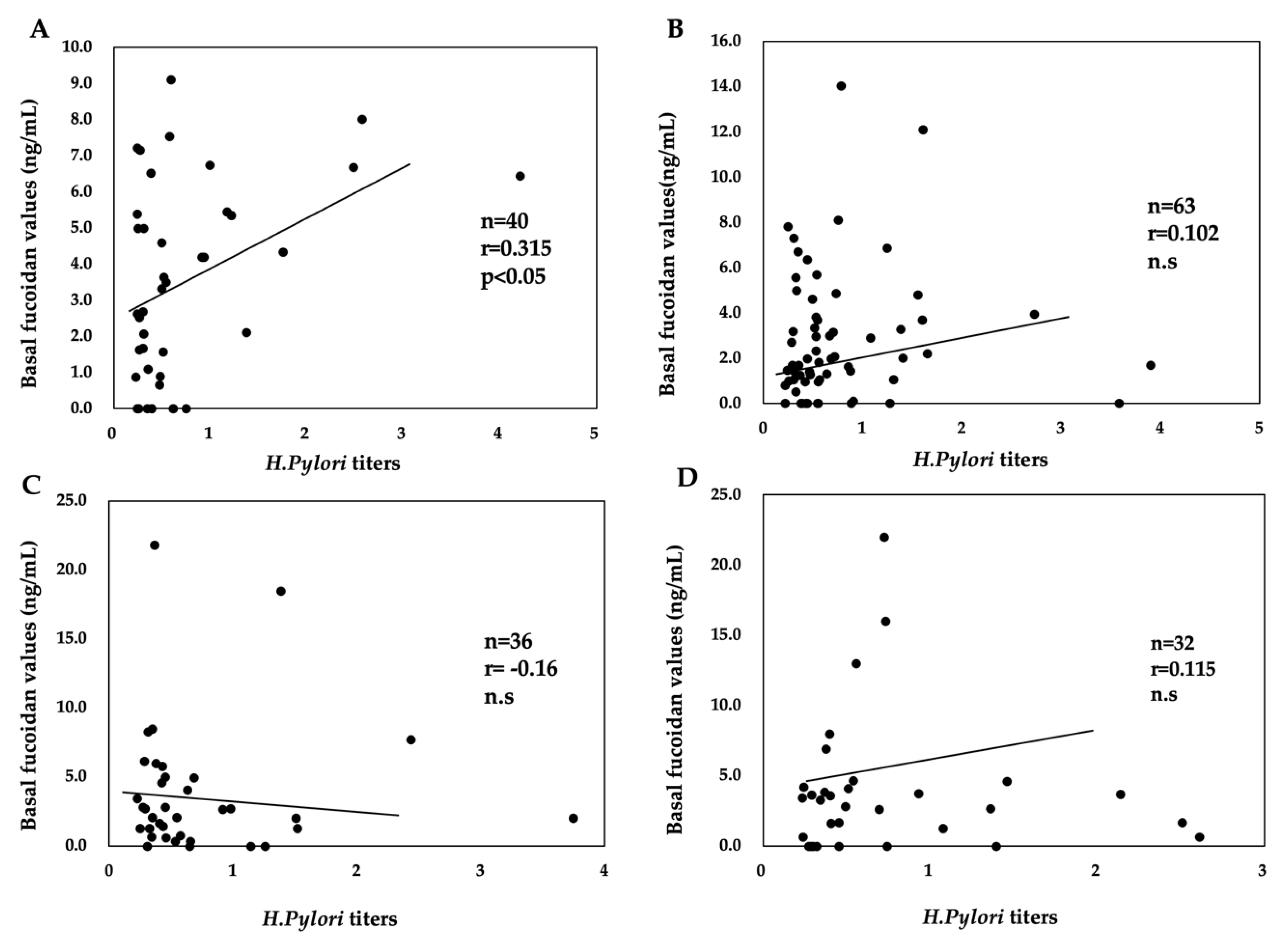
2.4. Relationship between H. pylori Titers and Basal Fucoidan Levels
2.5. Maximum Absorption of Fucoidan (ΔMax Fucoidan Value)
2.6. Relevance of H. Pylori Infection and Mozuku Consumption to Fucoidan Absorption
2.7. Relationship between the Basal and Δmax Fucoidan Values in H. Pylori-Positive Subjects
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Oral Intake of Fucoidan and Collection of Urine Samples
4.3. Assay for Fucoidan Levels in Urine Samples
4.4. Assay for Anti-H. pylori Antibody Titers in Urine
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef]
- Wu, L.; Sun, J.; Su, X.; Yu, Q.; Yu, Q.; Zhang, P.; Suna, J. A review about the development of fucoidan in antitumor activity: Progress and challenges. Carbohydr. Polym. 2016, 10, 96–111. [Google Scholar] [CrossRef]
- Fitton, J.H.; Park, A.Y.; Stringer, D.N.; Karpiniec, S.S. Therapies from fucoidan: New developments. Mar. Drugs 2019, 17, 571. [Google Scholar] [CrossRef]
- Van Weelden, G.; Bobiński, M.; Okła, K.; Van Weelden, W.J.; Romano, A.; Pijnenborg, J.M.A. Fucoidan structure and activity in relation to anti-cancer mechanisms. Mar. Drugs 2019, 17, 32. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological activities of fucoidan and the factors mediating its therapeutic effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef]
- Shibata, H.; Kimura-Takagi, I.; Nagaoka, M.; Hashimoto, S.; Sawada, H.; Ueyama, S.; Yokokura, T. Inhibitory effect of cladosiphon fucoidan on the adhesion of helicobacter pylori to human gastric cells. J. Nutr. Sci. Vitaminol. (Tokyo) 1999, 45, 325–336. [Google Scholar] [CrossRef]
- Shibata, H.; Iimuro, M.; Uchiya, N.; Kawamori, T.; Nagaoka, M.; Ueyama, S.; Hashimoto, S.; Yokokura, T.; Sugimura, T.; Wakabayashi, K. Preventive effects of cladosiphon fucoidan against Helicobacter pylori infection in mongolian gerbils. Helicobacter 2003, 8, 59–65. [Google Scholar] [CrossRef]
- Besednova, N.N.; Zaporozhets, T.S.; Somova, L.M.; Kuznetsova, T.A. Review: Prospects for the use of extracts and polysaccharides from marine algae to prevent and treat the diseases caused by Helicobacter pylori. Helicobacter 2015, 20, 89–97. [Google Scholar] [CrossRef]
- Chua, E.G.; Verbrugghe, P.; Perkins, T.T.; Tay, C. Fucoidans disrupt adherence of Helicobacter pylori to AGS cells in vitro. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–6. [Google Scholar] [CrossRef]
- Nagamine, T.; Hayakawa, K.; Nakazato, K.; Iha, M. Determination of the active transport of fucoidan derived from Okinawa Mozuku across the human intestinal Caco-2 cells as assessed by size-exclusion chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 997, 187–193. [Google Scholar] [CrossRef]
- Zhang, E.; Chu, F.; Xu, L.; Liang, H.; Song, S.; Ji, A. Use of fluorescein isothiocyanate isomer Ι to study the mechanism of intestinal absorption of fucoidan sulfate in vivo and in vitro. Biopharm. Drug. Dispos. 2018, 39, 298–307. [Google Scholar] [CrossRef]
- Imbs, T.I.; Zvyagintseva, T.N.; Ermakova, S.P. Is the transformation of fucoidans in human body possible? Int. J. Biol. Macromol. 2020, 142, 778–781. [Google Scholar] [CrossRef]
- Kadena, K.; Tomori, M.; Iha, M.; Nagamine, T. Absorption study of Mozuku Fucoidan in Japanese volunteers. Mar. Drugs 2018, 16, 254. [Google Scholar] [CrossRef]
- Hehemann, J.-H.; Correc, G.; Barbeyron, T.; Helbert, W.; Czjzek, M.; Michel, G. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature 2010, 464, 908–912. [Google Scholar] [CrossRef]
- Sipponen, P.; Helske, T.; Järvinen, P.; Hyvarinen, H.; Seppälä, K.; Siurala, M. Fall in the prevalence of chronic gastritis over 15 years: Analysis of outpatient series in Finland from 1977, 1985, and 1992. Gut 1994, 35, 1167–1171. [Google Scholar] [CrossRef]
- Kamada, T.; Haruma, K.; Akiyama, T.; Tanaka, S.; Shiotani, A.; Graham, D.Y.; Ito, M.; Inoue, K.; Manabe, N.; Matsumoto, H.; et al. Time trends in Helicobacter pylori infection and atrophic gastritis over 40 Years in Japan. Helicobacter 2015, 20, 192–198. [Google Scholar] [CrossRef]
- Pichon, M.; Burucoa, C. Impact of the gastro-intestinal bacterial microbiome on Helicobacter-associated diseases. Healthcare 2019, 7, 34. [Google Scholar] [CrossRef]
- Pero, R.; Brancaccio, M.; Laneri, S.; Biasi, M.; Lombardo, B.; Scudiero, O. A novel view of human Helicobacter pylori infections: Interplay between microbiota and beta-defensins. Biomolecules 2019, 9, 237. [Google Scholar] [CrossRef]
- Franceschi, F.; Annalisa, T.; Teresa, D.R.; Giovanna, D.; Ianiro, G.; Franco, S.; Viviana, G.; Valentina, T.; Riccardo, L.L.; Antonio, G. Role of Helicobacter pylori infection on nutrition and metabolism. World J. Gastroenterol. 2014, 20, 12809–12817. [Google Scholar] [CrossRef]
- Aditi, A.; Graham, D.Y. Vitamin C, Gastritis, and gastric disease: A historical review and update. Dig. Dis. Sci. 2012, 57, 2504–2515. [Google Scholar] [CrossRef]
- Ackam, M. Helicobacter pylori and micronutrients. Indian Pediatr. 2010, 47, 119–126. [Google Scholar]
- Tokita, Y.; Nakajima, K.; Mochida, H.; Iha, M.; Nagamine, T. Development of a fucoidan-specific antibody and measurement of fucoidan in serum and urine by Sandwich ELISA. Biosci. Biotechnol. Biochem. 2010, 74, 350–357. [Google Scholar] [CrossRef]
- Amornlerdpison, D.; Peerapompisal, Y.; Taesoticul, T.; Noiraraksar, T.; Kanjanapothi, D. Gastroprotective activity of Padina minor Yamada. Chiang Mai J. Sci. 2009, 36, 92–103. [Google Scholar]
- Biesalski, H.K. Nutrition meets the microbiome: Micronutrients and the microbiota. Ann. N. Y. Acad. Sci. 2016, 1372, 53–64. [Google Scholar] [CrossRef]
- Ticinesi, A.; Lauretani, F.; Milani, C.; Nouvenne, A.; Tana, C.; Del Rio, D.; Maggio, M.; Ventura, M.; Meschi, T. Aging gut microbiota at the cross-road between nutrition, physical frailty, and sarcopenia: Is there a gut-muscle axis? Nutrients 2017, 9, 1303. [Google Scholar] [CrossRef]
- Sandhu, K.V.; Sherwin, E.; Schellekens, H.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Feeding the microbiota-gut-brain axis: Diet, microbiome, and neuropsychiatry. Transl. Res. 2017, 179, 223–244. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Moschen, A.R.; Wieser, V.; Tilg, H. Dietary factors: Major regulators of the gut’s microbiota. Gut Liver 2012, 6, 411–416. [Google Scholar] [CrossRef]
- Das, A.; Pereira, V.; Saxena, S.; Ghosh, T.S.; Anbumani, D.; Bag, S.; Das, B.; Nair, G.B.; Abraham, P.; Mande, S.S. Gastric microbiome of Indian patients with Helicobacter pylori infection, and their interaction networks. Sci. Rep. 2017, 7, 15438. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Contreras, A.; Goldfarb, K.C.; Godoy-Vitorino, F.; Karaoz, U.; Contreras, M.; Blaser, M.J.; Brodie, E.L.; Dominguez-Bello, M.G. Structure of the human gastric bacterial community in relation to Helicobacter pylori status. ISME J. 2010, 5, 574–579. [Google Scholar] [CrossRef]
- Schulz, C.; Schütte, K.; Koch, N.; Vilchez-Vargas, R.; Wos-Oxley, M.L.; Oxley, A.P.A.; Vital, M.; Malfertheiner, P.; Pieper, D.H. The active bacterial assemblages of the upper GI tract in individuals with and withoutHelicobacterinfection. Gut 2016, 67, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Asaka, M.; Saito, M.; Sekine, H.; Ohara, S.; Toyota, T.; Akamatsu, T.; Kaneko, T.; Kiyosawa, K.; Nishizawa, O.; et al. Clinical usefulness of urine-based enzyme-linked immunosorbent assay for detection of antibody to Helicobacter pylori: A collaborative study in nine medical institutions in Japan. Helicobacter 2000, 5, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Miwa, H.; Hirose, M.; Kikuchi, S.; Terai, T.; Iwazaki, R.; Kobayashi, O.; Takei, Y.; Ogihara, T.; Sato, N. How useful is the detection kit for antibody to Helicobacter pylori in urine (URINELISA) in clinical practice? Am. J. Gastroenterol. 1999, 94, 3460–3463. [Google Scholar] [CrossRef] [PubMed]
- Katsuragi, K.; Noda, A.; Tachikawa, T.; Azuma, A.; Mukai, F.; Murakami, K.; Fujioka, T.; Kato, M.; Asaka, M. Highly sensitive urine-based enzyme-linked immunosorbent assay for detection of antibody to Helicobacter pylori. Helicobacter 1998, 3, 289–295. [Google Scholar] [CrossRef]

| Age Group | 1–3 Times Weekly | Once Every 2 Weeks | Once Monthly | Once Every 2–3 Months | Hardly Eat | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| H. Pylori | H. Pylori | H. Pylori | H. Pylori | H. Pylori | ||||||
| (-) | (+) | (-) | (+) | (-) | (+) | (-) | (+) | (-) | (+) | |
| 20’s (n = 50) | 2 | 5 | 6 | 7 | 5 | 5 | 4 | 5 | 3 | 8 |
| 30’s (n = 75) | 2 | 3 | 3 | 10 | 6 | 11 | 10 | 11 | 9 | 10 |
| 40’s (n = 63) | 3 | 4 | 2 | 10 | 6 | 11 | 10 | 7 | 3 | 7 |
| 50’s (n = 45) | 2 | 6 | 2 | 7 | 2 | 11 | 3 | 4 | 1 | 7 |
| ≥60’s (n = 26) | 0 | 6 | 0 | 2 | 2 | 7 | 1 | 5 | 0 | 3 |
| Odds Ratio | 95%CI | ||
|---|---|---|---|
| Habit of eating mozuku | 1.12 | 0.89–1.42 | |
| Age | 40y.o.< | 1.00 | 1.01–2.85 |
| ≥40y.o. | 1.70 | ||
| 0 | 3 h | 6 h | 9 h | |
|---|---|---|---|---|
| ng/mL | ||||
| Subjects (n = 259) | 3.7 ± 3.4 | 15.3 ± 18.8 a | 24.4 ± 35.1 a,b | 24.2 ± 35.2 a,b |
| Habit of Eating Mozuku | H. pylori (-) | H. pylori (+) | P-Value H. pylori (-) vs H. pylori (+) |
|---|---|---|---|
| 1–3 times weekly | 4.1 ± 1.3 (n =9) a | 3.2 ± 3.0 (n = 24) | 0.29 |
| Once every 2 weeks | 2.7 ± 3.2 (n = 14) | 3.2 ± 2.6 (n = 35) | 0.58 |
| Once monthly | 3.1 ± 3.0 (n = 21) b | 2.8 ± 2.8 (n = 44) | 0.69 |
| Once every 2–3 months | 3.3 ± 3.3 (n = 28) c | 4.3 ± 5.7 (n = 33) | 0.40 |
| hardly eat | 1.4 ± 1.5 (n = 16) | 3.3 ± 3.4 (n = 35) | 0.01 |
| H.pylori (-) | H.pylori (+) | H.pylori (-) vs H.pylori (+) | |
|---|---|---|---|
| Total | 29.4 ± 40.1 (n = 88) | 24.2 ± 37.1 (n = 171) | P = 0.300 |
| 40 y.o.< | 26.4 ± 38.8 (n = 52) | 35.3 ± 47.6 (n = 76) | P = 0.323 |
| ≥40 y.o. | 33.8 ± 59. 8 (n = 36) | 21.9 ± 35.3 (n = 95) a | P = 0.135 |
| Habit of Eating Mozuku | H.pylori (-) | H.pylori (+) | P-Value H. pylori(+) Aged <40 y.o. vs. H. pylori(+) Aged ≥40 y.o. | ||
|---|---|---|---|---|---|
| 40 y.o.< | ≥40 y.o. | 40 y.o.< | ≥40 y.o. | ||
| 1–3 times weekly | 25.0 ± 8.2 (n = 4) | 37.8 ± 20.3 (n = 5) | 20.4 ± 16.1 (n = 8) | 17.4 ± 25.6 (n = 16) | n.s |
| Once every 2 weeks | 24.5 ± 25.2 (n = 10) | 36.4 ± 14.8 (n = 4) a | 34.8 ± 52.7 (n = 17) | 12.9 ± 14.7 (n = 18) | 0.08 |
| Once monthly | 29.1 ± 71.6 (n = 11) | 27.7 ± 17.8 (n = 10) | 41.9 ± 54.5 (n = 16) | 18.9 ± 21.4 (n = 28) | 0.06 |
| Once every 2-3 months | 31.1 ± 31.8 (n = 15) | 39.3 ± 67.0 (n = 13) | 36.2 ± 46.7 (n = 18) | 42.5 ± 73.1 (n = 15) | n.s |
| hardly eat | 24.4 ± 22.0 (n = 12) | 15.8 ± 22.9 (n = 4) | 38.1 ± 53.3 (n = 18) | 22.2 ± 17.9 (n = 17) | n.s |
| Habit of Eating Mozuku | H.pylori (-) | H.ylori (+) | ||
|---|---|---|---|---|
| 40 y.o < | ≥40 y.o | 40 y.o < | ≥40 y.o | |
| Regularly consumed mozuku 1) | 26.6 ± 48.7 (n = 25) | 32.1 ± 17.6 (n = 19) | 34.5 ± 48.1 (n = 40) | 16.8 ± 20.8 a,b,c (n = 63) |
| Rarely ate mozuku 2) | 28.1 ± 27.6 (n = 27) | 33.8 ± 59.8 (n = 17) | 33.9 ± 47.7 (n = 36) | 30.5 ± 51.9 (n = 32) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomori, M.; Nagamine, T.; Iha, M. Are Helicobacter pylori Infection and Fucoidan Consumption Associated with Fucoidan Absorption? Mar. Drugs 2020, 18, 235. https://doi.org/10.3390/md18050235
Tomori M, Nagamine T, Iha M. Are Helicobacter pylori Infection and Fucoidan Consumption Associated with Fucoidan Absorption? Marine Drugs. 2020; 18(5):235. https://doi.org/10.3390/md18050235
Chicago/Turabian StyleTomori, Makoto, Takeaki Nagamine, and Masahiko Iha. 2020. "Are Helicobacter pylori Infection and Fucoidan Consumption Associated with Fucoidan Absorption?" Marine Drugs 18, no. 5: 235. https://doi.org/10.3390/md18050235
APA StyleTomori, M., Nagamine, T., & Iha, M. (2020). Are Helicobacter pylori Infection and Fucoidan Consumption Associated with Fucoidan Absorption? Marine Drugs, 18(5), 235. https://doi.org/10.3390/md18050235





