Natural Product Chemistry of Gorgonian Corals of Genus Junceella–Part III
Abstract
:1. Introduction
2. Junceella
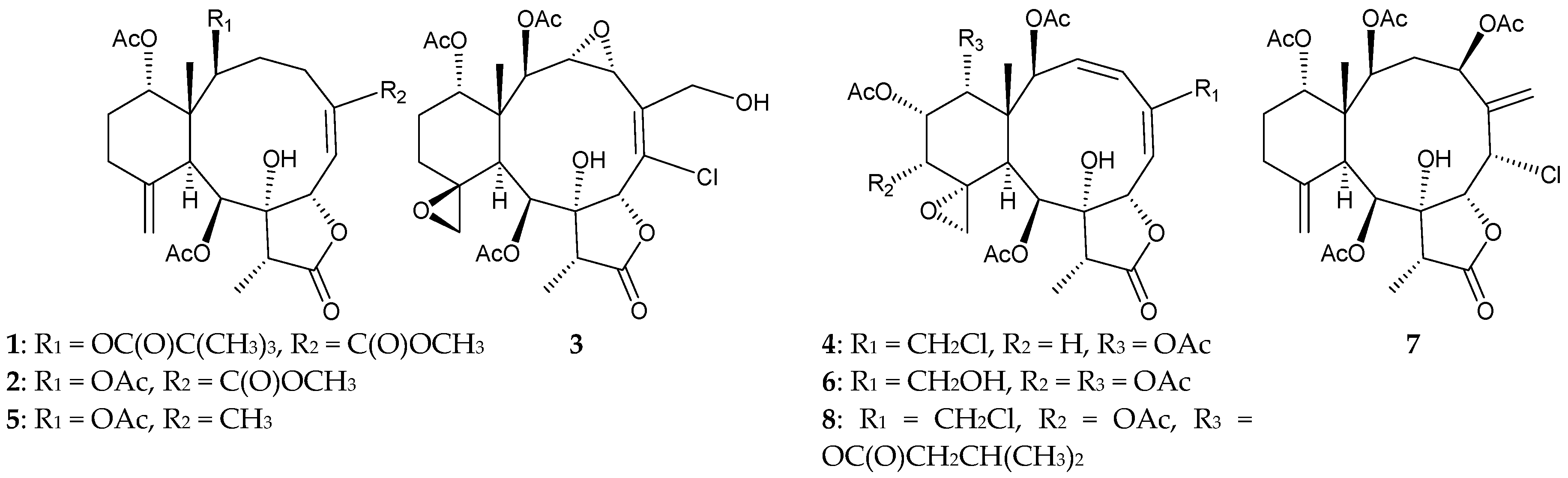
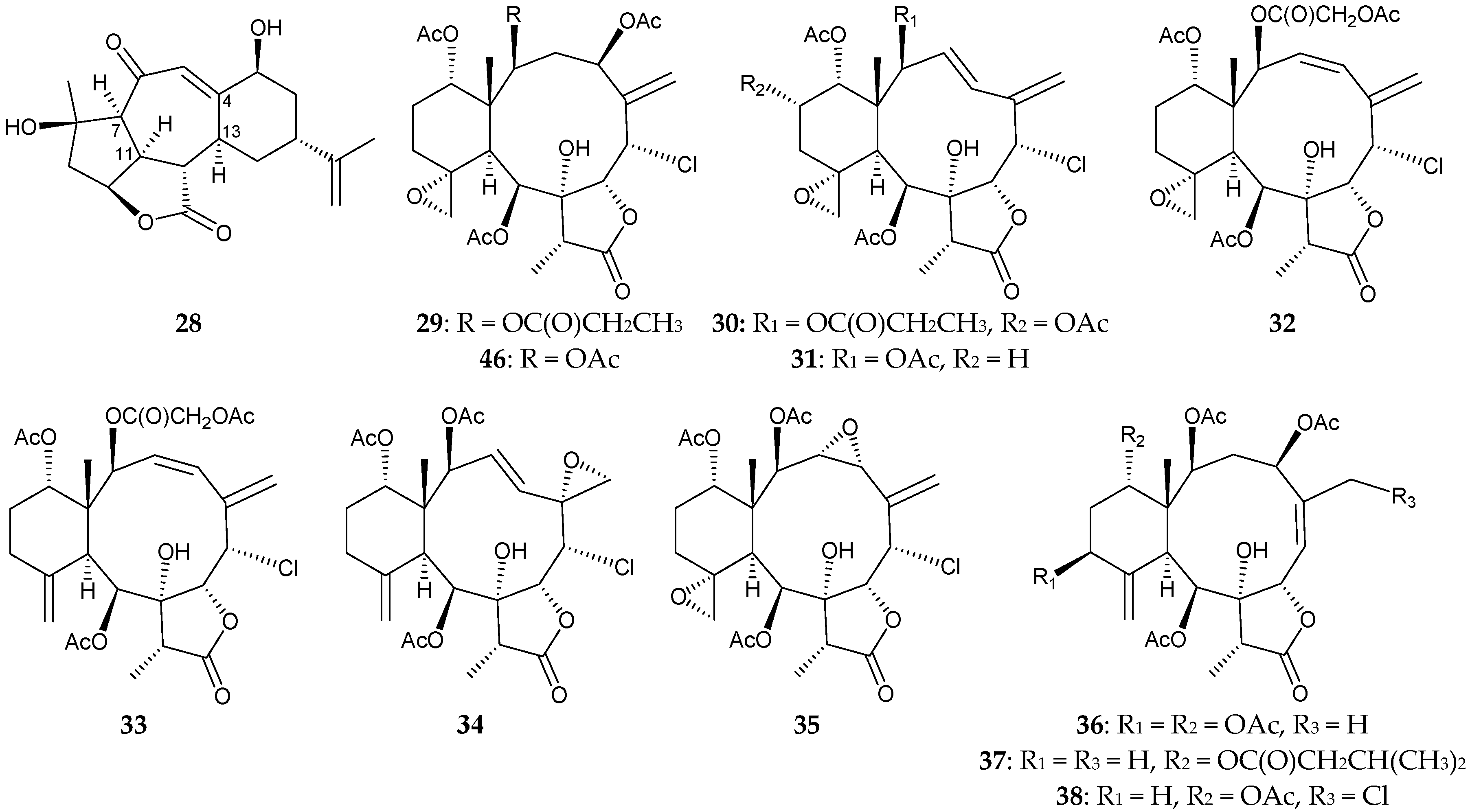
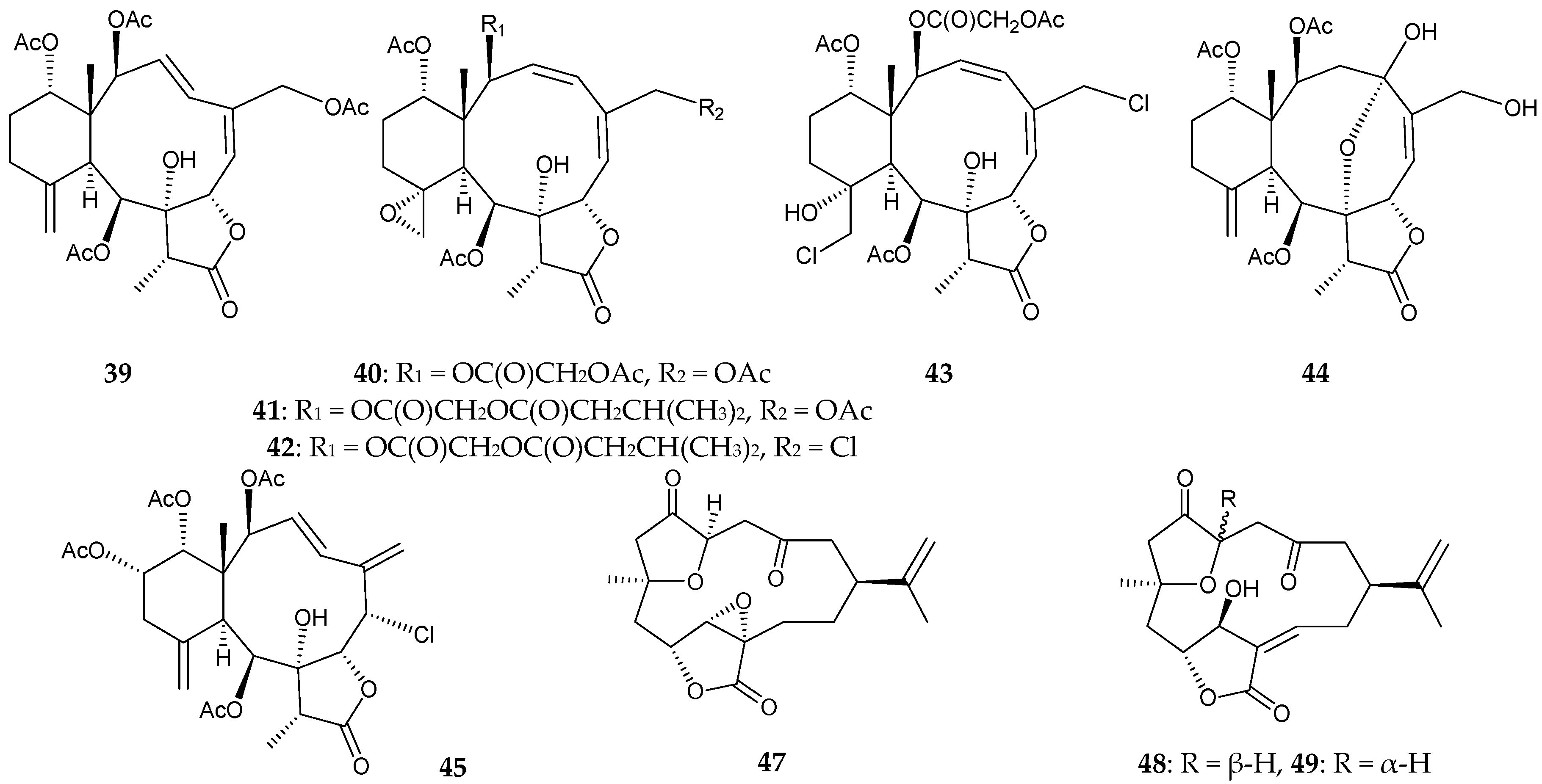
2.1. Junceella Fragilis
2.2. Junceella Gemmacea
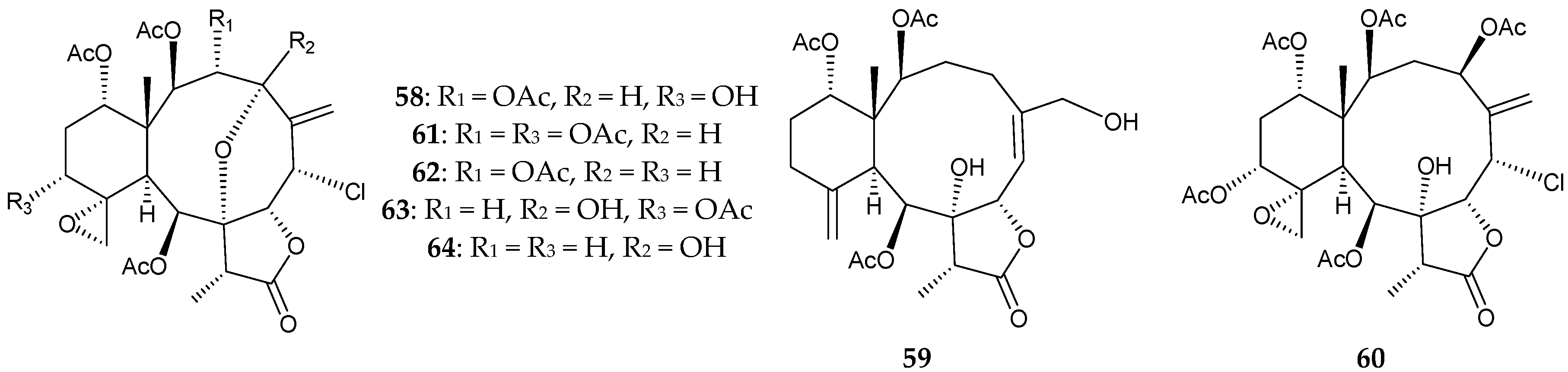
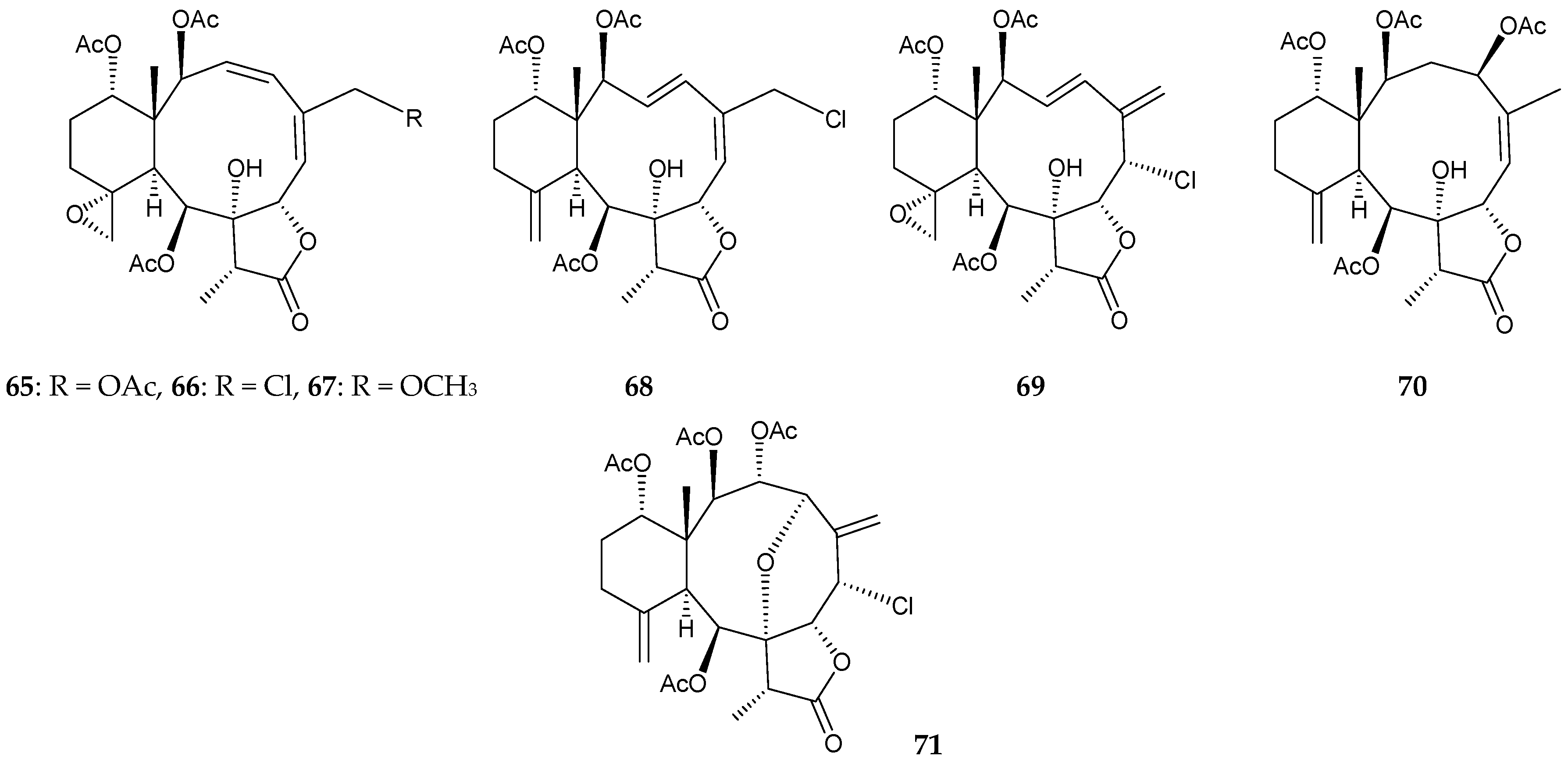
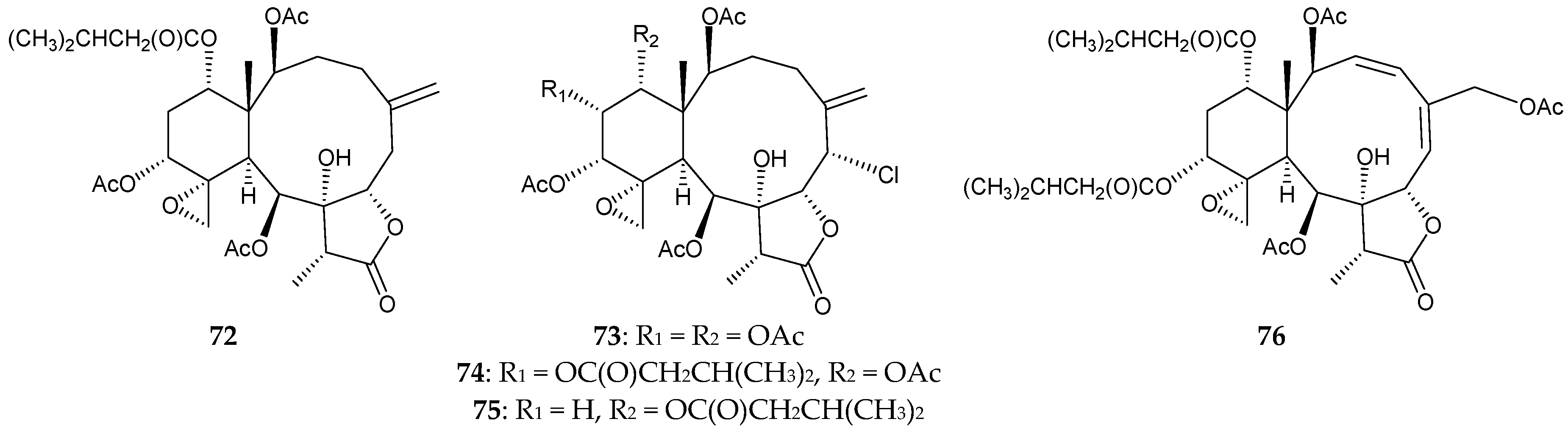
2.3. Junceella Juncea
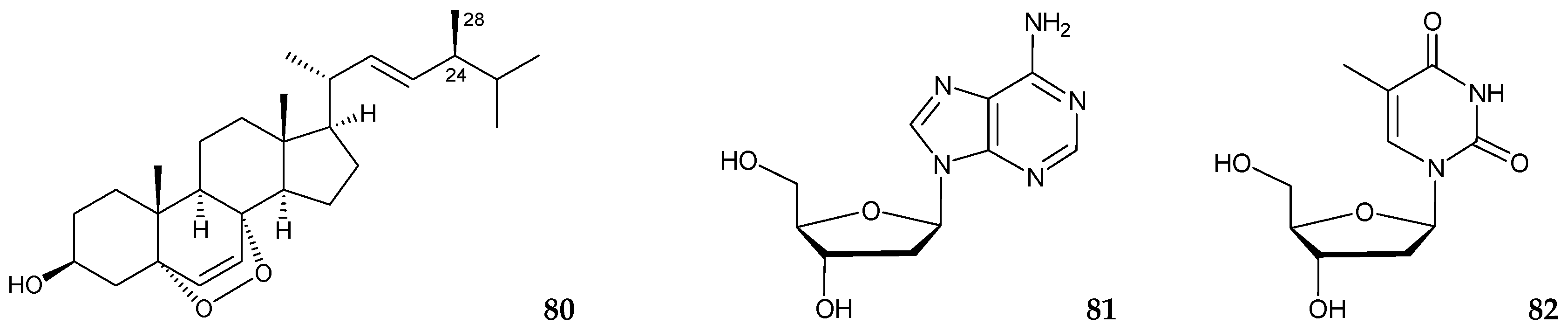
2.4. Junceella sp.
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bayer, F.M. Key to the genera of Octocorallia exclusive of Pennatulacea (Coelenterata: Anthozoa), with diagnoses of new taxa. Proc. Biol. Soc. Wash. 1981, 94, 902–947. [Google Scholar]
- Bayer, F.M.; Grasshoff, M. The genus group taxa of the family Ellisellidae, with clarification of the genera established by J. E. Gray (Cnidaria: Octocorallia). Senckenberg. Biol. 1994, 74, 21–45. [Google Scholar]
- Chen, C.-C.; Chang, K.-H. Gorgonacea (Coelenterata: Anthozoa: Octocorallia) of Southern Taiwan. Bull. Inst. Zool. Acad. Sin. 1991, 30, 149–181. [Google Scholar]
- Fabricius, K.; Alderslade, P. Soft Corals and Sea Fans—A Comprehensive Guide to the Tropical Shallow-Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea, 1st ed.; Australian Institute of Marine Science: Queensland, Australia, 2001; pp. 62, 230–231.
- Sung, P.-J.; Gwo, H.-H.; Fan, T.-Y.; Li, J.-J.; Dong, J.; Han, C.-C.; Wu, S.-L.; Fang, L.-S. Natural product chemistry of gorgonian corals of the genus Junceella. Biochem. Syst. Ecol. 2004, 32, 185–196. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Su, J.-H.; Chou, T.-T.; Cheng, Y.-P.; Weng, C.-F.; Lee, C.-H.; Fang, L.-S.; Wang, W.-H.; Li, J.-J.; Lu, M.-C.; et al. Natural product chemistry of gorgonian corals of genus Junceella. Mar. Drugs 2011, 9, 2773–2792. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, C.; Kulatheeswaran, R.; Ward, R.S. Briarane diterpenes from the Indian Ocean gorgonian Gorgonella umbraculum. J. Nat. Prod. 1998, 61, 1120–1122. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, C.; Ratnakumar, S.; Ward, R.S. Umbraculolides B–D, further briarane diterpenes from the gorgonian Gorgonella umbraculum. Tetrahedron 2000, 56, 4585–4588. [Google Scholar] [CrossRef]
- Shen, Y.-C.; Lin, Y.-C.; Ko, C.-L.; Wang, L.-T. New briaranes from the Taiwanese gorgonian Junceella juncea. J. Nat. Prod. 2003, 66, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Guo, Y.-W.; Mollo, E.; Cimino, G. Junceellonoids A and B, two new briarane diterpenoids from the Chinese gorgonian Junceella fragilis Ridley. Helv. Chim. Acta 2004, 87, 2341–2345. [Google Scholar] [CrossRef]
- Qi, S.-H.; Zhang, S.; Qian, P.-Y.; Xiao, Z.-H.; Li, M.-Y. Ten new antifouling briarane diterpenoids from the South China Sea gorgonian Junceella juncea. Tetrahedron 2006, 62, 9123–9130. [Google Scholar] [CrossRef]
- Liaw, C.-C.; Lin, Y.-C.; Lin, Y.-S.; Chen, C.-H.; Hwang, T.-L.; Shen, Y.-C. Four new briarane diterpenoids from Taiwanese gorgonian Junceella fragilis. Mar. Drugs 2013, 11, 2042–2053. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Sun, J.-F.; Han, Z.; Zhou, X.-F.; Yang, B.; Liu, Y. Fragilisinins A–L, new briarane-type diterpenoids from gorgonian Junceella fragilis. RSC Adv. 2014, 4, 5261–5271. [Google Scholar] [CrossRef]
- Shin, J.; Park, M.; Fenical, W. The junceellolides, new anti-inflammatory diterpenoids of the briarane class from the Chinese gorgonian Junceella fragilis. Tetrahedron 1989, 45, 1633–1638. [Google Scholar] [CrossRef]
- Sung, P.-J.; Pai, C.-H.; Su, Y.-D.; Hwang, T.-L.; Kuo, F.-W.; Fan, T.-Y.; Li, J.-J. New 8-hydroxybriarane diterpenoids from the gorgonians Junceella juncea and Junceella fragilis (Ellisellidae). Tetrahedron 2008, 64, 4224–4232. [Google Scholar] [CrossRef]
- Qi, S.-H.; Zhang, S.; Wen, Y.-M.; Xiao, Z.-H.; Li, Q.-X. New briaranes from the South China Sea gorgonian Junceella fragilis. Helv. Chim. Acta 2005, 88, 2349–2354. [Google Scholar] [CrossRef]
- Sung, P.-J.; Wang, S.-H.; Chiang, M.Y.; Su, Y.-D.; Chang, Y.-C.; Hu, W.-P.; Tai, C.-Y.; Liu, C.-Y. Discovery of new chlorinated briaranes from Junceella fragilis. Bull. Chem. Soc. Jpn. 2009, 82, 1426–1432. [Google Scholar] [CrossRef]
- Sung, P.-J.; Lin, M.-R.; Su, Y.-D.; Chiang, M.Y.; Hu, W.-P.; Su, J.-H.; Cheng, M.-C.; Hwang, T.-L.; Sheu, J.-H. New briaranes from the octocorals Briareum excavatum (Briareidae) and Junceella fragilis (Ellisellidae). Tetrahedron 2008, 64, 2596–2604. [Google Scholar] [CrossRef]
- Shen, Y.-C.; Chen, Y.-H.; Hwang, T.-L.; Guh, J.-H.; Khalil, A.T. Four new briarane diterpenoids from the gorgonian coral Junceella fragilis. Helv. Chim. Acta 2007, 90, 1391–1398. [Google Scholar] [CrossRef]
- Liaw, C.-C.; Shen, Y.-C.; Lin, Y.-S.; Hwang, T.-L.; Kuo, Y.-H.; Khalil, A.T. Frajunolides E–K, briarane diterpenes from Junceella fragilis. J. Nat. Prod. 2008, 71, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Liaw, C.-C.; Kuo, Y.-H.; Lin, Y.-S.; Hwang, T.-L.; Shen, Y.-C. Frajunolides L–O, four new 8- hydroxybriarane diterpenoids from the gorgonian Junceella fragilis. Mar. Drugs 2011, 9, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.-H.; Ahmed, A.F.; Shiue, R.-T.; Dai, C.-F.; Kuo, Y.-H. Scabrolides A–D, four new norditerpenoids isolated from the soft coral Sinularia scabra. J. Nat. Prod. 2002, 65, 1904–1908. [Google Scholar] [CrossRef] [PubMed]
- Shoji, N.; Umeyama, A.; Arihara, S. A novel norditerpenoid from the Okinawan soft coral Sinularia sp. J. Nat. Prod. 1993, 56, 1651–1653. [Google Scholar] [CrossRef]
- Bowden, B.F.; Coll, J.C.; Mitchell, S.J.; Mulder, J.; Stokie, G.J. Studies of Australian soft corals. IX A novel nor-diterpene from the soft coral Sinularia leptoclados. Aust. J. Chem. 1978, 31, 2049–2056. [Google Scholar] [CrossRef]
- Turner, K.E.; Bowden, B.F.; Stokie, G.J. (4R*,8S*,11R*,13S*,14R*)-8,11-Epoxy-14-hydroxy-11-methyl-4-(1-methylvinyl)-6,9-dioxocyclotetradec-1-ene-1,13-carbolactone. Acta Cryst. 1979, B35, 1283–1284. [Google Scholar] [CrossRef]
- Sato, A.; Fenical, W.; Zheng, Q.-T.; Clardy, J. Norcembrene diterpenoids from Pacific soft-corals of the genus Sinularia (Alcyonacea: Octocorallia). Tetrahedron 1985, 41, 4303–4308. [Google Scholar] [CrossRef]
- Cheng, W.; Ji, M.; Li, X.; Ren, J.; Yin, F.; van Ofwegen, L.; Yu, S.; Chen, X.; Lin, W. Fragilolides A–Q, norditerpenoid and briarane diterpenoids from the gorgonian coral Junceella fragilis. Tetrahedron 2017, 73, 2518–2528. [Google Scholar] [CrossRef]
- Wang, S.-H.; Chang, Y.-C.; Chiang, M.Y.; Chen, Y.-H.; Hwang, T.-L.; Weng, C.-F.; Sung, P.-J. Chlorinated briarane diterpenoids from the sea whip gorgonian corals Junceella fragilis and Ellisella robusta (Ellisellidae). Chem. Pharm. Bull. 2010, 58, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Kubota, N.K.; Kobayashi, Y.; Iwamoto, H.; Fukazawa, Y.; Uchio, Y. Two new halogenated briarane diterpenes from the Papuan gorgonian coral Junceella fragilis. Bull. Chem. Soc. Jpn. 2006, 79, 634–636. [Google Scholar] [CrossRef]
- Cheng, W.; Li, X.; Yin, F.; van Ofwegen, L.; Lin, W. Halogenated briarane diterpenes with acetyl migration from the gorgonian coral Junceella fragilis. Chem. Biodivers. 2017, 14, e1700053. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; La, M.-P.; Tang, H.; Pan, W.-H.; Sun, P.; Krohn, K.; Yi, Y.-H.; Li, L.; Zhang, W. Bioactive briarane diterpenoids from the South China Sea gorgonian Dichotella gemmacea. Bioorg. Med. Chem. Lett. 2012, 22, 4368–4372. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Long, K.; Fang, Z. Studies of the chemical constituents of the Chinese gorgonia (III). Isolation and identification of a new polyacetoxy chlorine-containing diterpene lactone (praelolide). Acta Sci. Nat. Univ. Sunyatseni 1983, 1, 83–92. [Google Scholar]
- Dai, J.; Wan, Z.; Rao, Z.; Liang, D.; Fang, Z.; Luo, Y.; Long, K. Molecular structure and absolute configuration of the diterpene lactone, praelolide. Sci. Sin. B 1985, 28, 1132–1142. [Google Scholar] [PubMed]
- Sung, P.-J.; Fan, T.-Y.; Fang, L.-S.; Wu, S.-L.; Li, J.-J.; Chen, M.-C.; Cheng, Y.-M.; Wang, G.-H. Briarane derivatives from the gorgonian coral Junceella fragilis. Chem. Pharm. Bull. 2003, 51, 1429–1431. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.-H.; Zhang, S.; Huang, H.; Xiao, Z.-H.; Huang, J.-S.; Li, Q.-X. New briaranes from the South China Sea gorgonian Junceella juncea. J. Nat. Prod. 2004, 67, 1907–1910. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Rodríguez, J.; Jiménez, C. Absolute structures of new briarane diterpenoids from Junceella fragilis. J. Nat. Prod. 1999, 62, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.-J.; Fan, T.-Y.; Chen, M.-C.; Fang, L.-S.; Lin, M.-R.; Chang, P.-C. Junceellin and praelolide, two briaranes from the gorgonian corals Junceella fragilis and Junceella juncea (Ellisellidae). Biochem. Syst. Ecol. 2004, 32, 111–113. [Google Scholar] [CrossRef]
- Zheng, L.-G.; Chang, Y.-C.; Hu, C.-C.; Wen, Z.-H.; Wu, Y.-C.; Sung, P.-J. Fragilides K and L, new briaranes from the gorgonian coral Junceella fragilis. Molecules 2018, 23, 1510. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Long, K. Studies of the chemical constituents of the Chinese gorgonia (IV)-Junceella, a new chlorine-containing diterpenoid from Junceella squamata. Acta Sci. Nat. Univ. Sunyatseni 1983, 2, 46–51. [Google Scholar]
- Yao, J.; Qian, J.; Fan, H.; Shin, K.; Huang, S.; Lin, Y.; Long, K. The structure of crystal and molecule of junceellin. Acta Sci. Nat. Univ. Sunyatseni 1984, 1, 83–87. [Google Scholar]
- Zhou, W.; Li, J.; E, H.-C.; Liu, B.-S.; Tang, H.; Gerwick, W.H.; Hua, H.-M.; Zhang, W. Briarane diterpenes from the South China Sea gorgonian coral, Junceella gemmacea. Mar. Drugs 2014, 12, 589–600. [Google Scholar] [CrossRef] [PubMed]
- He, H.-Y.; Faulkner, D.J. New chlorinated diterpenes from the gorgonian Junceella gemmacea. Tetrahedron 1991, 47, 3271–3280. [Google Scholar] [CrossRef]
- Hamann, M.T.; Harrison, K.N.; Carroll, A.R.; Scheuer, P.J. Briarane diterpenes from Micronesian gorgonians. Heterocycles 1996, 42, 325–331. [Google Scholar]
- Anjaneyulu, A.S.R.; Rao, N.S.K. Juncins G and H: New briarane diterpenoids of the Indian Ocean gorgonian Junceella juncea Pallas. J. Chem. Soc. Perkin Trans. 1997, 1, 959–962. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Rao, V.L.; Sastry, V.G.; Venugopal, M.J.R.V.; Schmitz, F.J. Juncins I–M, five new briarane diterpenoids from the Indian Ocean gorgonian Junceella juncea Pallas. J. Nat. Prod. 2003, 66, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Murthy, Y.L.N.; Rajack, D.M.A.; Reddy, G.D. A new antifungal briarane diterpenoid from the gorgonian Junceella juncea Pallas. Bioorg. Med. Chem. Lett. 2011, 21, 7522–7525. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-Y.; Liaw, C.-C.; Fazary, A.E.; Hwang, T.-L.; Shen, Y.-C. New briarane diterpenoids from the gorgonian coral Junceella juncea. Mar. Drugs 2012, 10, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Gauvin, A.; Smadja, J.; Aknin, M.; Faure, R.; Gaydou, E.-M. Isolation of bioactive 5α,8α-epidioxy sterols from the marine sponge Luffariella cf. variabilis. Can. J. Chem. 2000, 78, 986–992. [Google Scholar] [CrossRef]
- Kapustina, I.I.; Kalinovskii, A.I.; Dmitrenok, P.S.; Kuz’mich, A.S.; Nedashkovskaya, O.I.; Grebnev, B.B. Diterpenoids and other metabolites from the Vietnamese gorgonians Lophogorgia sp. and Junceella sp. Chem. Nat. Comp. 2014, 50, 1140–1142. [Google Scholar] [CrossRef]
- Wright, J.L.C.; McInnes, A.G.; Shimizu, S.; Smith, D.G.; Walter, J.A.; Idler, D.; Khalil, W. Identification of C-24 alkyl epimers of marine sterols by 13C nuclear magnetic resonances spectroscopy. Can. J. Chem. 1978, 56, 1898–1903. [Google Scholar]
- Orji, C.C.; Kelly, J.; Ashburn, D.A.; Silks, L.A., III. First synthesis of β-2′-deoxy[9-15N]adenosine. J. Chem. Soc. Perkin Trans. 1996, 1, 595–597. [Google Scholar] [CrossRef]
- Moyroud, E.; Biala, E.; Strazewski, P. Synthesis and enzymatic digestion of an RNA nonamer in both enantiomeric forms. Tetrahedron 2000, 56, 1475–1484. [Google Scholar] [CrossRef]
- Su, Y.-D.; Su, J.-H.; Hwang, T.-L.; Wen, Z.-H.; Sheu, J.-H.; Wu, Y.-C.; Sung, P.-J. Briarane diterpenoids isolated from octocorals between 2014 and 2016. Mar. Drugs 2017, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- Kokke, W.C.M.C.; Epstein, S.; Look, S.A.; Rau, G.H.; Fenical, W.; Djerassi, C. On the origin of terpenes in symbiotic associations between marine invertebrates and algae (zooxanthellae). J. Biol. Chem. 1984, 259, 8168–8173. [Google Scholar] [PubMed]
- Putra, M.Y.; Wibowo, J.T.; Murniasih, T. A review of chemistry and biological activities of the Indonesian octocorallia. J. Appl. Pharm. Sci. 2017, 7, 219–227. [Google Scholar]
- Yan, H.-Y. Harvesting drugs from the seas and how Taiwan could contribute to this effort. Changhua J. Med. 2004, 9, 1–6. [Google Scholar]
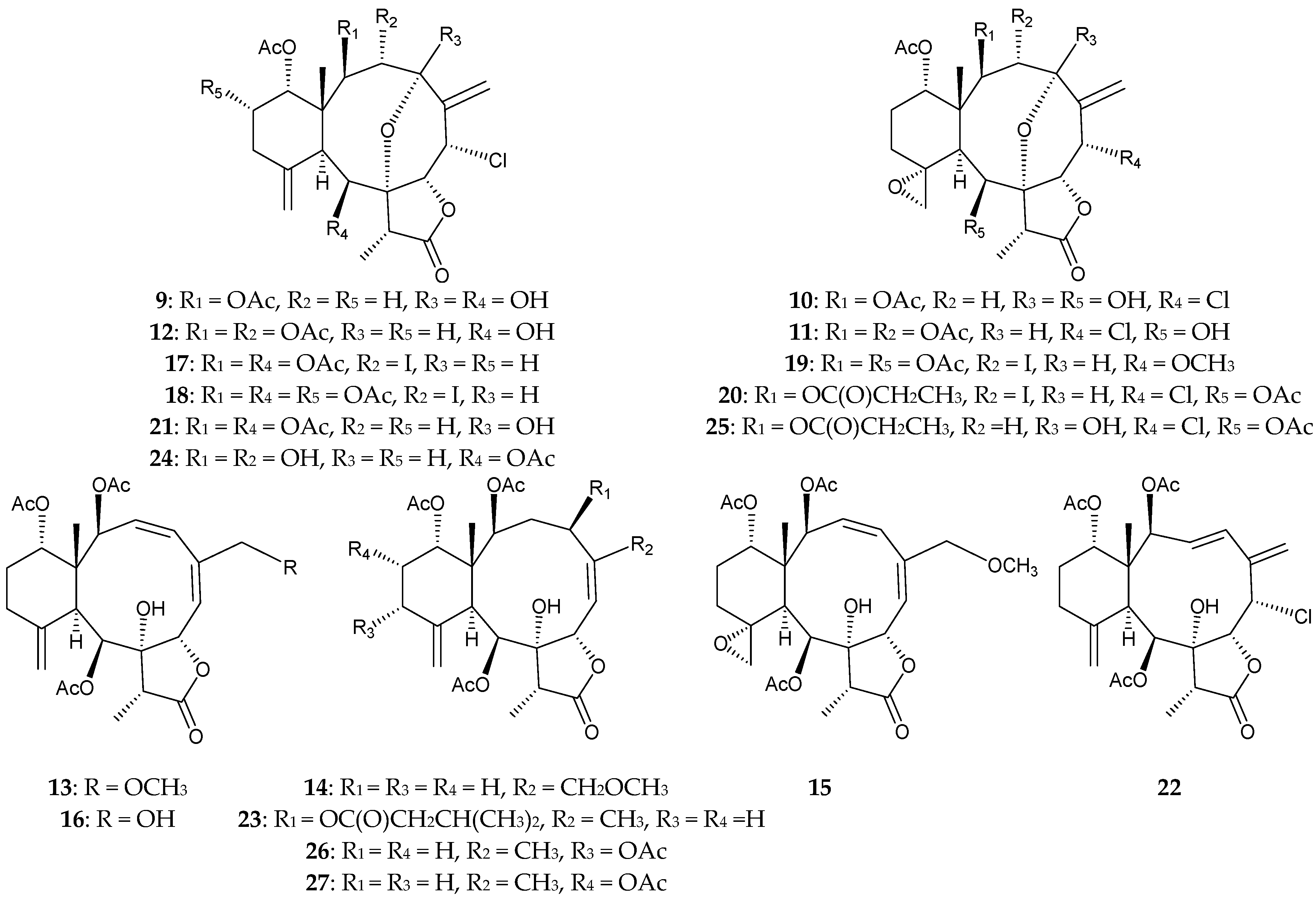
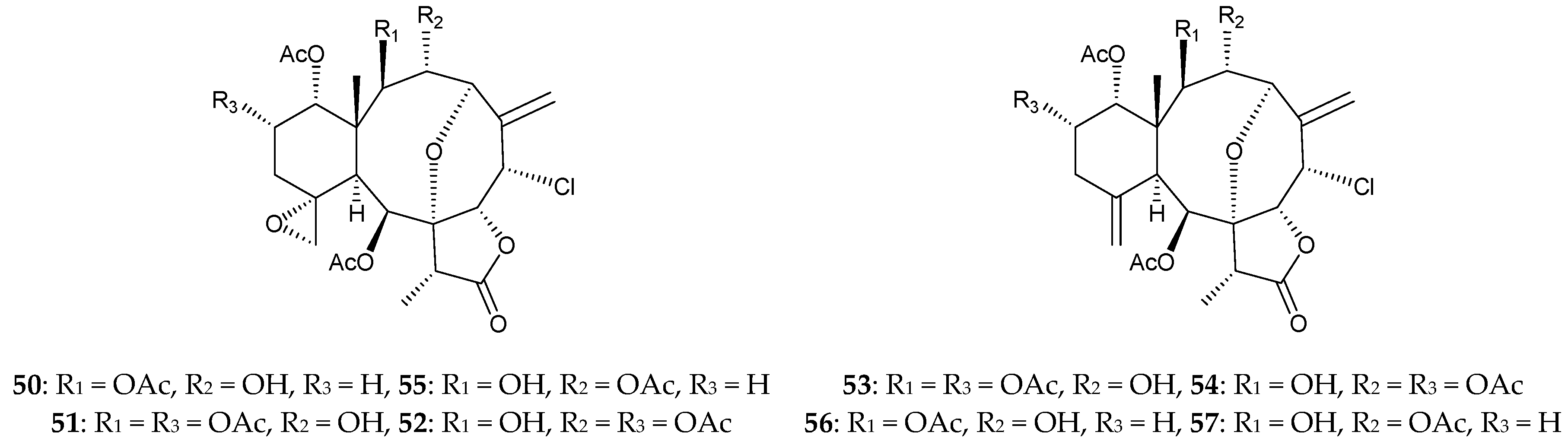

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, H.-M.; Wang, Y.-C.; Tseng, C.-C.; Chen, N.-F.; Wen, Z.-H.; Fang, L.-S.; Hwang, T.-L.; Wu, Y.-C.; Sung, P.-J. Natural Product Chemistry of Gorgonian Corals of Genus Junceella–Part III. Mar. Drugs 2018, 16, 339. https://doi.org/10.3390/md16090339
Chung H-M, Wang Y-C, Tseng C-C, Chen N-F, Wen Z-H, Fang L-S, Hwang T-L, Wu Y-C, Sung P-J. Natural Product Chemistry of Gorgonian Corals of Genus Junceella–Part III. Marine Drugs. 2018; 16(9):339. https://doi.org/10.3390/md16090339
Chicago/Turabian StyleChung, Hsu-Ming, Yi-Chen Wang, Chung-Chih Tseng, Nan-Fu Chen, Zhi-Hong Wen, Lee-Shing Fang, Tsong-Long Hwang, Yang-Chang Wu, and Ping-Jyun Sung. 2018. "Natural Product Chemistry of Gorgonian Corals of Genus Junceella–Part III" Marine Drugs 16, no. 9: 339. https://doi.org/10.3390/md16090339
APA StyleChung, H.-M., Wang, Y.-C., Tseng, C.-C., Chen, N.-F., Wen, Z.-H., Fang, L.-S., Hwang, T.-L., Wu, Y.-C., & Sung, P.-J. (2018). Natural Product Chemistry of Gorgonian Corals of Genus Junceella–Part III. Marine Drugs, 16(9), 339. https://doi.org/10.3390/md16090339






